Abstract
Whether low dose alteplase is comparable to standard dose in efficacy and safety for intravenous thrombolysis (IVT) in Asian stroke patients remains unverified. PubMed, EMBASE, and Cochrane Library Database from the beginning to June 30, 2017 were searched. IVT efficacy was measured by favorable outcome (modified Rankin Scale scores of 0–1) at 3 months, and safety measured by mortality within 3 months and symptomatic intracerebral hemorrhage (SICH). Pooled estimates were conducted using fixed- or random-effects model depending on heterogeneity. For SICH, studies were pooled separately according to different definitions. Twelve studies involving 7,905 participants were included. No association was found between alteplase dose and favorable outcome (OR = 0.94, 95% CI 0.78–1.14, P = 0.5; heterogeneity: P hetero = 0.01, I2 = 57.3%) and mortality (OR = 0.87, 95% CI 0.74–1.02, P = 0.08; P hetero = 0.83, I2 = 0) using random- and fixed-effects models, respectively. Low dose alteplase was associated with lower SICH as defined by the National Institute of Neurological Disorders and Stroke study (OR = 0.79, 95% CI 0.64–0.99, P = 0.04; P hetero = 0.57, I2 = 0) using fixed-effects model. Subgroup and sensitivity analysis could change the results significantly. Current limited evidence was insufficient to support the speculation that low dose alteplase was comparable to standard dose in thrombolytic efficacy and safety in Asian stroke patients.
Similar content being viewed by others
Introduction
Intravenous thrombolysis (IVT) with alteplase (recombinant tissue plasminogen activator, rt-PA) has been the standard of care for acute ischemic stroke (AIS) since it was approved by the Food and Drug Administration (FDA) in 19961,2. The current recommended dose of rt-PA in the United States and Europe is 0.9 mg/kg with a maximum dose of 90 mg1. This recommendation was based on two small dose-escalation trials done in Western countries in the early 1990s3,4. However, rt-PA is not without potential adverse consequences, one of the most serious being an increased risk of intracerebral hemorrhage1,5,6. And compared to patients from Western countries, Asian patients may have higher risk of intracerebral hemorrhage after thrombolysis7,8. Based on the results from the Safe Implementation of Thrombolysis in Stroke-Monitoring study (SITS-MOST) in 2007 and the Safe Implementation of Thrombolysis in Stroke-Non-European Union World (SITS-NEW) study in 2014, the incidence of SICH ranged from 1.66% to 7.27% in European population and from 1.87% to 8.70% in Asian population according to different criteria9,10. This increased risk may be attributed to a difference in blood coagulation-fibrinolysis factors, intracranial atherosclerotic diseases, or other physical conditions8,11.
Following the Japan Alteplase Clinical Trial (J-ACT), a series of single-arm trials were conducted in Japan and demonstrated that AIS patients receiving rt-PA at a dose of 0.6 mg/kg could obtain comparable efficacy and safety to historical controls given 0.9 mg/kg rt-PA12,13,14,15,16. Consequently, the Japanese drug safety authority has approved rt-PA to be administered at a dose of 0.6 mg/kg. It has also become increasingly accepted in other Asian countries to administer a lower dose of rt-PA to AIS patients. However, although many cohort studies directly comparing the efficacy and safety of rt-PA at different doses have been conducted since 2006, results were inconsistent among studies17,18,19,20,21,22,23,24,25,26,27,28,29,30,31. A meta-analysis published in 2015 demonstrated that low dose rt-PA (<0.85 mg/kg) was comparable to standard dose (0.85 mg/kg) in terms of efficacy and safety for IVT32. But since then, a randomized study conducted by Anderson et al. using data from international multicenter Enhanced Control of Hypertension and Thrombolysis Stroke (ENCHANTED) study, which is the largest randomized trial published to date involving predominantly Asian patients, demonstrated that IVT at a dose of 0.6 mg/kg caused significantly lower risk of symptomatic intracerebral hemorrhages (SICH), but also showed a downward trend in disability-free survival at 3 months when compared to IVT at a dose of 0.9 mg/kg19. Thus, whether low dose rt-PA has a better or at least comparable efficacy and safety profile to the standard dose remains unclear.
Almost all studies on this topic were conducted in Asia. Although many studies from Western countries have concentrated on IVT outcomes in patients who are overweight or obesity, or explored the impact of inaccurate weight assessment on IVT outcome33,34,35,36,37,38,39,40,41,42,43,44, there is a lack of studies that perform a head-to-head comparison of the efficacy and safety of different doses of rt-PA. We therefore conducted a meta-analysis to assess the association between rt-PA dose and IVT outcomes for AIS patients, particularly in Asian population.
Methods
Ethical approval was not necessary for the present study due to no patient involvement. The study was conducted in accordance with the MOOSE (meta-analysis of observational studies in epidemiology protocol) guidelines45.
Literature Search Strategy
A systematic search of the existing literature was performed using PubMed (1976 to June 30, 2017), EMBASE (1982 to June 30, 2017), and the Cochrane Library Database (1987 to June 30, 2017) with no language restriction. Search terms included “stroke”, “ischemic stroke”, “cerebral infarction”, “brain infarction”, “thrombolysis”, “thrombolytic”, “tissue plasminogen activator”, “alteplase”, “rt-PA”, “t-PA”, “dose” and “dosing”. The references from all included studies or relevant reviews were screened to avoid accidental omission.
Inclusion Criteria
Included studies met the following criteria: (1) type of study: prospective or retrospective study; (2) study population: patients with AIS receiving IVT with rt-PA; (3) comparison: head-to-head comparison in outcomes between different doses; (4) outcome measures: IVT efficacy measured by favorable outcome (modified Rankin Scale [mRS] scores of 0–1) at 3 months, and IVT safety measured by 3-months mortality and SICH after thrombolysis. SICH was defined by criteria from the European Cooperative Acute Stroke Study (ECASS) II46, ECASS III2, National Institute of Neurological Disorders and Stroke study (NINDS)1, and Safe Implementation of Thrombolysis in Stroke-Monitoring study (SITS-MOST)9, or by criteria defined by the authors themselves in their researches22,29,30.
Data Extraction and Quality Assessment
A standardized data collection sheet was used to extract data. The following information was collected: publication reference, first author, year of publication, region, number of study centers, design, method of data collection, gender distribution of participants, loss to follow-up, dose of rt-PA, number of cases, age at stroke onset, baseline National Institute of Health Stroke Scale (NIHSS) score, time interval from stroke onset to IVT, and outcome measures (favorable outcome, mortality, and SICH) (Supplementary Table 1). As for SICH, when one study reported the results according to both ECASS II and ECASS III definitions, we would only extract the data of SICH as defined by ECASS II criteria.
Quality of the included studies was assessed using the Newcastle-Ottawa Scales (NOS) items (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
Two investigators (G.T., H.W.) independently selected eligible studies, extracted data, and assessed the quality of articles. Any disagreement between the two investigators was resolved by discussion with the help of a third investigator (L.L.). If there was unavailable data or uncertain information in any of the included studies, the authors would be contacted.
Group Assignment
IVT with rt-PA at a dose of 0.85 mg/kg was reported to have similar efficacy as that at a dose of 0.95 mg/kg, and SICH seldom occurred with rt-PA doses of ≤0.85 mg/kg3,4. We therefore selected 0.85 mg/kg as the cut-off point and divided patients into the low dose (<0.85 mg/kg) or the standard dose group (≥0.85 mg/kg).
Statistical Analysis
The numbers of patients with the outcomes of interest in the low dose and standard dose groups were extracted. The odds ratio (OR) of low to standard dose with 95% confidential interval (CI) was calculated for each included study. Pooled ORs with 95% CI were calculated from fixed- or random-effects model depending on the heterogeneity among studies. The between-study heterogeneity was assessed by Cochran Q test and I2. For Cochran Q test, heterogeneity was considered to be statistically significant when P hetero < 0.1. For I2, no evidence of heterogeneity was defined as I2 of 0, low level of heterogeneity as I2 of <25%, moderate level of heterogeneity as I2 of 25–50%, and high level of heterogeneity as I2 of >50%. A fixed-effects model was applied when P hetero > 0.1 or I2 < 50%, and a random-effects model was also conducted to evaluate the stability of the results. For SICH, a pooled estimate was conducted by separately synthesizing studies according to different definitions (i.e. ECASS, NINDS, or SITS-MOST).
A subgroup analysis was conducted based on the number of recruited centers, i.e. multicenter (>3 centers) versus non-multicenter. Another subgroup analysis with non-Asian patients excluded were also conducted. Sensitivity analyses, in addition to the switching between a fixed- and random-effects model, were performed as follows: assessing the influence of a single study on the pooled estimate by eliminating one study each time, or assessing the stability of the pooled estimate by removing some of the studies from the meta-analysis according to different exclusion criteria each round.
Potential publication biases were roughly assessed by visual inspection of funnel plots and further identified by Egger’s linear regression test. A P-value < 0.05 was considered statistically significant.
All statistical analyses were conducted using the STATA software package (version 14.0, Stata Corporation, College Station, TX, USA).
Results
Literature Search and Selection
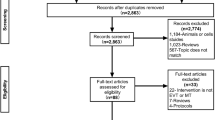
The literature search and selection process are depicted in the flow diagram (Fig. 1). A total of 5,826 citations were initially retrieved. Of them, 5,787 studies were removed by reviewing the title or abstract, leaving 39 studies to be reviewed by the full-text article. Of the 39 studies, 21 were eliminated for not fulfilling the inclusion criteria. Of the remaining 18 studies, 7 were excluded (1 for focusing on patients with only mild stroke47, 1 for only including 2 patients who received low dose rt-PA48, 2 for having unavailable data even after contacting the author49,50, and 3 for using the data that were completely included in another one with a larger sample size17,19,20,24,28). Eleven studies were included in the final meta-analysis18,19,21,22,23,24,25,26,27,29,30.
Among authors contacted for data unavailable or information uncertain, Anderson and his colleague assured us that both Robinson’s study in 2017 and their study in 2016 were conducted using the same data from ENCHANTED study, and participants in Robinson’s study were entirely included in their study17,19. They also supplied us the data of favorable outcomes at 3 months for Asian patients. Chao et al. replied and acknowledged that data from their 2010 publication was included in whole in another paper published in 201424,28. And the participants in Ho’s study was identified to be the same as that in Chao’s 2010 publication by comparing the data between them20,28. Ong et al. supplied us data for outcomes of interest and rate of loss to follow-up at 3 months18. Abraham et al. responded to our data request, but could not supply the missing information on outcomes due to the retrospective design of the study49. No reply was obtained from the rest of authors contacted.
Study Characteristics and Quality
General characteristics of included studies published from 2010 to 2017, are summarized in Supplementary Table 1. Ten studies were conducted in Asia, with 7 from China, and 1 each from Korean, Singapore and Vietnam. The remaining one was performed using an international, multicenter database with 63.2% of the patients being from Asia. Sample size among the studies ranged from 83 to 3,297, with a total of 7,905 participants. The dose of rt-PA across the studies ranged from 0.5 mg/kg to >0.95 mg/kg. The cut-off points of rt-PA dose and groups that participants were divided into were various across included studies. There was a significant between-group difference in gender in 5 studies22,23,25,27,29, age in 3 studies18,24,30, time interval from stroke onset to IVT in 2 studies21,24, and baseline NIHSS score in 1 study30. One study had no significant differences in baseline information between the groups being compared19.
NOS scores of the included studies are summarized in Supplementary Table 2 and, ranged from 6 to 9 with a mean of 7.7.
Pooled Estimates for Outcomes
Favorable outcome at 3 months was reported in 11 studies, 7 of which showed a fewer favorable outcome in those receiving the low dose rather than standard dose rt-PA, with one study reaching statistical significance. The remaining 4 studies showed results to the contrary with two study reaching statistical significance. Heterogeneity across the studies was high (P hetero = 0.01, I2 = 57.3%). Using a random-effects model, there was no significant difference in favorable outcome between low dose and standard dose (OR = 0.94, 95% CI 0.78–1.14, P = 0.5), as shown in Table 1 and Supplementary Figure 1.
Eleven studies reported mortality within 3 months. None of them showed a significant difference between low dose and standard dose rt-PA. Heterogeneity across the studies was low (P hetero = 0.83, I2 = 0), and no significant difference was found from a fixed-effects model (OR = 0.87, 95% CI 0.74–1.02, P = 0.08), as shown in Table 1 and Supplementary Figure 2.
As shown in Supplementary Table 1, SICH was identified in 6 studies per ECASS definitions, with 4 per ECASS II definition18,19,23,24, and 2 per ECASS III definition25,27. SICH was also identified in 6 studies per NINDS definition19,23,24,25,26,27 and 4 studies per SITS-MOST definition19,23,24,26. In 3 other studies, SICH was identified per author-defined criteria22,29,30, which was nearly the same as the ECASS II definition. One study had zero cases of intracerebral hemorrhage occur21. Thus, pooled analyses for SICH were conducted separately based on ECASS (ECASS II, ECASS III, and author-defined criteria), SITS-MOST, and NINDS (Table 1, Fig. 2, and Supplementary Figures 3 and 4). When adopting the NINDS definition, no evidence of heterogeneity was found (P hetero = 0.57, I2 = 0), and SICH was significantly lower in those receiving low dose rt-PA than in those receiving standard dose from both fixed- and random-effects models (both: OR = 0.79, 95% CI 0.64–0.99, P = 0.04).
Subgroup Analysis
When only combining results from multicenter studies, the pooled estimate for favorable outcome had no heterogeneity (P hetero = 0.61, I2 = 0), and was statistically significant from both fixed- and random-effects models (both: OR = 0.88, 95% CI 0.80–0.98, P = 0.02). For SICH defined by NINDS, there was no heterogeneity (P hetero = 0.39, I2 = 0), and the pooled estimate was statistically significant using both fixed- and random-effects models (OR = 0.77, 95% CI 0.61–0.96, P = 0.02; OR = 0.77, 95% CI 0.61–0.97, P = 0.02, respectively). But no statistical significance was found for any outcome in non-multicenter studies (Table 1). When removing the non-Asian patients in Anderson’s study19 from meta-analysis, no significant difference was found between low dose and standard dose group in all outcomes (Table 2).
Sensitivity Analysis
The variation and range of the pooled ORs after removing a single study from the meta-analysis and repeating the process multiple times are listed in Supplementary Table 3.
The stability of the pooled estimate was assessed by removing some studies from the meta-analysis based on various exclusion criteria each time; this stability analysis is summarized in Supplementary Table 4. The pooled estimate for favorable outcome, mortality and SICH as defined by NINDS changed significantly depending on some of the exclusion criteria that was used.
Publication Bias
No substantial asymmetry was found in the funnel plot (Fig. 3). The Egger’s linear regression test indicated no publication bias for favorable outcome, mortality and SICH defined by ECASS (P = 0.592, 0.996 and 0.558, respectively). Publication bias for SICH as defined by NINIDS or SITS-MOST was not assessed, due to the small number of included studies (far less than 10).
Discussion
We herein present a meta-analysis assessing the difference in IVT efficacy and safety between low dose and standard dose rt-PA, and found that low dose rt-PA may be related to fewer favorable outcomes at 3 months, and a lower incidence of SICH as defined by the NINDS. These results, however, lack stability.
A prior meta-analysis with 10 cohort studies involving 4,348 cases was conducted by Liu et al. in 2015 and, demonstrated that there was no significant difference in favorable outcome between low dose (<0.85 mg/kg) and standard dose (0.85–0.95 mg/kg) rt-PA using a random-effects model32. Two of the 10 cohort studies presented in Liu’s study were excluded from our meta-analysis: one for data overlapping entirely with another study of larger sample size24,28, and one for having only 2 patents receive low dose rt-PA48. The addition of 3 studies published recently18,19,21 allowed us to combine 11 studies involving 7,598 patients to evaluate the effect of rt-PA dose on favorable outcome. The pooled estimate showed that there was no significant difference in favorable outcome between low dose and standard dose rt-PA using a random-effects model, which was consistent with the prior meta-analysis.
Although only 4 studies met the criteria of multicenter research for favorable outcome, participants in these studies accounted for 84.7% (6438/7598) of all19,22,23,24. We therefore conducted a subgroup analysis that considered only multicenter studies, and found that standard dose rt-PA was significantly associated with an increased chance of favorable outcome using both fixed- and random-effects models. Additionally, sensitivity analysis demonstrated that pooled estimates for favorable outcome could reach a statistical significance according to some exclusion criteria (Supplementary Tables 3 and 4). Thus, it seemed that the association between standard dose rt-PA and increasing favorable outcome could not be denied by present results. But when interpreting these finding, we need to take into account that most of the included studies were conducted with nonrandomized design, which would cause a non-ignorable selection bias, where patients in low dose may have a fewer chance of favorable outcome. Since in clinical practices, patients at a higher risk of hemorrhage transformation on admission are more prone to be allocated to receiving lower dose rt-PA by the neurologists. And these patients usually have a larger infarct area or other adverse factors, which may decrease the chance of favorable outcome at 3 months. Therefore, the probable beneficial effect of standard dose on favorable outcome at 3 months need to be validated by more randomized controlled trials in future.
With respect to SICH, Liu’s meta-analysis found no significant difference in the incidence of SICH between low dose and standard dose rt-PA using a random-effects model32. Since there were many definitions of SICH among the various studies, which may challenge the reliability of pooled results, we in present meta-analysis combined results and conducted pooled estimates separately for SICH according to different definitions (ECASS, NINDS, and SITS-MOST). When using the definition from the NINDS, the incidence of SICH was found to be significantly higher in standard dose group than in low dose group using both fixed- and random-effects models. Among the included studies, only Anderson’s study involved parts of non-Asian patients19. When removing these non-Asian patients from meta-analysis, there was no significant difference between low dose and standard dose in incidence of SICH as defined by NINDS. Moreover, only Anderson’s study showed that incidence of SICH as defined by NINDS was significantly different between low dose (0.6 mg/kg) and standard dose (0.9 mg/kg) groups, with those in the standard dose group having a higher incidence of SICH19. Given that the sample size of Anderson’s study was much larger than the other studies, accounting for 58.5% (3,297/5,634) of all participants, the pooled estimate was undoubtedly dominated by this study. Thus, we also calculated the pooled estimate after removing Anderson’s study from the analysis despite the quality of this study being high with a NOS score of 9; removing it did not change the heterogeneity of the pooled analysis. And no significant difference in incidence of SICH as defined by NINDS was found after Anderson’s study removed (data not shown). In addition, when removing studies based on some exclusion criteria, the pooled estimate for SICH as defined by the NINDS failed to reach statistical significance (Supplementary Table 4). Thus the results regarding the association between rt-PA dose and SICH as defined by the NINDS need to be interpreted with caution, and further research is warranted to explore this topic.
The NINDS definition was thought to be overly inclusive, less precise, and of lowest inter-rater agreement in identifying SICH when compared to the ECASS II, ECASS III, and SITS-MOST definitions51,52. Even though the standard dose rt-PA may actually increase the incidence of SICH as defined by the NINDS, the implications of this on later outcomes at 3 months were inconclusive. Gumbinger’s study demonstrated that SICH as defined by the NINDS, as well as by the ECASS II, ECASS III, and SITS-MOST, was significantly associated with mortality at 3 months52. In Rao’s study, however, SICH as defined by the NINDS was not associated with the distribution of mRS scores or mortality at 3 months51. Furthermore, the prior meta-analysis32 and our present study found that mortality was not significantly different between low dose and standard dose rt-PA. These results suggest that low dose rt-PA may reduce the risk of SICH as defined by the NINDS, but may not decrease mortality at 3 months when compared to standard dose treatment. Given the possibility of the beneficial effects of standard dose treatment on favorable outcome at 3 months, a lower risk of SICH as defined by the NINDS may not alone be a sound reason to administer rt-PA at a low dose.
In developing Asian countries, in addition to worrying about the potentially higher risk of SICH compared to Western patients, financial burden on a family is another frequently-encountered barrier compelling the next-of-kin and neurologist to choose a low dose (i.e. one vial) of rt-PA. As Sila suggested, however, “using less effective therapies to save short-term costs will only increase the costs of long-term care for disabled stroke survivors”53. Therefore, until additional evidence is available, thrombolysis with low dose rt-PA for the purpose of being cost-conscious may not be an ideal choice.
Some limitations to our study must be acknowledged. Firstly, in some studies, low dose rt-PA administered because of a patient hitting the limit for maximum dose or inaccurate weight assessment can range widely, besides, parts of the participants in several studies may have been assigned to the wrong group based on a slightly different cut-off point than 0.85 mg/kg, of which both may obscure a true difference in outcomes between low dose and standard dose treatment. Future studies with pre-specified doses (i.e. 0.6 mg/kg versus 0.9 mg/kg) are warranted to avoid this limitation. Secondly, we conducted the pooled estimate for SICH by separately combining and analyzing studies based on different definitions of SICH (ECASS, NINDS, and SITS-MOST). This could decrease the clinical heterogeneity across studies, but also reduced the number of studies included in any given sub-analysis, which may weaken the reliability of the results. Thirdly, patients receiving endovascular recanalization treatment during thrombolysis was referred to in Kim’s study22, accounting for 26.5% of all patients, and was not clearly indicated in the other studies included in the meta-analysis. Given the disparity in medical and economic conditions and resources across countries and regions, the percentage of patients receiving both thrombolysis and endovascular recanalization treatment may differ substantially between studies, which may cause a non-negligible clinical heterogeneity. Fourthly, the nonrandomized design of most studies would cause a selection bias, then challenge the reliability of outcomes. But we did not conduct a subgroup analysis for randomized studies, due to the few number of studies. Finally, subgroup analysis by age, gender, baseline NIHSS score, time interval from stroke onset to IVT, or other clinical characteristics would possibly supply some additional meaningful results, but was not conducted in the present meta-analysis due to the diversity in the way the information was presented and reported across studies.
Conclusion
The current limited published evidence was insufficient to support the speculation that low dose rt-PA was comparable to the standard dose in thrombolytic efficacy and safety in Asian stroke patients. However, results from the present meta-analysis lacked stability. Large-scale randomized controlled trials, or multicenter studies including a large sample of patients and pre-specified dosing are warranted in the future.
References
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333, 1581–1587 (1995).
Hacke, W., Kaste, M. & Bluhmki, E. et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359, 1317–1329 (2008).
Brott, T. G., Haley, E. C. Jr. & Levy, D. E. et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke 23, 632–640 (1992).
Haley, E. C. Jr., Levy, D. E. & Brott, T. G. et al. Urgent therapy for stroke. Part II. Pilot study of tissue plasminogen activator administered 91–180 minutes from onset. Stroke 23, 641–645 (1992).
Emberson, J., Lees, K. R. & Lyden, P. et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 384, 1929–1935 (2014).
Wardlaw, J. M., Murray, V. & Berge, E. et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 379, 2364–2372 (2012).
van Asch, C. J. et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9, 167–176 (2010).
Ueshima, S. & Matsuo, O. The differences in thrombolytic effects of administrated recombinant t-PA between Japanese and Caucasians. Thromb Haemost 87, 544–546 (2002).
Wahlgren, N., Ahmed, N. & Davalos, A. et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 369, 275–282 (2007).
Rha, J. H., Shrivastava, V. P. & Wang, Y. et al. Thrombolysis for acute ischaemic stroke with alteplase in an Asian population: results of the multicenter, multinational Safe Implementation of Thrombolysis in Stroke-Non-European Union World (SITS-NEW). Int J Stroke 9(Suppl A100), 93–101 (2014).
Wong, L. K. Global burden of intracranial atherosclerosis. Int J Stroke 1, 158–159 (2006).
Takayanagi, S., Ochi, T., Hanakita, S., Suzuki, Y. & Maeda, K. The safety and effectiveness of low-dose recombinant tissue plasminogen activator (0.6 mg/kg) therapy for elderly acute ischemic stroke patients (>/=80 years old) in the pre-endovascular era. Neurol Med Chir (Tokyo) 54, 435–440 (2014).
Mori, E. et al. Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II). Stroke 41, 461–465 (2010).
Nakagawara, J., Minematsu, K. & Okada, Y. et al. Thrombolysis with 0.6 mg/kg intravenous alteplase for acute ischemic stroke in routine clinical practice: the Japan post-Marketing Alteplase Registration Study (J-MARS). Stroke 41, 1984–1989 (2010).
Toyoda, K., Koga, M. & Naganuma, M. et al. Routine use of intravenous low-dose recombinant tissue plasminogen activator in Japanese patients: general outcomes and prognostic factors from the SAMURAI register. Stroke 40, 3591–3595 (2009).
Yamaguchi, T., Mori, E. & Minematsu, K. et al. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke 37, 1810–1815 (2006).
Robinson, T. G., Wang, X. & Arima, H. et al. Low- Versus Standard-Dose Alteplase in Patients on Prior Antiplatelet Therapy: The ENCHANTED Trial (Enhanced Control of Hypertension and Thrombolysis Stroke Study). Stroke 48, 1877–1883 (2017).
Ong, C. T., Wong, Y. S., Wu, C. S. & Su, Y. H. Outcome of stroke patients receiving different doses of recombinant tissue plasminogen activator. Drug Des Devel Ther 11, 1559–1566 (2017).
Anderson, C. S., Robinson, T. & Lindley, R. I. et al. Low-Dose versus Standard-Dose Intravenous Alteplase in Acute Ischemic Stroke. N Engl J Med 374, 2313–2323 (2016).
Ho, B. L., Chen, C. F., Lin, R. T., Liu, C. K. & Chao, A. C. Clinical implication of hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator. Neurol Sci 37, 1799–1805 (2016).
Zheng, M. et al. Clinical efficacy and safety of hypernormal shortened door to needle time (DNT) plus individualized low-dose alteplase therapy in treating acute ischemic stroke. Pak J Med Sci 32, 811–816 (2016).
Kim, B. J., Han, M. K. & Park, T. H. et al. Low-Versus Standard-Dose Alteplase for Ischemic Strokes Within 4.5 Hours: A Comparative Effectiveness and Safety Study. Stroke 46, 2541–2548 (2015).
Liao, X., Wang, Y. & Pan, Y. et al. Standard-dose intravenous tissue-type plasminogen activator for stroke is better than low doses. Stroke 45, 2354–2358 (2014).
Chao, A. C., Liu, C. K. & Chen, C. H. et al. Different doses of recombinant tissue-type plasminogen activator for acute stroke in Chinese patients. Stroke 45, 2359–2365 (2014).
Pan, S. M., Liu, J. F. & Liu, M. et al. Efficacy and safety of a modified intravenous recombinant tissue plasminogen activator regimen in Chinese patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 22, 690–693 (2013).
Chen, C. H. et al. Optimal dose for stroke thrombolysis in Asians: low dose may have similar safety and efficacy as standard dose. J Thromb Haemost 10, 1270–1275 (2012).
Zhou, X. Y., Wang, S. S., Collins, M. L., Davis, S. M. & Yan, B. Efficacy and safety of different doses of intravenous tissue plasminogen activator in Chinese patients with ischemic stroke. J Clin Neurosci 17, 988–992 (2010).
Chao, A. C., Hsu, H. Y. & Chung, C. P. et al. Outcomes of thrombolytic therapy for acute ischemic stroke in Chinese patients: the Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) study. Stroke 41, 885–890 (2010).
Nguyen, T. H., Truong, A. L. & Ngo, M. B. et al. Patients with thrombolysed stroke in Vietnam have an excellent outcome: results from the Vietnam Thrombolysis Registry. Eur J Neurol 17, 1188–1192 (2010).
Sharma, V. K., Tsivgoulis, G. & Tan, J. H. et al. Feasibility and safety of intravenous thrombolysis in multiethnic Asian stroke patients in Singapore. J Stroke Cerebrovasc Dis 19, 424–430 (2010).
Ramaiah, S. S. & Yan, B. Low-dose tissue plasminogen activator and standard-dose tissue plasminogen activator in acute ischemic stroke in Asian populations: a review. Cerebrovasc Dis 36, 161–166 (2013).
Liu, M. D., Ning, W. D. & Wang, R. C. et al. Low-Dose Versus Standard-Dose Tissue Plasminogen Activator in Acute Ischemic Stroke in Asian Populations: A Meta-Analysis. Medicine (Baltimore) 94, e2412 (2015).
Garavaglia, J., Sherman, J., Yetzer, H., Regier, M. & Smith, M. Impact of Tissue Plasminogen Activator Dosing on Patients Weighing More Than 100 kg on 3-Month Outcomes in Acute Ischemic Stroke. J Stroke Cerebrovasc Dis 26, 1041–1046 (2017).
Sarikaya, H., Arnold, M. & Engelter, S. T. et al. Outcome of intravenous thrombolysis in stroke patients weighing over 100 kg. Cerebrovasc Dis 32, 201–206 (2011).
Lou, M. & Selim, M. Does body weight influence the response to intravenous tissue plasminogen activator in stroke patients? Cerebrovasc Dis 27, 84–90 (2009).
Hassan, A. E., Chaudhry, S. A. & Jani, V. et al. Is there a decreased risk of intracerebral hemorrhage and mortality in obese patients treated with intravenous thrombolysis in acute ischemic stroke? J Stroke Cerebrovasc Dis 22, 545–549 (2013).
Diedler, J., Ahmed, N. & Glahn, J. et al. Is the maximum dose of 90 mg alteplase sufficient for patients with ischemic stroke weighing >100 kg? Stroke 42, 1615–1620 (2011).
Chatzikonstantinou, A., Ebert, A. D. & Wolf, M. E. The Impact of Body Mass Index on the Thrombolytic Treatment of Acute Ischemic Stroke. Cerebrovasc Dis 42, 240–246 (2016).
Sahlas, D. J., Gould, L. & Swartz, R. H. et al. Tissue plasminogen activator overdose in acute ischemic stroke patients linked to poorer functional outcomes. J Stroke Cerebrovasc Dis 23, 155–159 (2014).
Barrow, T., Khan, M. S., Halse, O., Bentley, P. & Sharma, P. Estimating Weight of Patients With Acute. Stroke When Dosing for Thrombolysis. Stroke 47, 228–231 (2016).
Breuer, L., Nowe, T. & Huttner, H. B. et al. Weight approximation in stroke before thrombolysis: the WAIST-Study: a prospective observational “dose-finding” study. Stroke 41, 2867–2871 (2010).
Garcia-Pastor, A., Diaz-Otero, F. & Funes-Molina, C. et al. Tissue plasminogen activator for acute ischemic stroke: calculation of dose based on estimated patient weight can increase the risk of cerebral bleeding. J Thromb Thrombolysis 40, 347–352 (2015).
Messe, S. R., Kasner, S. E. & Cucchiara, B. L. et al. Dosing errors did not have a major impact on outcome in the NINDS t-PA stroke study. J Stroke Cerebrovasc Dis 20, 236–240 (2011).
Aulicky, P., Rabinstein, A., Seet, R. C., Neumann, J. & Mikulik, R. Dosing of tissue plasminogen activator often differs from 0.9 mg/kg, but does not affect the outcome. J Stroke Cerebrovasc Dis 22, 1293–1297 (2013).
Stroup, D. F., Berlin, J. A. & Morton, S. C. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283, 2008–2012 (2000).
Hacke, W., Kaste, M. & Fieschi, C. et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 352, 1245–1251 (1998).
Yang, J. et al. A Retrospective Study of thrombolysis with 0.6 mg/kg Recombinant Tissue Plasminogen Activator (rt-PA) in Mild Stroke. Sci Rep 6, 31344 (2016).
Suwanwela, N. C., Phanthumchinda, K. & Likitjaroen, Y. Thrombolytic therapy in acute ischemic stroke in Asia: The first prospective evaluation. Clin Neurol Neurosurg 108, 549–552 (2006).
Abraham, S. V. et al. The need for a population-based, dose optimization study for recombinant tissue plasminogen activator in acute ischemic stroke: A study from a tertiary care teaching hospital from South India. Ann Indian Acad Neurol 20, 36–40 (2017).
Salam, K. A. et al. Intravenous thrombolysis for acute ischemic stroke: the Malabar experience 2003 to 2008. J Clin Neurosci 16, 1276–1278 (2009).
Rao, N. M., Levine, S. R., Gornbein, J. A. & Saver, J. L. Defining clinically relevant cerebral hemorrhage after thrombolytic therapy for stroke: analysis of the National Institute of Neurological Disorders and Stroke tissue-type plasminogen activator trials. Stroke 45, 2728–2733 (2014).
Gumbinger, C., Gruschka, P. & Bottinger, M. et al. Improved prediction of poor outcome after thrombolysis using conservative definitions of symptomatic hemorrhage. Stroke 43, 240–242 (2012).
Sila, C. Finding the Right t-PA Dose for Asians with Acute Ischemic Stroke. N Engl J Med 374, 2389–2390 (2016).
Author information
Authors and Affiliations
Contributions
G.T. and L.L. conceived the study. G.T. and H.W. retrieved, selected and assessed studies, and extracted data. S.C., D.C. and L.Z. participated in data extraction and statistical analysis. G.T. and H.W. drafted the manuscript. D.X. and Y.Z. revised the manuscript and language. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, G., Wang, H., Chen, S. et al. Efficacy and safety of low dose alteplase for intravenous thrombolysis in Asian stroke patients: a meta-analysis. Sci Rep 7, 16076 (2017). https://doi.org/10.1038/s41598-017-16355-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16355-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.