Abstract
Two genera of terrestrial slugs (Arion and Geomalacus) display a striking disproportion in species richness in the Iberian Peninsula. While there are 17 Iberian endemic species in Arion, morphological criteria only recognize four species within Geomalacus. Sequence data were used to test whether these differences could result from: (1) cryptic diversity within Geomalacus; (2) an earlier origin for Arion (older clades are expected to accumulate more species); (3) distinct patterns of diversification rates (higher initial speciation rates in Arion), and (4) some combination of the above factors (e.g., an older clade with higher speciation rates). Species delimitation tests based on mitochondrial and nuclear data revealed eight cryptic lineages within Geomalacus that lessened the asymmetry; nevertheless, the disparity required further investigation. No meaningful differences in crown group ages of each recovered clade were found. Regardless the different premises of the two equally plausible diversification models (similar initial speciation rates vs. higher initial speciation rates in Geomalacus), both coincide on diversity-dependent diversification for the two groups but weaker rate declines in Arion best explains the observed asymmetry in species richness. Also, the broader environmental tolerance combined with a faster dispersal and wider distribution may have represented an evolutionary advantage for Arion.
Similar content being viewed by others
Introduction
Patterns of species richness are often unbalanced throughout the tree of life1. These disproportions may reflect differences in clade ages given the expectation that the number of species will increase through time, and therefore, older clades are expected to display more diversity, as long as extinction rates remain constant. Another possibility is the existence of shifts across phylogenies with clades evolving at different rates2. Differences in species richness across clades may, however, not reflect a real biodiversity bias. The existence of hidden diversity within an apparently species-poor clade may create an artificial disequilibrium. Cryptic species, defined as two morphologically indistinguishable taxa that are classified as a single nominal species3, represent a significant component of total biodiversity that is often underestimated when taxonomy relies exclusively on morphological characters4.
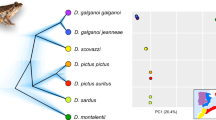
Mountain ecosystems offer vertical environmental gradients and frequently harbour higher levels of endemism than adjacent lowland areas5, particularly in species with reduced tolerance to desiccation and low dispersal abilities. In southern Europe, the Iberian Peninsula shows a mountain range orientation (east to west) that offers a wide range of climates (Alpine, Atlantic, Mediterranean and Desert) that promote speciation. The Iberian Peninsula shows an exceptionally high level of endemism among arionid slugs6. The genus Arion includes species with a Palearctic distribution throughout Europe with currently no less than 17 morphologically-defined, Iberian endemic species7,8,9,10. In contrast, the genus Geomalacus is endemic to the Iberian Peninsula and only four species are currently recognized viz. G. oliveirae, G. anguiformis, G. malagensis and G. maculosus 11,12,13,14 (Fig. 1). Geomalacus maculosus also occurs in Ireland but its presence is considered of anthropogenic origin15; therefore, its status as an Iberian endemic is retained in the present study. No other species of Geomalacus are found outside Iberia. The 17 Iberian endemic Arion represent about 53% of the European diversity; 32 species currently listed9,16 but this number is most likely underestimated.
Map showing sample locations of Geomalacus (further details on Supplementary material S3). Figure generated using the worldHires (http://CRAN.R-project.org/package=mapdata) function implemented in R language (R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/) (version 3.3.1) and edited in Adobe Illustrator CS6 (version 16.0.0) (http://www.adobe.com/products/illustrator.html).
The taxonomy of arionids is based on plastic characters such as body size and colour that in part may depend on diet, environment, and age17. Other characters include the anatomy of the reproductive apparatus, the interpretation of which is often difficult if not impossible in immature stages, often preventing the correct identification of juveniles18. While for the genus Arion there is a molecular phylogeny based on mitochondrial (NADH1) and nuclear (ITS1) sequence data7,19, the only molecular analyses of Geomalacus are focused on G. maculosus 15 or G. malagensis 20, with no data available for the remaining species within the genus.
In this study, we explored the following hypotheses to explain the differences in endemic species richness between two slug genera, Geomalacus and Arion: (1) cryptic diversity within Geomalacus; (2) differences in the crown group age of each genus; (3) distinct patterns of diversification rates (e.g. higher initial speciation rates in Arion), and (4) some combination of the above factors (e.g. an older clade with higher initial speciation rates). To do so, we performed tests to delimit species within Geomalacus using mitochondrial cytochrome oxidase subunit I (COI) and nuclear ribosomal small subunit (18 S rRNA) sequence data to assure the exact number of species within the clade. To estimate the crown group ages of both genera we included available COI sequences of Iberian endemic and non-endemic Arion. We analysed diversification rates over time for each genus, also testing which model of diversification fitted best the data.
Results
Species delimitation, phylogeny and dating
ABGD identified eight putative species within Iberian endemic Geomalacus: G. maculosus, G. anguiformis, G. malagensis and five within G. oliveirae. The spedeSTEM analysis (theta = 0.08) supported the existence of eight putative species within Iberian endemic Geomalacus (ln L = −10380.97, model likelihood = 1.0; Supplementary Table S1), the same as recovered by ABGD. Values for single and multiple AIC calculations are identical.
Within-group estimates of average divergence within Geomalacus based on COI data ranged between 0% (G. oliveirae - Peña de Francia) and 6.8% (G. maculosus) Net divergence between groups ranged from 11.4% (between G. oliveirae - Peña de Francia and G. oliveirae - Gredos) to 20.6% (between G. maculosus and G. oliveirae - Montes de Toledo) (Table 1).
The maximum likelihood phylogeny based on the Geomalacus COI data set revealed five well-supported clades within the nominal species Geomalacus oliveirae that correspond to the species defined by both ABGD and spedeSTEM species delimitation methods (Fig. 2). G. oliveirae is recovered as paraphyletic due to the inclusion of G. anguiformis (Fig. 2). The BI topology was similar to the ML tree, except that includes all five G. oliveirae lineages on a single clade albeit with relatively low statistical support (BPP: 0.7; Fig. 2). BI analyses of the combined data set yielded similar results to the BI COI-based topology (Supplementary Figure S1).
Phylogenetic relationships of Geomalacus based on a maximum likelihood analysis of 100 unique COI haplotypes from 21 sampling sites. Tip labels are haplotype codes and the number of sequences of each haplotype. Numbers at the nodes represent ML bootstrap values (top) and Bayesian posterior probabilities (bottom) for major clades. Colour bars indicate geographic origins. Maps indicate sample locations for each Geomalacus species and the corresponding colour for the geographic origin of each sample. Phylogenetic tree generated by FigTree http://tree.bio.ed.ac.uk/software/figtree and edited in Adobe Illustrator CS6 (version 16.0.0) (http://www.adobe.com/products/illustrator.html). Maps generated using the worldHires (http://CRAN.R-project.org/package=mapdata) function implemented in R language (R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/) (version 3.3.1) and edited in Adobe Illustrator CS6 (version 16.0.0) (http://www.adobe.com/products/illustrator.html).
The BI tree depicting the phylogenetic relationships within Arionidae based on COI sequence data is shown in the Supplementary Figure S2. While there is a single clade of Geomalacus, the 26 species of Arion grouped in two main clades that did not cluster together. One of two subclades of Arion (clade I; Supplementary Figure S2) is sister to Geomalacus. Iberian endemic Arion are not monophyletic as clade I included all Iberian endemic Arion plus six non-endemic species. The other clade of Arion (clade II) comprised non-endemic species only.
The estimated crown group ages of each clade recovered in the Beast analysis are the following: Geomalacus −9.85 [95% HPD: 7.8–12.34] myr; Arion clade I - 11.02 [95% HPD: 8.58–13.85] myr; Arion clade II - 11.28 [95% HPD: 8.63–14.34] myr (Fig. 3A).
(A) Beast maximum clade credibility chronogram showing main cladogenetic events within two genera of terrestrial slugs (Arion and Geomalacus) based on a fragment of the mitochondrial COI gene. Black circles represent Iberian endemic species. Clade I includes all 11 Iberian endemic Arion plus six non-endemic Arion species whereas clade II includes eight non-endemic Iberian Arion species. Age estimates in million years and corresponding 95% highest posterior density intervals (values in square brackets) are depicted; (B) Lineage through time plots (LTT) obtained from the empirical Beast timetree after pruning clade II that included non-endemic Arion only. Solid line represents the LTT plot. Orange lines represent the LTT plots from 1000 simulations using the CorSim approach taking into account missing species. Dashed green line represents the mean LTT plot and the area enclosed by stippled lines indicated the 95% CI for 1000 trees simulated under a pure-birth model; (C) Lineage through time plots (LTT) from the empirical Beast timetree including Geomalacus only. Solid line represents the LTT plot. The area enclosed by stippled lines indicated the 95% CI for 1000 trees simulated under a pure-birth mode. No CorSim simulations were performed because the genus was completely sampled; (D) Net diversification rate (r) vs. carrying-capacity (K) plots from the best-fit diversity dependent model on top (r 0A ≈ r 0G, KA, KG) and the second best-fit model on bottom (r 0A, r 0G, KA, KG). Tree figure generated by FigTree http://tree.bio.ed.ac.uk/software/figtree, LTT plots and r vs K produced in R (https://cran.r-project.org/ and edited in Adobe Illustrator CS6 (version 16.0.0) (http://www.adobe.com/products/illustrator.html).
Macroevolutionary dynamics
The convex shape of the semi-logarithmic LTT plots derived from the Arion and Geomalacus trees (Fig. 3B and C) suggest a non-constant diversification rate. The γ-statistic for the Arion tree differed significantly from a model of constant rate diversification (γ = −2.99, p < 0.001). Moreover, to discard the influence of incomplete sampling due to the existence of Arion species not included in the phylogeny, we used the γ-statistic to simulate a null distribution of γ (assuming a constant rate pure-birth process) with 1000 replicates and assuming an incompletely sampled phylogeny with a possible total number of 25, 35 and 40 Arion species. The simulation returned a γ-critical value of −2.05 (p = 0.005) for 25 missing species, −2.31 (p = 0.010) for 35 missing species, and −2.47 (p = 0.015) for 40 missing species. The endemic Geomalacus phylogeny, including Letourneuxia numidica, and with no missing species, yielded a γ-value of −2.38 (p = 0.009). Therefore, all results rejected the hypothesis of a constant rate of diversification with p < 0.05, indicating that the negative γ values are significant when compared with the null model of the constant diversification rate, irrespective of the effect of taxon sampling.
To assess if diversification within the two clades of arionids (Geomalacus and the clade corresponding to all Iberian endemic Arion plus six non-endemic species, clade I) conformed with a constant rate or has changed over time, we used BAMM and maximum likelihood to fit the branching times derived from our dated tree to a variety of lineage diversification models. Bamm analyses using the ultrametric tree obtained with Beast showed no significant rate shifts (Supplementary Figure S3). Shift posterior distributions for 901 analysed samples (after burn-in) were the following: shift posterior distributions: zero shifts, p = 0.83; one shift, p = 0.13; two shifts, p = 0.03; three shifts, p = 0.004; four shifts, p = 0.002. The model that fitted best the data was the pure-birth constrained model (λ Arion = λ Geomalacus) (Table 2). To test for the potential effects of diversity-dependence, we compared the fit of pure-birth, birth-death with linear diversity-dependent (DDL), exponential diversity-dependent (DDX) and yule-2-rate models. We tested these models first, which do not account for missing species, as a preliminary test for diversity-dependence. Results showed that DDL was the model that fitted best for both Arion and Geomalacus clades (Tables 3 and 4). Furthermore, the diversity-dependent diversification model (DDD) is the model that fitted best, when including incomplete taxon sampling in Arion diversification (Table 5).
Given the high support obtained for diversity-dependent models against the null hypothesis of diversity independent speciation rate (with or without sampling fraction), we evaluated six linear diversity-dependent, null extinction models accounting for incomplete sampling. The best-supported model was model 3 (Table 6) with diversity-dependent cladogenesis, identical initial diversification rates for both clades (r 0A = r 0G), and independently estimated carrying capacities (K) for both clades. The second-best model (∆ AIC = 1.46) indicates diversity-dependent cladogenesis, different initial diversification rates for both clades (r 0A ≠ r 0G), and independently estimated carrying capacities (K) for both clades. Models 4 to 6 with clade-dependent K values received substantially less support (∆ AIC > 20) than models with genera-independent K’s (∆ AIC < 6). To account for a larger number of missing taxa, we have performed all estimations increasing the missing species to 23, yielding a total of 40 Arion species. The best performing model is also model 3 (Supplementary Table S2).
Discussion
Current information about species richness of Iberian endemic terrestrial slugs suggests an apparent striking disparity between the two sister clades, Geomalacus and Arion clade I that includes all 11 Iberian endemics plus six non-endemic species. While 17 Iberian endemic species are listed within Arion, only four species are reported for Geomalacus. Establishing the number of Iberian endemic Arion was not trivial because species classification is largely based on morphology that is most probably affected by the existence of uncovered diversity and possibly conspecifics, not evaluated in the present work. Given these caveats, we judge the 17 Iberian endemic species to be a balanced list, subject however to future changes. Our species delimitation tests indicated the existence of five, morphologically similar, putative species within Geomalacus oliveirae, which raises to eight the number of lineages within the genus. Seven out of the eight putative Geomalacus species show a distribution predominantly associated with mountain regions. Geomalacus anguiformis is reported only from mountain areas of southern Iberia (Caldeirão, Monchique and Aracena), and each of the five lineages recovered within G. oliveirae are restricted to a single mountain area in central Iberia (Serra da Estrela, Peña de Francia, Gredos, Guadalupe or Montes de Toledo). Mountain areas have long been recognized as biodiversity hotspots due to their ecological heterogeneity21. For instance, a recent study showed a radiation of freshwater gastropods inhabiting the Iberian mountain ranges strongly connected to the geographic evolution of river basins22. Our results suggest that G. oliveirae has undergone an allopatric speciation process most likely driven by fragmentation of the suitable habitat that restricted its distribution to mountain areas. Species with fragmented distributions typically show reduced gene flow among populations23, which may promote speciation. We did not detect cryptic diversity within G. anguiformis but the relatively deep divergence between specimens of Aracena (Fig. 2) and the remaining mountain locations suggests that this species may be undergoing a speciation process.
The disparity between the Iberian endemic arionids is lessened if the cryptic diversity within Geomalacus is interpreted to represent valid species; nevertheless, the differences are still significant (Arion: 17 species vs. Geomalacus: 8 species) and other factors must be exerting control on species diversity. Although there are no quantitative observations on their ecological behaviour, anecdotal observations (C. Patrão, pers. obsv.) revealed the existence of several characteristics of Arion slugs that may represent an evolutionary advantage: (1) are more generalist than Geomalacus in their feeding; (2) some Arion species seem to disperse faster; (3) have wider environmental tolerance (unlike Geomalacus, Arion may survive in areas with no running water), and (4) show a wider geographic distribution. Ecological niche modelling further supports the narrower environmental tolerance of Geomalacus 14.
It is a reasonable expectation to assume that in extant clades higher species richness should correlate with clade age because older clades had more time to accumulate species24. Nevertheless, the three clades of arionid slugs retrieved in the dating analysis did not show differences in their crown-group ages that justify such disproportion in endemic species richness (Geomalacus: 9.85 myr; Arion clade I that includes all Iberian endemics plus six non-endemic lineages: 11.02 myr; Arion clade II that comprises non-endemic species only: 11.28 myr).
Another hypothesis that could explain the observed differences could be distinct diversification rate dynamics25. BAMM indicated absence of rate shifts within the three clades and macroevolutionary analyses supported two equally plausible models (ΔAIC ≤ 2) both showing diversity-dependent diversification and null extinction within Arion (clade I) and Geomalacus that do not explain differences in species richness. The diversity-dependent models imply a decrease in the speciation rate towards the present, as the number of species increases26 and niche space becomes saturated. However, the two equally-supported models indicated variable carrying capacities for both genera and a weaker rate decline for Arion that may provide an explanation for the higher number of species shown by this genus. The only difference between the two equally-plausible models concerns the initial speciation rate; while model 2 shows higher initial rates for Geomalacus and a stronger rate decline throughout time, model 3 specifies similar initial speciation rates for both groups. Nevertheless, both models clearly justify the higher number of Arion (and/or the lower number of Geomalacus species).
The higher diversity originated in the early history of Arion and Geomalacus is most likely an important reason for the observed slowdown towards the present. The two genera may overlap in food requirements, but the broader environmental tolerance combined with a faster dispersal and wider distribution may have represented an evolutionary advantage for Arion. The fragmented geographic distribution of Geomalacus in the mountain areas might have promoted a higher initial speciation rate because absence of gene flow between isolated populations but as niche space becomes saturated, a stronger decline in speciation rates is expected, which is supported by model 2. The interspersed inclusion of all Iberian endemic Arion with other non-endemic species in clade I suggests multiple colonizations of the Iberia Peninsula that could ultimately lead to more Arion species (Fig. 3).
Differences in Iberian endemic species richness between two genera of terrestrial slugs were not caused by a significantly earlier origin of the more specious genus (Arion). Regardless the differences between the two equally plausible diversification models (similar initial speciation rates vs. higher initial speciation rates in Geomalacus), both coincide in a weaker rate decline in Arion and diversity-dependent diversification for both groups. A broader environmental tolerance combined with faster dispersal and wider distribution allowed multiple colonizations of the Iberian Peninsula that further explain the higher species richness of Arion in this geographical area.
Methods
Taxon sampling
For the present study, we have chosen species endemic from the Iberian Peninsula biogeographical province, including the Pyrenees endemics. The choice of Geomalacus species was straightforward, as apart from G. maculosus, which apparently was recently introduced in Ireland accidentally and human assisted15, the genus only exists in Iberia. A total of 492 specimens representing the four nominal Geomalacus species were collected from 49 localities between 2007 and 2010 (Fig. 1 and Supplementary Tables S3 and S5). Geomalacus was identified following Castillejo et al.12. Foot tissue was preserved in 96% ethanol and stored at −20 °C. After perusing the existing literature7,27, elected species within Arion were chosen. The list of species includes 17 Arion species (Electronic supplementary Table S4), for which molecular data only exists for 10 of those species. Additionally, the Moroccan species Letourneuxia numidica was included for the dating analysis (see section Dating analysis).
DNA extraction, amplification and sequencing
Total genomic DNA of the 492 individuals was extracted with a chelex buffer using a modified protocol of Walsh et al.28: foot tissue was added to 250 µL of 5% (w/v) solution of chelex with 5 μL of Proteinase K, and incubated with thorough mixing at 55 °C for 60 min, followed by a 20 min incubation at 95 °C. The samples were then placed on ice for 2 min and centrifuged at 11,000 rpm for 3 min. The supernatant containing genomic DNA was removed and used directly as a template in downstream polymerase chain Reaction (PCR) analysis. Amplifications of a 750 bp fragment of the mitochondrial DNA COI gene were obtained using primers LCO1490 and HCO219829. Additionally, for a subset of individuals representing the major evolutionary lineages inferred by species delimitation tests, a 700 bp-fragment of the small subunit nuclear ribosomal gene (18 S) was amplified and sequenced with the primers 4F18S and 1R18S30. PCR profiles were: one cycle of 5 min at 95 °C, 40 cycles of 40 s at 95 °C, 40 s at 40 °C and 1 min at 72 °C and a last elongation step of 5 min at 72 °C. PCR amplifications were performed in a total 25 µl reaction volume of 1X buffer, 2 mM MgCl2, 0.2 mM DNTP’s, 0.2 µM of each primer and 1U Taq DNA polymerase Promega (Madison, USA). Amplification products were purified by ethanol/sodium acetate precipitation and directly sequenced using the corresponding PCR primers. Sequencing was performed on an ABI 3130xl (Applied Biosystems) automated sequencer using the BigDye Deoxy Terminator cycle-sequencing kit (Applied Biosystems) following manufacturer’s instructions. All new sequences were deposited in GenBank (Supplementary Tables S3 and S5).
Phylogenetic analyses
Geomalacus
DNA sequences were aligned with Mafft v. 7.24531 using the “—auto” option that automatically selects the appropriate strategy according to data size. Uncorrected, pairwise COI nucleotide diversities within and between the Geomalacus species were estimated in the software package MEGA 6.0.6 32. Identical haplotypes were collapsed with DNACOLLAPSER (http://users-birc.au.dk/biopv/php/fabox/dnacollapser.php). Two data sets were used in all phylogenetic analyses: (1) COI data set including all unique Geomalacus haplotypes (100 sequences, 594 bp); (2) the combined data set that included only individuals sequenced both for COI and the nuclear 18 S fragment (74 sequences, COI: 594 bp and 18 S: 524 bp).
Bayesian Inference (BI) analyses based on the COI and the combined data sets were performed with MrBayes v. 3.2.133. The BI analysis of the COI data set was analysed under the GTR + I + Γ, the best-fit model selected by Modeltest 34. BI analysis of the combined data set included two data partitions: COI and 18 S. Each partition was analysed according to the best-fit model selected by Modeltest (COI: GTR + I + Γ; 18 S: JC + I). Model and model parameters were estimated independently for each of the data partitions using the unlink command in MrBayes. Analyses accommodated among-partition rate variation through use of the “prset applyto = (all) ratepr = variable;” command in MrBayes. All analyses were run for 1 × 107 generations (four simultaneous Markov chains; 1 × 103 sample frequency) following a discarded burn-in of 10%. The convergence to the stationary distributions was confirmed by inspection of the MCMC samples using Tracer v. 1.635. Arion ater was selected as outgroup.
Maximum Likelihood (ML) analyses based on the COI and the combined data sets were performed with RAxML v. 8.2.836. COI-based analyses were run under the GTRGAMMA evolutionary model. Individual α-shape parameters, GTR-rates, and empirical base frequencies were estimated and optimized for each partition of the combined data set (COI and 18 S) used in this analysis. For both analyses, the best-scoring ML tree was determined from 100 randomized maximum-parsimony starting trees using the rapid hill-climbing algorithm and 1 × 103 bootstrap replicates were drawn on the best-scored ML tree using the exhaustive bootstrap algorithm. Arion ater was selected as outgroup.
Arionidae
Bayesian Inference (BI) analysis was performed in Beast v1.8.437 to analyse the phylogenetic relationships within the family Arionidae. We used COI sequences (42 taxa; 523 bp) that represent one individual per species of Geomalacus recovered by ABGD and spedeSTEM, 26 Arion species from which 11 are Iberian endemic, and all genera of Arionidae available in the GeneBank (accession numbers in Table S4). MCMC analyses were run for 100,000,000 generations with a sample frequency of 10,000, following a discarded burn-in of 10,000,000 steps. The convergence to the stationary distributions was confirmed by inspection of the MCMC samples using Tracer v1.635.
Species delimitation in Geomalacus
Given the asymmetry in species richness between Arion and Geomalacus we suspected of the existence of cryptic diversity within the latter; therefore, we used ABGD (Automatic Barcode Gap Discovery)38 and the COI data set to delimit species within Geomalacus. ABGD is based on the identification of the barcode gap, i.e. a larger divergence among individuals of different species than among conspecific individuals. We selected the Jukes-Cantor model and the X - value of 0.9 (minimum relative barcoding gap width), and prior intraspecific divergences ranging from Pmin = 0.001 to Pmax = 0.12 to run the barcode gap analysis.
We also used spedeSTEM39,40 to further analyse the number of putative species within Geomalacus. This method calculates the maximum likelihood species tree from all hierarchical arrangements of the sampled alleles and uses information theory to quantify the model probability of each permutation. Specifically, the probabilities of multiple permutations of putative evolutionary lineages are calculated using STEM 2.041. It returns a table of models ranked according to their probability. Ultrametric trees for each gene partition are required and a user-supplied estimate of θ. We used DnaSP v.542 to compute the average theta for the two loci, and Beast2.443 to infer both trees (separately) based on the COI and 18 S data sets. MCMC analyses were run for 1 × 108 generations with a sample frequency of 10,000, following a discarded burn-in of 1 × 107 steps. The convergence to the stationary distributions was confirmed by inspection of the MCMC samples using Tracer v1.6.
Evolutionary rates through time
To explore how the variation of diversification rates (speciation and extinction) affected the observed differences in species richness, we must evaluate three main hypotheses: (1) differences in the crown group age of each genera; (2) distinct patterns of diversification rates (e.g. higher initial speciation rates in Arion and/or diversity-dependence), and (3) a combination of the two (e.g. an older clade with higher initial and diversity dependence with weaker decline in diversification rates).
A Bayesian relaxed molecular-clock approach implemented in Beast v1.8.437 addresses the first hypothesis by estimating the crown group ages of Geomalacus and Arion. To perform this analysis, we used COI sequences (37 taxa; 543 bp) from one individual per species of Geomalacus recovered by ABGD and spedeSTEM and COI sequences from all Arion species available in the GeneBank (accession numbers in Table S1). We also included the Moroccan species Letourneuxia numidica because estimates of divergence are usually based upon well-known historical events, geologic or fossil, which can be used as calibration points to estimate taxon-specific mutation rates44. Yet, as there are no reliable arionid fossils, we used the opening of the Strait of Gibraltar [5.96–5.35 million years (myr) ago45], to calibrate the divergence between G. malagensis and L. numidica. Lehmannia valentiana and Limacus flavus, two Stylommatophorans from the superfamily Limacoidea were selected as outgroups46.
After testing different tree (Yule and Birth-Death) and clock (uncorrelated lognormal and strict) priors we have chosen a Birth-Death Incomplete Sampling tree prior and placed a normal distribution in the divergence of G. malagensis and L. numidica, considered more appropriate when using biogeographic events to calibrate the tree47. Parameters were: mean in real space, M = 5.645 and standard deviation, S = 0.315. MCMC analyses were run for 100,000,000 generations with a sample frequency of 10,000, following a discarded burn-in of 10,000,000 steps. The convergence to the stationary distributions was confirmed by inspection of the MCMC samples using Tracer v1.6.
To address hypotheses (2) and (3) we used the ultrametric tree obtained from the Beast analysis from which the Moroccan species Letourneuxia numidica was pruned and only Arion clade I (includes all 11 Iberian endemic Arion plus six non-endemic Arion species) was considered because we were interested in analysing through time variation of the diversification rates of Iberian endemic arionids. We used the R package48 laser 49 to compute the γ-statistic50 and phytools 51 to construct the lineage through time (LTT) plots, for each clade. For complete phylogenies, γ = ±1.645 represents the critical value of the constant-rate test50. Values outside this interval reject the null hypothesis of the Yule pure-birth model (no extinction). This approach yields a null distribution of values against which the empirical γ is compared using the Monte Carlo constant rates (MCCR) test statistic50. However, the result of the “gamStat” function (laser package) does not take into account incomplete sampling. Therefore, we used the estimated γ-statistic and simulated 10,000 replicates to account for these missing taxa, using “mccrTest” function also available in laser 49. This procedure, randomly prunes the number of missing taxa from each replicated tree, to simulate incomplete taxa sampling. A null distribution of the γ-statistic is generated from these replicates, and the observed γ-statistic from the previous test is compared to this distribution.
We used BAMM-Bayesian analysis of Macroevolutionary Mixture52 to detect heterogeneity in speciation rates across the time-calibrated tree obtained with Beast while accounting for non-random incomplete sampling (we used useGlobalSamplingProbability = 0 and then set the corresponding sampling fractions in the file sample_probs.txt). We set four rj-MCMC runs for 200 × 106 generations sampled every 200 × 103 generations. We used the function “setBAMMpriors” in R to select more appropriate prior values and ESS (effective sample size) to assess the convergence of the runs and considered values above 200 as indicating convergence53.
Additionally, to handle with incomplete taxon sampling in Arion clade I, we applied the “CorSiM” (“Correction by Simulating Missing splits”) approach54 using the TreeSim R-package55 to simulate 1000 trees under a constant speciation and extinction rate. “CorSiM” requires as input data a speciation and extinction rates that we obtained from the best-fit lineage diversification model (see Results).
To further evaluate hypotheses (2) and (3), we compared constant genera-decoupled rate models, i.e., models that evaluate simultaneously separate parameters for each clade, to assess if constant speciation rates (λ), and constant extinction rates (μ) are homogeneous: pure-birth (λ Arion ≠ λ Geomalacus), constrained pure-birth (λ Arion ≈ λ Geomalacus) and birth–death models (λ, μ Arion ≠ λ, μ Geomalacus)56 (see Supplementary Text S1 for details). Because the selected best-fit model based on these parameters (constrained pure birth, see Results and Box 1 from Supplementary information for further details) did not explain difference in species richness, we had to evaluate models that contemplate variable speciation rates such as diversity-dependence while assuming extinction rate to be zero: (1) linear diversity-dependent speciation rate (DDL) and exponential diversity-dependent declining speciation rates as a function of extant lineages (DDX)49; (2) single shift in speciation rate at a certain time (yule2rate); (3) diversity-dependent diversification model (DDD)57 and (4) time-varying speciation only (SPVAR)56 (see Supplementary Text S1 for details).
As the best-fit model indicates a diversity-dependent diversification (see Results and Box 1 from Supplementary information), we proceeded to evaluate clade decoupling models, i.e., models which evaluate simultaneously separate parameters for each genus. We used the R package DDD 57 that evaluates six different diversity-dependent models (see details in Supplementary Text S1). These models were tested using the following R-packages48: laser v2.449 (function: “fitdAICrc”); diversitree V.0.956 (functions: “make.bd.split”, “make.bd.t”) and DDD v.3.426,57,58 (functions: “dd_ML”, “dd_KI_ML”). (see Supplementary Text S1 for details). The best supported models were those with low Akaike scores, and that deviate from the best model by less than two units (i.e., ∆AIC < 259.
All analyses were performed on the R2C2 research group cluster facility, provided by the IT department of the University of Algarve.
References
Xu, H. et al. Disentangling the determinants of species richness of vascular plants and mammals from national to regional scales. Sci Rep 6, 21988, https://doi.org/10.1038/srep21988 (2016).
Rabosky, D. L. et al. Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat Commun 4 (2013).
Bickford, D. et al. Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22, 148–155 (2007).
Linkem, C. W., Hesed, K. M., Diesmos, A. C. & Brown, R. M. Species boundaries and cryptic lineage diversity in a Philippine forest skink complex (Reptilia; Squamata; Scincidae: Lygosominae). Mol Phylog Evol 56, 572–585 (2010).
Spehn, E. M., Rudmann-Maurer, K. & Körner, C. Mountain biodiversity and global change, http://agris.fao.org/agris-search/search.do?recordID=XF2015000742 (2010).
Castillejo, J. B. & de la Peninsula Ibérica, I. Los arionidos. Catalogo crítico y Mapas de Distribución. Iberus 9, 331–345 (1990).
Quinteiro, J., Rodríguez‐Castro, J., Castillejo, J., Iglesias‐Piñeiro, J. & Rey‐Méndez, M. Phylogeny of slug species of the genus Arion: evidence of monophyly of Iberian endemics and of the existence of relict species in Pyrenean refuges. J Zool Syst Evol Res 43, 139–148 (2005).
Backeljau, T. The original diagnoses of Arion simrothi Künkel and Arion magnus Torres Minguez (Mollusca, Pulmonata). Annls Soc R Zool Belg 2, 199–211 (1989).
Backeljau, T., Winter, A. J., Martin, R., Rodriguez, T. & Bruyn, L. D. Genital and allozyme similarity between Arion urbiae and A. anguloi (Mollusca: Pulmonata). Zool J Linn Soc 110, 1–18 (1994).
Bank, R. Fauna Europaea Project. Checklist of the land and freshwater Gastropoda of the Iberian Peninsula (Spain, Portugal, Andorra, Gibraltar) http://www.nmbe.ch/sites/default/files/uploads/pubinv/fauna_europaea_-_gastropoda_of_iberian_peninsula.pdf (2011).
Rodríguez, T., Ondina, P., Outeiro, A. & Castillejo, J. Slugs of Portugal. III. revision of the Genus Geomalacus Allman, 1843 (Gastropoda: Pulmonata: Arionidae). The Veliger 36, 145–159 (1993).
Castillejo, J., Garrido, C. & Iglesias, J. The slugs of the genus Geomalacus Allman, 1843, from the Iberian Peninsula (Gastropoda: Pulmonata: Arionidae). Basteria 58, 15–26 (1994).
Wiktor, A. & Norris, A. Geomalacus malagensis sp. n. and some remarks concerning generic level systematics of Arionidae (Gastropoda, Pulmonata). J Conchol 34, 19–29 (1991).
Patrão, C. et al. Habitat suitability modelling of four terrestrial slug species in the Iberian Peninsula (Arionidae: Geomalacus species). J Moll Stud 81, 427–434, https://doi.org/10.1093/mollus/eyv018 (2015).
Reich, I. et al. Genetic study reveals close link between Irish and Northern Spanish specimens of the protected Lusitanian slug Geomalacus maculosus. Biol J Linn Soc 116, 156–168 (2015).
Bank, R. et al. Fauna europaea project. Malacologia 47, 397 (2001).
Jordaens, K., van Riel, P., Geenen, S., Verhagen, R. & Backeljau, T. Food-induced body pigmentation questions the taxonomic value of colour in the self-fertilizing slug Carinarion spp. J Moll Stud 67, 161–167 (2001).
Backeljau, T. & De Bruyn, L. On the infrageneric systematics of the genus Arion Férussac, 1819 (Mollusca, Pulmonata). Bull l’Inst R Sci Nat Belg 60, 35–68 (1990).
Rowson, B., Anderson, R., Turner, J. A. & Symondson, W. O. The slugs of Britain and Ireland: undetected and undescribed species increase a well-studied, economically important fauna by more than 20%. PloS one 9, e91907 (2014).
Patrão, C., Jordaens, K., Castilho, R. & Backeljau, T. In World Congress of Malacology (ed Somsak Panha) 180 (Chulalongkorn University, Phuket, Thailand, 2010).
Orme, C. D. L. et al. Global hotspots of species richness are not congruent with endemism or threat. Nature 436, 1016–1019 (2005).
Delicado, D., Machordom, A. & Ramos, M. A. Living on the mountains: patterns and causes of diversification in the springsnail subgenus Pseudamnicola (Corrosella)(Mollusca: Caenogastropoda: Hydrobiidae). Mol Phylogenet Evol 68, 387–397 (2013).
Willi, Y., Van Buskirk, J. & Hoffmann, A. A. Limits to the adaptive potential of small populations. Annu Rev Ecol Evol Syst 433–458 (2006).
McPeek, M. A. & Brown, J. M. Clade age and not diversification rate explains species richness among animal taxa. Am Nat 169, E97–E106 (2007).
Ricklefs, R. E. Estimating diversification rates from phylogenetic information. Trends Ecol Evol 22, 601–610 (2007).
Etienne, R. S., Pigot, A. L. & Phillimore, A. B. How reliably can we infer diversity‐dependent diversification from phylogenies? Methods Ecol. Evol. 7, 1092–1099 (2016).
Castillejo, J. Guía de las babosas Ibéricas. 154 (Real Academia Galega de Ciências, 1998).
Walsh, P. S., Metzger, D. A. & Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10, 506–513 (1991).
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech 3, 294–297 (1994).
Winnepenninckx, B., Backeljau, T. & Dewachter, R. Small ribosomal-subunit rna and the phylogeny of mollusca. Nautilus 108, 98–110 (1994).
Katoh, K. & Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26, 1899–1900 (2010).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30, 2725–2729 (2013).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61, 539–542 (2012).
Posada, D. & Crandall, E. D. Modeltest: testing the model of DNA substitution. Bioinformatics 14, 817–818 (1998).
Tracer version 1. 6, Available from http://beast.bio.ed.ac.uk/Tracer (2014).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012).
Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol 21, 1864–1877 (2012).
Ence, D. D. & Carstens, B. C. SpedeSTEM: a rapid and accurate method for species delimitation. Mol Ecol Res 11, 473–480 (2011).
Satler, J. D., Carstens, B. C. & Hedin, M. Multilocus species delimitation in a complex of morphologically conserved trapdoor spiders (Mygalomorphae, Antrodiaetidae, Aliatypus). Syst Biol 62, 805–823 (2013).
Kubatko, L. S., Carstens, B. C. & Knowles, L. L. STEM: species tree estimation using maximum likelihood for gene trees under coalescence. Bioinformatics 25, 971–973 (2009).
Librado, P. & Rozas, J. DnaSPv5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009).
Bouckaert, R. et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comp Biol 10, e1003537 (2014).
McCormack, J. E., Heled, J., Delaney, K. S., Peterson, A. T. & Knowles, L. L. Calibrating divergence times on species trees versus gene trees: implications for speciation history of Aphelocoma jays. Evolution 65, 184–202 (2011).
Duggen, S., Hoernle, K., Van Den Bogaard, P., Rüpke, L. & Morgan, J. P. Deep roots of the Messinian salinity crisis. Nature 422, 602–606 (2003).
Wade, C. M., Mordan, P. B. & Clarke, B. A phylogeny of the land snails (Gastropoda: Pulmonata). P Roy Soc Lond B Bio 268, 413–422 (2001).
Heath, T. A. Divergence Time Estimation using BEASTv2.2. 0, https://taming-the-beast.github.io/tutorials/FBD-tutorial/FBD-tutorial.pdf (2015).
R: a language and environment for statistical computing. R Foundation for Statistical Computing (Vienna, Austria, 2016).
Rabosky, D. L. LASER: a maximum likelihood toolkit for detecting temporal shifts in diversification rates from molecular phylogenies. Evolutionary bioinformatics online 2, 247 (2006).
Pybus, O. G. & Harvey, P. H. Testing macro-evolutionary models using incomplete molecular phylogenies. Proc R Soc Lond B Biol Sci 267, 2267–2272 (2000).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol. 3, 217–223 (2012).
Rabosky, D. L. BAMMtools: an R package for the analysis of evolutionary dynamics on phylogenetic trees. J Am Stat Assoc 90, 773–795 (2014).
Drummond, A. & Bouckaert, R. R. Bayesian evolutionary analysis with Beast 33 p. (Cambridge University Press, 2015).
Cusimano, N., Stadler, T. & Renner, S. S. A new method for handling missing species in diversification analysis applicable to randomly or nonrandomly sampled phylogenies. Syst. Biol. 61, 785–792 (2012).
Stadler, T. Simulating trees with a fixed number of extant species. Syst Biol 60, 676–684 (2011).
FitzJohn, R. Diversitree: comparative phylogenetic analyses of diversification in R. Methods Ecol Evol. 3, 1084–1092 (2012).
Etienne, R. S. & Haegeman, B. A conceptual and statistical framework for adaptive radiations with a key role for diversity dependence. The American Naturalist 180, E75–E89 (2012).
Etienne, R. S. et al. Diversity-dependence brings molecular phylogenies closer to agreement with the fossil record. Proc R Soc Lond B Biol Sci 279, 1300–1309 (2012).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference. A Practical Information-Theoretic Approach. (Springer Verlag, 2002).
Acknowledgements
We are very thankful to the anonymous reviewers #1 and #3 that greatly contributed for improving the earlier versions of this manuscript. We are particularly grateful to reviewer #1 for providing a script that allowed the analysis of diversification rates through time. We thank Thierry Backeljau and Kurt Jordaens for intense and fruitful discussions on earlier versions of this manuscript. We are very grateful to Andrés Parada for providing R scripts for the CorSim approach. RLC was supported by a post-doctoral fellowship (SFRH/BPD/109685/2015) and CP was supported by a doctoral fellowship (SFRH/BD/30024/2006) respectively, from FCT (Fundação para a Ciência e Tecnologia, Portugal) and FSE (Fundo Social Europeu). The work was funded by RC funds from FCT strategic plan UID/Multi/04326/2013 granted to CCMAR.
Author information
Authors and Affiliations
Contributions
C.P. and R.C. conceived the study. C.P. collected the samples and conducted molecular work. R.L.C. and R.C. analysed data. R.C. prepared figures and C.P. prepared Tables. R.L.C. and R.C. wrote the manuscript. All authors contributed with their ideas and reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cunha, R.L., Patrão, C. & Castilho, R. Different diversity-dependent declines in speciation rate unbalances species richness in terrestrial slugs. Sci Rep 7, 16198 (2017). https://doi.org/10.1038/s41598-017-16417-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16417-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.