Abstract
The use of rhythmic auditory cueing to enhance gait performance in parkinsonian patients’ is an emerging area of interest. Different theories and underlying neurophysiological mechanisms have been suggested for ascertaining the enhancement in motor performance. However, a consensus as to its effects based on characteristics of effective stimuli, and training dosage is still not reached. A systematic review and meta-analysis was carried out to analyze the effects of different auditory feedbacks on gait and postural performance in patients affected by Parkinson’s disease. Systematic identification of published literature was performed adhering to PRISMA guidelines, from inception until May 2017, on online databases; Web of science, PEDro, EBSCO, MEDLINE, Cochrane, EMBASE and PROQUEST. Of 4204 records, 50 studies, involving 1892 participants met our inclusion criteria. The analysis revealed an overall positive effect on gait velocity, stride length, and a negative effect on cadence with application of auditory cueing. Neurophysiological mechanisms, training dosage, effects of higher information processing constraints, and use of cueing as an adjunct with medications are thoroughly discussed. This present review bridges the gaps in literature by suggesting application of rhythmic auditory cueing in conventional rehabilitation approaches to enhance motor performance and quality of life in the parkinsonian community.
Similar content being viewed by others
Introduction
Susceptibility to fall grows rapidly amongst elderly and patients with neurological deficits1,2,3. The impairments in neuromuscular functioning promotes instability4,5, weakness6, reduces physical activity7, further leading to musculoskeletal deformities and a higher predisposition to fall8. Injuries related to such instabilities inflict heavy costs at both individual and economic levels9, increase dependency, social isolation and affects the quality of life10,11. Neurological disorders such as parkinsonism presents itself with impairments in motor functions exhibiting characteristics such as, akinesia, chorea, hypokinesia, bradykinesia, motor blocks, rigidity, and problems with generation of cyclic movements, further leading to “freezing” instances12,13. Jankovic and Tolosa14 suggests the degeneration of dopaminergic cells in substantia nigra in basal ganglia, which might result in its impaired excitatory output and affect its functioning (autonomic control of movement planning, scaling and initiation)15. Likewise, ageing together with parkinsonism results in rigorous denervation and re-innervation due to progressive reduction in functional motor units in spinal cord and myelinated ventral root fibers16. Together, these neurological dysfunctions impair the ability to execute and maintain autonomic motor tasks such as, posture and gait17.
Research also suggests a “fear” related stability modification in gait, postural performance for such fall prone population groups18, possibly leading to a range of spatiotemporal and kinematic modifications19. For instance, reduction in gait velocity, stride length, increase in double limb support8,18,20, and stride-to-stride fluctuation21 have been extensively reported. This compensatory effort to develop gait patterns that are resistant to external perturbations leads to poor static and dynamic stability8,22. Practically, these changes might impair an individual’s ability to pass safely through high stress situations constrained by space or time, such as escalators, traffic signals4,23, leading to an increased predisposition to fall. Studies have suggested that this modification in gait patterns is due to an alleviation in “internal” conscious attention towards autonomic control, which adversely impacts proprioception and autonomic functioning possibly because of movement specific re-investment1,24,25,26. The theory suggests that directing attention internally to control autonomic movements such as gait, can have an adverse impact on its performance1. The theory further adds that aging23, neurological ailment and injuries1,3 are common conditions that promote movement specific reinvestment. Such fall-prone population groups have a differential cortical activation pattern, which could possibly be linked with changes in task prioritization and conscious attention while carrying out cognitive or motor tasks27. Moreover, electromyographic analysis has revealed enhanced variability in motor unit recruitments that adversely impacts the execution of automated and voluntary motor tasks28. Likewise, limitations in execution of functional activities of daily living tasks have also been extensively reported29,30,31,32.
Common treatment strategies to curb motor dysfunctions in parkinsonism include training with virtual-reality33, biofeedback34, physical/occupational therapy35, physical exercise36, dance37, treadmill38, external sensory feedback39, and dual-task training4. Likewise, pharmacological intervention with psychoactive drug such as levodopa, dopamine agonists, monoamine oxidase type B inhibitors40, have been reported to be effective short-term for managing motor symptoms, such as bradykinesia, tremors41. However their effectiveness in managing gait and postural dysfunctions in long-term is largely debated42,43.
Spaulding et al.43 argued that the lack of adequate sensory information in patients with parkinsonism plays a destructive role in autonomic motor functioning. Therefore, motor performance in parkinsonian patients might benefit from additional sensory information. Several studies have analyzed the effects of augmented external auditory, visual and tactile feedback on performance44,45,46,47. Nevertheless, studies have suggested the predominant role of auditory information as compared to its counterparts39,43. Predominantly auditory cortex has been reported to perceive stimuli with shorter reaction times (20–50 ms) as compared to its visual or tactile counterparts45,48,49,50. Further, the auditory cortex possesses rich connectivity to motor centers from spinal cord extending towards brainstem, cortical and subcortical structures51,52,53. Thereby, allowing strong cross-sensory impacts of auditory signal characteristics, such as frequency54, timbre55, on motor execution. Consequently, several types of external auditory feedback techniques have been analyzed in the literature, such as rhythmic auditory cueing53, patterned sensory enhancement46,56, and real-time auditory feedback57. However, rhythmic auditory cueing is most widely studied, with respect to motor performance post parkinsonism45, stroke58, cerebral palsy59,60, and more20,61,62,63. Rhythmic auditory cueing is defined as a medium of repetitive isosynchronous beats applied with an aim to synchronize motor execution with a rhythm53,58. The underlying mechanisms for attaining benefits in the motor domain are suggested to be multifactorial45,64. The auditory cueing has been suggested to modulate neuromagnetic β oscillations65, enhance biological motion perception57,66, promote motor imagery67,68, reducing shape variability in musculoskeletal activation patterns69, mediate cortical reorganization, neural-plasticity70, supressing movement specific re-investment71, and more72,73.
We identified high quality systematic reviews analysing the effects of external auditory cueing on Parkinsonism42,43,74. However, the meta-analysis due to extremely strict inclusion criteria allowed the inclusion of only randomized controlled trials for statistical analysis, and not for a joint qualitative analysis42,43. Moreover, findings from the meta-analysis of Spaulding et al.43 were interpreted without the presence of any heterogeneity test in between the studies. Similarly, limitations concerning statistical analysis were observed for Rocha et al.42. Lim et al.74 and Nombela et al.45 performed excellent quality narrative reviews, but the lack of statistical analysis doesn’t allow to draw firm conclusions. Moreover, none of the review studies analysed the effects of different types of tempo, different signal characteristics, training dosage, and dual-task performance with rhythmic auditory cueing. Therefore, we attempted to develop a state of knowledge for the benefit of parkinsonian patients and medical practitioners, where both qualitative and quantitative data from good quality studies can be interpreted. Moreover, to the best of our knowledge, up to now, no review has elucidated the effects of dual tasks, fast/slow paced stimuli, and the precise training dosage of rhythmic auditory cueing on spatiotemporal gait parameters in Parkinson’s disease. This present review for the first time, conducted a systematic review in combination with a meta-analysis to determine the effects of rhythmic auditory cueing among parkinsonian patients.
Methods
This review was conducted according to the guidelines outlined in Preferred Reporting Items for Systematic Reviews and Meta-analysis: The PRISMA statement75.
Data sources and search strategy
Academic databases Web of science, PEDro, EBSCO, MEDLINE, Cochrane, EMBASE and PROQUEST were searched from inception until July 2017. A sample search strategy has been provided in (Table 1).
Data extraction
Upon selection for review, the following data were extracted from each article; author, date of publication, sample size, sample description (gender, age, health status), disease duration, intervention, characteristics of auditory cueing, dual-task, outcome measures, results, and conclusions. The data were then summarized and tabulated (Supplementary Table 1).
The inclusion criteria for the studies was (i) Randomized controlled trials, cluster randomized controlled trials or controlled clinical trials; (ii) Studies reporting reliable and valid spatiotemporal gait parameters (iii) Studies including static/dynamic aspects of gait/postural stability (iv) Studies scoring ≥4 in PEDro methodological quality scale; (v) Experiments conducted on human participants; (vi) Published in a peer-reviewed academic journal; (vii) Articles published in English, German and Korean languages.
Quality and risk of bias assessment
The quality of the studies was assessed using the PEDro methodological quality scale76. The scale consists of 11 items addressing external validity, internal validity, and interpretability and can detect potential bias with fair to good reliability80, and validity76. A blinded rating of the methodological quality of the studies was carried out by the primary reviewer (SG). Ambiguous issues were discussed with second and third reviewers (IG, GS, AOE) and consensus was reached. Included studies were rated according to scoring of 9–10, 6–8 and 4–5, and were interpreted as “excellent”, “good” and “fair” quality studies78, respectively. Inadequate randomization, non-blinding of assessors, no intention to treat analysis and no measurement of compliance were considered as major threats for biasing79.
Data Analysis
This systematic review also included a meta-analysis approach to develop a better understanding of the incorporated interventions80. The presence and lack of heterogeneity asserted the use of either random or fixed effect meta-analysis81. A narrative synthesis of the findings structured around the intervention, population characteristics; methodological quality (Supplementary Table 1) and the type of outcome are provided. Likewise, summaries of intervention effects for each study were provided in a tabular format (Supplementary Table 1). A meta-analysis was conducted between pooled homogenous studies using CMA (Comprehensive meta-analysis V 2.0, USA). Heterogeneity between the studies was assessed using I2 statistics. The data in this review were systematically distributed and for each available variable pooled, dichotomous data were analyzed and forest plots with 95% confidence intervals are reported. The effect sizes were adjusted and reported as Hedge’s g82. Thresholds for interpretation of effect sizes were as follows; a standard mean effect size of 0 means no change, negative effect size means a negative change, mean effect size of 0.2 considered a small effect, 0.5 a medium effect and 0.8 a large effect83. Interpretation of heterogeneity via I2 statistics was as; 0%, 25%, 75% as negligible, moderate and substantial heterogeneity, respectively. Meta-analysis reports indicating heterogeneity among studies were evaluated to determine the reason of heterogeneity, and the included studies were then pooled separately and analyzed again. The alpha level of 0.05 was adopted.
Results
Characteristics of included studies
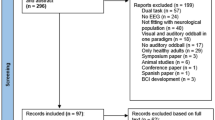
Our initial search yielded a total of 4794 studies, which on implementing our inclusion/exclusion criteria, were reduced to fifty (Fig. 1). Data from the included studies have been summarized in (Supplementary Table 1). Of the fifty included studies, seven were randomized controlled trials, and forty-four were controlled clinical trials.
Participants
A total of 1892 participants were analyzed in the incorporated studies. In the included studies, forty-eight studies incorporated mix gender patients. Two studies incorporated only male participants84,85. Two studies didn’t specify the gender of the included participants86,87. The included studies provided data on 1892 participants (n = 745 females/1089 males). Descriptive statistics relating to the age (mean ± standard deviation) of the participants were tabulated across the studies. In addition, the age of participants was mentioned in range by six studies88,89,90,91,92,93, and only a mean value was provided by two studies31,94. Disease duration of parkinsonian patients have been mentioned (see Supplementary Table 1).
Risk of bias
To reduce the risks of bias, studies scoring ≥4 on PEDro were included in the review. Moreover, the limitation of research protocols to be included in the review was limited to gold standard randomized controlled trials, cluster randomized controlled trials and controlled clinical trials. The individual scores attained by the studies using the PEDro scale have been reported (Supplementary Tables 1 and 2). The average PEDro score for the fifty included studies was computed to be 5.4 out of 10, indicating fair-quality of the overall studies. Seven studies scored 8, three scored 7, twelve studies scored 6, thirteen studies scored 5, and seventeen studies scored 4. Publication bias was analyzed by plotting a Hedge’s g against standard error (Fig. 2). Asymmetries concerning mean in the funnel plot might suggest bias (either positive or negative). Risk of bias across the studies has been demonstrated in (Fig. 3).
Funnel plot for Hedge’s g and standardized effect for each value in the meta-analysis. Each of the effect is represented in the plot as a circle. Funnel boundaries represent area where 95% of the effects are expected to lie if there were no publication biases. The vertical line represents the mean standardized effect of zero. Absence of publication bias is represented by symmetrically distributed effects around the line.
Meta-Analysis
Outcomes
The results suggest clear evidence for a positive impact of rhythmic auditory cueing on spatiotemporal gait parameters amongst parkinsonian patients. An enhancement in gait parameters were also observed when rhythmic auditory cueing was introduced with biological variability94,95, and music56,93,96,97,98. In the included fifty studies, one study reported enhancements (p > 0.05)96, two studies reported negligible effects100,101, one study reported significant reduction of rhythmic auditory cueing on spatiotemporal gait parameters102. Forty-six studies reported significant enhancements in primary spatiotemporal gait parameters while receiving rhythmic auditory cueing.
Meta-analysis report
The evaluation of research studies via meta-analysis requires strict inclusion criteria to efficiently limit the heterogeneity103. However, among the pooled group of studies post strict inclusion criteria, some amount of unexplained heterogeneity was still observed. Thereafter, sub-group analyses were performed among homogenous studies to exclude and evaluate the cause of heterogeneity. The evaluated parameters were the spatio-temporal gait parameters such as, cadence, stride length, gait velocity, double limb support duration, and turn time. Analyses were also conducted to evaluate the effects of dual-task conditions, the effects of different training durations, presence/lack of medication, early/late phase of treatment, presence of treadmill, and different tempi at which rhythmic auditory cueing was provided on gait parameters. We included a generalized group analysis combined for all the pooled studies. A separate analysis in addition to clinical controlled trials was performed for high quality randomized controlled trails, for allowing a better interpretation of the direction and magnitude of effects. The main reason for not including the statistical approach within the studies was due to major differences in between assessment methods, patient characteristics, auditory stimuli and lack of descriptive statistics within the manuscript. However, attempts were made to retrieve data from respective corresponding co-authors.
Gait velocity
Gait velocity was analyzed among thirty-five studies. Additional sub-group data were extracted from thirteen included studies71,86,87,89,94,99,104,105,106,107,108. In the additional analyses, two studies analyzed early and late treatment groups89,99. Two studies analyzed normal and treadmill gait performance82,103. Six studies compared cueing between fast and slow tempo86,93,104,105,109,110. Five studies analyzed the effects with only fast paced111,112,113,114,115, and two with only slow paced tempo116,117. Seven studies analyzed cueing at slow tempo86,93,104,105,109,116,117. The fast/slow tempo in the included studies was determined by keeping the patient’s preferred cadence as reference. Three studies analyzed patients in “on” and “off” stages of medications71,107,110, signifying the presence and absence of medications, respectively. A positive effect here refers to enhancement in gait velocity, and a negative effect refers to reduction in gait velocity.
The analysis of studies revealed (Fig. 4) a small effect size in the positive domain (g: 0.23, 95% C.I: 0.1 to 0.3). Substantial heterogeneity was observed in between the studies (I2: 87.4%, p > 0.01). Further, sub-group analyses were conducted among homogenous studies to explore heterogeneity. An analysis between “on” and “off” medications patients (Supplementary Figures 1 and 2), revealed a positive small effect size for “off” group with negligible heterogeneity (g: 0.43, 95% C.I: 0.11 to 0.75, I2: 18.8%, p = 0.29), and positive medium effect size for “on” group with negligible heterogeneity (g: 0.55, 95% C.I: 0.23 to 0.87, I2: 0.0%, p = 0.44). A sub-group analysis for treadmill training groups (Supplementary Figure 3) revealed a positive large effect size with negligible heterogeneity (g: 1.0, 95% C.I: 0.33 to 1.67, I2: 24.6%, p = 0.24). A sub group analysis between “fast” and “slow” externally paced auditory cueing (Supplementary Figure 4), revealed a positive medium effect for the “fast” group with negligible heterogeneity (g: 0.7, 95% C.I: 0.50 to 0.89, I2: 0.0%, p = 0.44), and a negative small effect for the “slow” group (Supplementary Figure 5) with negligible heterogeneity (g: −0.24, 95% C.I: 10.51 to 0.19, I2: 23.53%, p = 0.24). Further, twenty-one studies analyzing the effects of a simple rhythmic auditory cueing were analyzed (Supplementary Figure 6). The sub-analysis revealed a positive small effect size (g: 0.05, 95% C.I: −0.07 to 0.17, I2: 86.4%, p < 0.01) with substantial heterogeneity. The analysis revealed two main types of sub-groups analyzing the effects of rhythmic auditory cueing with and without training. The analysis of ten studies analysing the direct effects of rhythmic auditory cueing i.e. without training (Supplementary Figure 7) revealed a negative small effect size (g: −0.34, 95% C.I: −0.5 to −0.18, I2: 85.9%, p < 0.01) with substantial heterogeneity. The studies were then categorized according to the disease duration of parkinsonian patients in the studies i.e. >9 years or <9 years. Six studies evaluated the effects of rhythmic auditory cueing on gait performance, with patients having mean disease duration <9 years. The analysis revealed a positive small effect size (g: 0.16, 95% C.I: −0.12 to 0.44, I2: 0%, p = 0.56) with negligible heterogeneity. The studies analysing severe parkinsonian patients i.e. >9 years of disease duration revealed a negative small effect size (g: −0.37, 95% C.I: −0.62 to −0.13, I2: 91%, p < 0.01) with substantial heterogeneity. Upon further evaluation of heterogeneity in the sub-group we observed that the experimental procedures differed considerably between each other. For instance, Chen et al.118 analysed gait performance during gait turning, Arias and Cudeiro104 utilized a varied range of frequency that differed from other studies, and Rochester et al.119 utilized a complex functional task that required the patients to perform a sitting to stand and carrying a tray. Therefore, a further sub-analysis was not carried out.
Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on gait velocity among parkinsonian patients. Weighted effect sizes; Hedge’s g (boxes) and 95% C.I (whiskers) are presented, demonstrating repositioning errors for individual studies. The (Diamond) represents pooled effect sizes and 95% CI. A negative effect size indicated reduction in gait velocity; a positive effect size indicated enhancement in gait velocity. (FP: Fast paced, SP: Slow paced, Fz: Freezers, NFz: Non-Freezers, ON: with medications, OFF: without medications, INC: Inclined training, T: Treadmill training, FP T: Fast paced training, I: Isosynchrounous cueing, Rn: Random, BL Biological variability, RAC: Rhythmic auditory cueing).
A sub-group analysis for thirteen studies analysing the effects of training with rhythmic auditory cueing (Supplementary Figure 8), revealed a positive medium effect size (g: 0.63, 95% C.I: 0.49 to 0.76, I2: 74.1%, p < 0.01) with substantial heterogeneity. Further, upon sub-group analysis to reveal the cause of heterogeneity we excluded Frazzitta et al.106 because the patients in their study had on average a longer disease duration (13.2 ± 4.1 years) than the patients from other studies. Additionally, Dalla Bella et al.99 was excluded from further analysis. The study’s training regime differed from the other studies i.e. rhythmic auditory cueing with ±10% modulation of pace according to preferred cadence. Moreover, the training included a hand tapping task concurrently with gait training. The analysis after excluding these studies revealed a positive medium effect size with moderate heterogeneity (g: 0.64, 95% C.I: 0.37 to 0.92, I2: 36.08%, p = 0.34). Moreover, an additional analysis of training duration was performed (more or less than 45 min). The analysis for thirteen studies included a treatment duration for less than 45 minutes (Supplementary Figure 9) revealed a positive medium effect size with substantial heterogeneity (g: 0.61, 95% C.I: 0.48 to 0.74, I2: 69.3%, p < 0.01). Further, exclusion of Chaiwanichsiri et al.85, Frazzitta et al.106 was done as both the studies incorporated treadmill training and a training duration of 20 minutes, and Harro et al.113 as the authors only included only one training session per week, whilst the others included more than 3 sessions per week. The analysis for 30–45 min of duration (Supplementary Figure 10), revealed a positive medium effect size with moderate heterogeneity (g: 0.52, 95% C.I: 0.38 to 0.66, I2: 33.8%, p > 0.05). The analysis for 20 min of duration (Supplementary Figure 11), revealed a positive large effect size with substantial heterogeneity (g: 1.09, 95% C.I: 0.7 to 1.47, I2: 80.9%, p < 0.01). The studies however differed considerably from one another as, Frazzitta et al.106 included parkinsonian patients in advanced stage of disease, as compared Chaiwanichsiri et al.85 where patients were in early stages. The two studies analyzing the effects of training in ling duration i.e. more than 45 minutes were considerably different as del Olmo and Cudeiro112 utilized rhythmic auditory cueing with an instruction to perform faster gait, and while carrying out a manual task, whereas del Olmo et al.102 did not incorporate such technique. A sub-group analysis based on the number of weeks the patients received treatment i.e. less or more than 5 weeks was performed. Analysis for patients receiving treatment for less than 5 weeks (Supplementary Figure 12) revealed a positive medium effect size with substantial heterogeneity (g: 0.73, 95% C.I: 0.31 to 1.14, I2: 21.3%, p > 0.05). Likewise, for patients receiving treatment for more than 5 weeks (Supplementary Figure 13) revealed a positive small effect size with negligible heterogeneity (g: 0.46, 95% C.I: 0.2 to 0.72, I2: 0%, p > 0.05).
Randomized controlled trials
A sub-group analysis on the included randomized controlled trials was performed (Supplementary Figure 14). Two studies analyzed early and late intervention groups96,117. Three studies involved a training regime with rhythmic auditory cueing88,98,113. One study analyzed immediate effects of rhythmic auditory cueing on gait121. The analysis revealed a positive small effect for the group with substantial heterogeneity (g: 0.25, 95% C.I: 0.11 to 0.40, I2: 73.5%, p = 0.001).
A sub-group analysis between “early” and “late” treatment groups revealed a positive small effect size for “early” group (Supplementary Figure 15) with negligible heterogeneity (g: 0.11, 95% C.I: −0.16 to 0.39, I2: 0.0%, p = 0.88), and similar small effect size for “late” group (Supplementary Figure 16) with negligible heterogeneity (g: 0.11, 95% C.I: −0.16 to 0.39, I2: 0.0%, p = 0. 45). A sub-group analysis between de Bruin et al.98 and Harro et al.110 revealed a positive large effect size with substantial heterogeneity (g: 0.97, 95% C.I: 0.29 to 1.66, I2: 93.35%, p < 0.01). The training program differed between the studies, de Bruin et al.98 trained their patients for at least 3 sessions per week, whereas Harro et al.113 performed only one training session per week. Gait velocity under dual-task condition was analyzed amongst nine studies. The specifics of dual-tasks have been mentioned (Supplementary Table 1). The analysis (Supplementary Figure 17) revealed a positive small effect size (g: 0.38, 95% C.I: 0.09 to 0.66, I2: 9.95%, p > 0.05) with negligible heterogeneity.
Stride length
Stride length was analyzed amongst thirty-four studies. Additional sub-group data was extracted from fourteen included studies. A positive effect here refers to enhancement in stride length, and a negative effect refers to reduction in stride length. The combined analysis revealed (Fig. 5) a positive small effect size (g: 0.42, 95% C.I: 0.35 to 0.5, I2: 85.05%, p < 0.01) with substantial heterogeneity. A sub-group analysis in between “off” and “on” medication groups was performed among three studies71,107,110. The analysis for “on” group (Supplementary Figure 18), revealed a large effect size in positive domain (g: 0.77, 95% C.I: 0.45 to 1.1, I2: 43.6%, p = 0.16) with moderate heterogeneity. Likewise, analysis for “off” group (Supplementary Figure 19), revealed a large effect size in positive domain (g: 0.85, 95% C.I: 0.49 to 1.2, I2: 51%, p = 0.12) with marginally moderate heterogeneity. This heterogeneity could possibly be attributed to Chester et al.110, as the authors utilized a different tempo for rhythmic auditory cueing as compared to the other two counterparts. Post exclusion the meta-analysis revealed large effect size for both “on” and “off” in positive domain (g: 0.86, 95% C.I: 0.52 to 1.2, I2: 0%, p = 0.64), (g: 0.96, 95% C.I: 0.59 to 1.34, I2: 0%, p = 0.39), with negligible heterogeneity, respectively.
Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on stride length among parkinsonian patients. Weighted effect sizes; Hedge’s g (boxes) and 95% C.I (whiskers) are presented, demonstrating repositioning errors for individual studies. The (Diamond) represents pooled effect sizes and 95% CI. A negative effect size indicated reduction in stride length; a positive effect size indicated enhancement in stride length. (FP: Fast paced, SP: Slow paced, Fz: Freezers, NFz: Non-Freezers, ON: with medications, OFF: without medications, INC: Inclined training, T: Treadmill training, FP T: Fast paced training, I: Isosynchrounous cueing, Rn: Random, BL Biological variability, RAC: Rhythmic auditory cueing).
Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on cadence among parkinsonian patients. Weighted effect sizes; Hedge’s g (boxes) and 95% C.I (whiskers) are presented, demonstrating repositioning errors for individual studies. The (Diamond) represents pooled effect sizes and 95% CI. A negative effect size indicated reduction in step frequency; a positive effect size indicated enhancement in step frequency. (FP: Fast paced, SP: Slow paced, Fz: Freezers, NFz: Non-Freezers, ON: with medications, OFF: without medications, INC: Inclined training, T: Treadmill training, FP T: Fast paced training, I: Isosynchrounous cueing, Rn: Random, BL Biological variability, RAC: Rhythmic auditory cueing, step frequency: number of steps/minute).
A further analysis differentiated fast and slow-paced stimuli with respect to the patient’s preferred cadence. Seven studies compared the effects between fast and slow paced rhythmic cueing86,93,104,105,109,110,111, whereas four studies analyzed the effects of only fast paced cueing31,91,112,114. Analysis for fast-paced stimuli among eleven studies (Supplementary Figure 20), revealed a positive small effect size (g: 0.27, 95% C.I: 0.09 to 0.4, I2: 69%, p < 0.01) with substantial heterogeneity. Chester et al.110 was excluded from further analysis as the authors evaluated the effects of fast and slow-paced stimuli on patients with different stages of severity. Likewise, Lopez et al.91 utilized a tempo faster than the other studies i.e. +25% of preferred cadence, hence was also excluded. Thereafter, a positive small effect size (g: 0.3, 95% C.I: 0.08 to 0.52, I2: 0%, p = 0.7) with negligible heterogeneity was observed. Similarly, for the slow-paced stimuli five studies were evaluated (Supplementary Figure 21) a positive small effect size (g: 0.43, 95% C.I: 0.18 to 0.69, I2: 83.2%, p < 0.01) with substantial heterogeneity was observed. After excluding (Chester et al., 2006) we observed a positive medium effect size (g: 0.69, 95% C.I: 0.35 to 1.03, I2: 20.03%, p = 0.29) with negligible heterogeneity. Studies analyzing only the effects of un-modulated rhythmic auditory cueing were analyzed on twenty-three studies (Supplementary Figure 22) and they revealed a positive small effect size (g: 0.35, 95% C.I: 0.22 to 0.48, I2: 35.3%, p = 0.04) with moderate heterogeneity. Studies were then separated based on training or direct application of rhythmic auditory cueing. A sub-group analysis for nine studies analyzing direct application of rhythmic auditory cueing among nine studies (Supplementary Figure 23), revealed a positive small effect size (g: 0.12, 95% C.I: −0.11 to 0.35, I2: 0%, p = 0.72) with negligible heterogeneity. Moreover, for the training group thirteen studies (Supplementary Figure 24) were evaluated and the analysis revealed a positive small effect size (g: 0.37, 95% C.I: 0.23 to 0.51, I2: 26.2%, p = 0.16) with moderate heterogeneity. For training group with 30 minutes of training (Supplementary Figure 25), the analysis revealed a positive small effect size (g: 0.36, 95% C.I: 0.21 to 0.51, I2: 42.5%, p = 0.07) with moderate heterogeneity. Three studies with different training regimes i.e. 20 minutes85, and 1 hour duration were excluded102,112. A further analysis determining treatment duration across more than or less than 5 weeks revealed medium positive effect size (g: 0.61, 95% C.I: 0.44 to 0.78, I2: 71.2%, p > 0.1) with substantial heterogeneity. Further sub-group analysis for less than 5 session per week of training revealed small positive effect size (g: 0.39, 95% C.I: 0.08 to 0.7, I2: 0%, p < 0.11) with negligible heterogeneity122,123. For studies analyzing training for more than 5 sessions per week (Supplementary Figure 26), revealed small positive effect size (g: 0.4, 95% C.I: 0.1 to 0.68, I2: 0%, p > 0.5) with negligible heterogeneity.
Randomized controlled trials
A sub group analysis for 4 randomized controlled trials (Supplementary Figure 27) revealed a positive medium effect size (g: 0.56, 95% C.I: 0.42 to 0.69, I2: 98.04%, p < 0.01) with substantial heterogeneity. A sub group analysis for two randomized controlled trials analyzing the effects of rhythmic auditory cueing without training revealed no effect (g: 0.0, 95% C.I: −0.28 to 0.3, I2: 0%, p = 0.9) with negligible heterogeneity. Stride length under dual-task condition was analyzed amongst eight studies (Supplementary Figure 28). The analysis revealed a positive small effect size (g: 0.31, 95% C.I: 0.14 to 0.48, I2: 0%, p = 0.8) with negligible heterogeneity.
Cadence
Cadence was analyzed amongst thirty studies (Figure 6). Additional sub-group data was extracted from eleven included studies. The analysis of studies revealed a negative small effect size (g: −0.05, 95% C.I: −0.13 to 0.03, I2: 93.6%, p < 0.01) with substantial heterogeneity. A positive effect here refers to enhancement in step frequency i.e. number of steps per minute, and a negative effect refers to reduction in step frequency.
Two studies compared the effects of “on” and “off” phase of medications on the patient's affected from parkinsonism71,107. A sub-group analysis for “off” treatment groups (Supplementary Figure 29) revealed a negative small effect size (g: −0.1, 95% C.I: −0.46 to −0.25, I2: 81.97%, p = 0.01) with substantial heterogeneity. Likewise, for “on” treatment group (Supplementary Figure 30), a positive small effect size (g: −0.13, 95% C.I: −0.20 to 0.46, I2: 89.69%, p < 0.01) with substantial heterogeneity was observed. The heterogeneity could be attributed to the use of different tempi i.e. at preferred cadence, at tempo faster than preferred cadence, during rhythmic auditory cueing training by McIntosh et al.107. Moreover, sub-groups analyses were performed for gait performance with “fast” and “slow” paced tempi with respect to patient’s preferred cadence. Five studies compared effects of fast and slow paced stimuli86,93,104,105,109, whereas two studies evaluated the effects of only fast paced stimuli on gait performance111,115. Further, seven studies analyzed only fast-paced stimuli (Supplementary Figure 31), a positive large effect size (g: 1.0, 95% C.I: 0.78 to 1.34, I2: 87.05%, p < 0.01) with substantial heterogeneity was observed. A sub-group analysis lead to exclusion of three studies based on the severity of patients included i.e. >9 years of disease duration85,104,109. The analysis of fast-paced stimuli in less severe patients revealed a positive medium effect size (g: 0.61, 95% C.I: 0.25 to 0.94, I2: 0%, p = 0.49) with negligible heterogeneity. In severe patients, we observed a positive large effect size (g: 1.75, 95% C.I: 1.31 to 2.18, I2: 74.6%, p = 0.01) with substantial heterogeneity. However, we excluded Willems et al.86, from further analysis as the authors incorporated a faster tempo i.e. + 20% as compared to + 10% in the other studies104,109. We observed a positive large effect size (g 1.4, 95% C.I: 0.89 to 1.91, I2: 27.6%, p = 0.24) with marginally moderate heterogeneity. Six studies analyzed the effects of slow-paced stimuli on gait performance in patients (Supplementary Figure 32). We observed a negative large effect (g −1.25, 95% C.I: −1.59 to −0.92, I2: 89.34%, p < 0.01) with substantial heterogeneity. Further, on dividing the studies into two categories i.e. >9 years of disease duration (severe and less severe). We observed a negative medium effect (g −0.5, 95% C.I: −0.97 to −0.04, I2: 0%, p = 0.93) with negligible heterogeneity, in less severe group. Whereas, a negative large effect (g −2.05, 95% C.I: −2.53 to −1.57, I2: 92.45%, p < 0.01) with substantial heterogeneity was observed in the more severe group. Further, we excluded Willems et al.86 because the patients differed considerably in terms of age, disease duration and treatment. We then observed a negative large effect (g −1.54, 95% C.I: −2.06 to −1.02, I2: 0%, p = 0.37) with negligible heterogeneity. Twenty studies analyzing rhythmic auditory cueing at preferred cadence revealed a positive small effect size (g 0.17, 95% C.I: 0.01 to 0.32, I2: 90.5%, p < 0.01) with substantial heterogeneity (Supplementary Figure 33). On further sub-group analysis nine studies analyzing only rhythmic auditory cueing (Supplementary Figure 34), implementation without training revealed a positive small effect size (g 0.30, 95% C.I: 0.07 to 0.53, I2: 0%, p = 0.45) with negligible heterogeneity. Thereafter, studies analyzing only rhythmic auditory cueing implementation with training (Supplementary Figure 35), revealed a small negative effect size (g: 0.04, 95% C.I: −0.1 to 0.2, I2: 93.6%, p < 0.01) with substantial heterogeneity. Studies analyzing only rhythmic auditory cueing implementation with 30 min (Supplementary Figure 36), of training revealed a small effect size (g: 0.09, 95% C.I: −0.06 to 0.25, I2: 95.5%, p < 0.01) with substantial heterogeneity. Further, we excluded Song and Ryu124, Pau et al.122, and de Bruin et al.98 as the authors included the training for 8, 12 and 13 weeks, respectively. Furthermore, two studies differing considerably in training regimes were excluded from further analysis88,125. Studies analyzing only rhythmic auditory cueing implementation with training for a few sessions in less than 5 weeks (Supplementary Figure 37), revealed a positive medium effect size (g: 0.65, 95% C.I: 0.33 to 0.96, I2: 0%, p = 0.94) with negligible heterogeneity. An analysis of studies evaluating rhythmic auditory cueing with training more than 5 days per week (Supplementary Figure 38) revealed a negative small effect size (g: −0.22, 95% C.I: −1.16 to 0.71, I2: 23.6%, p > 0.05) with negligible heterogeneity.
Randomized controlled trials
We analyzed three randomized controlled trials which evaluated the effects of rhythmic auditory cueing on cadence (Supplementary Figure 39). Upon analysis, we observed a small effect (g 0.07, 95% C.I: −0.08 to 0.22, I2: 44.6%, p = 0.13) with moderate heterogeneity. Cadence under dual-task condition was analyzed amongst nine studies (Supplementary Figure 42). The analysis revealed a positive small effect size (g: 0.11, 95% C.I: −0.06 to 0.8, I2: 0%, p = 0.8) with negligible heterogeneity.
Double limb support phase
Double limb support phase was analyzed amongst eight studies56,86,110,111,114,120,122,126. Additional sub-group data was extracted from three included studies86,89,110. A positive effect here refers to increase in total duration when both feet are in contact with the ground, and vice versa for the negative effect. The analysis (Supplementary Figure 43) revealed a positive medium effect size (g: 0.5, 95% C.I: 0.34 to 0.67, I2: 93.46%, p < 0.01) with substantial heterogeneity. With a fast-paced stimulus the rhythmic auditory cueing (Supplementary Figure 44), reveals a small effect size in positive domain (g: 0.46, 95% C.I: 0.05 to 0.87, I2: 92.3%, p < 0.01) with negligible heterogeneity. Two studies with slow-paced stimuli (Supplementary Figure 45) yielded small positive effects (g: 0.33, 95% C.I: −0.18 to 0.85, I2: 92.8%, p < 0.01) with substantial heterogeneity. This heterogeneity could possibly be attributed to the range of severity i.e. stage II, III and IV of parkinsonism in110. Finally, analysis with rhythmic auditory cueing at preferred cadence (Supplementary Figure 46), revealed reduction in double limb support phase with medium effect size in negative domain (g: −0.56, 95% C.I: −0.9 to −0.22, I2: 0%, p = 0.72) with negligible heterogeneity.
Turn time
Three studies analyzed the effects of rhythmic auditory cueing on turn time86,110,111. A positive effect here refers to increase in total duration for performing a turn during gait, and vice versa for the negative effect. The analysis revealed a negative large effect size (g: −2.2, 95% C.I: −2.49 to −1.94, I2: 83.8%, p < 0.01) with substantial heterogeneity. Arias and Cudeiro31 were excluded from further analysis as the authors utilized a rhythmic auditory cueing with faster tempo. The studies were then segregated according to their patient’s characteristics as freezers and non-freezers. The meta-analysis for freezers revealed negative large effect size (g: −2.08, 95% C.I: −2.5 to −1.66, I2: 93.7%, p < 0.01) with substantial heterogeneity. Further, an analysis for non-freezers revealed negative large effect size (g: −2.3, 95% C.I: −2.71 to −1.88, I2: 87.67%, p < 0.01) with substantial heterogeneity. The heterogeneity cannot be further explained here.
Discussion
The primary objective of this present systematic review and meta-analysis was to develop a current state of knowledge for the effects of rhythmic auditory cueing on gait stability in parkinsonian patients. Out of fifty-included studies 88% studies reported beneficial effects of rhythmic auditory cueing on gait parameters. Further, the meta-analysis yielded significant small-to-large standardized effects for the benefits of rhythmic auditory cueing on spatiotemporal gait parameters for parkinsonian patients. Previous studies have reported substantial negative effects of parkinsonism on spatial parameters of gait for instance, stride length, and gait velocity. The current analysis revealed that both stride length (g: 0.48) and gait velocity (g: 0.27) can be enhanced by rhythmic auditory cueing. However, a generalized negative effect of rhythmic auditory cueing was observed on cadence (g: −0.13). Generally, patients with parkinsonism are characterized with reduced gait velocity, stride length, foot clearance, increased cadence, narrowed base of support, festination and in advanced cases freezing of gait127,128. The primary underlying physiological reason being inability to generate a substantial amplitude of motor movements128, possibly due to deficits in internal timing of movements45,129,130,131.
From a neurophysiological aspect, Spaulding et al.43 suggested discrepancies in sensory-motor interactions which might lead to such autonomic disruptions. Nombela et al.45 reattributed and mentioned the dysfunction of an internal cueing system which is associated with coordinating a information exchange between basal ganglia and supplementary motor area. Moreover, studies have also suggested degeneration of a widespread neural network in Parkinson’s disease including cerebellum, basal ganglia, somatosensory area and pre-somatosensory area during the degenerative process45,131. Kotz and Schwartze132 reported that during the preclinical stage, hyperactivity in pre-somatosensory area might be a compensatory mechanism for cerebellar dysfunctions. Likewise, in advanced stages selective loss of pyramidal neurons in pre-somatosensory area might result in its underactivity, followed by deficits in temporal processing45,132, possibly leading to motor block or freezing instances during gait.
The use of rhythmic auditory cueing has been discussed widely in published literature20,43,45,53,60,74. This medium of entrainment transfer has been speculated to bypass the affected basal ganglia network (pallidal-supplementary motor area) via another alternative pathway114,133,134. Moreover, Fujioka et al.65 reported modulation of neuromagnetic β oscillations with rhythmic auditory stimuli in auditory cortex, cerebellum, inferior frontal gyrus, somatosensory area and sensorimotor cortex. The stimuli has been suggested to activate inferior colliculi135, cerebellum, brainstem114,136, sensorimotor cortex137,138, further instigating reorganization in cortico-cerebellar circuits70. Rhythmic auditory cueing has also been suggested to reap the benefits of the preserved neural centres139, involved in perceiving externally cued and goal directed movements amongst parkinsonian patients (see also “kinesia paradoxica”140). The authors proposed that motor activities directed by external sensory cueing evoke pathways via cortical, premotor areas141, effectively bypassing the affected basal ganglia region95. Studies have suggested that rhythmic sensory cues can also replace deficient pallidal-cortical projections, activate the supplementary motor area and aid in motor tasks by mimicking feedforward input, thereby reducing bradykinesia, and associated motor deficits142. Similarly, the external cueing can supplement critical spatio-temporal information which is necessary for initiation or facilitating motor activities30,89, such as during gait or arm movements69,143. In context of gait execution the external rhythm can guide the patients to synchronize their ground contact and lift-off times144. The auditory patterns might also assist the planning of a motor command before executing a movement145. Moreover, the periodicity in rhythmic auditory cueing has also demonstrated to effectively reduce variability in musculoskeletal activation patterns, thereby allowing more economical and consistent motor unit recruitment46, further smoothing the velocity and acceleration profiles of joint motions by scaling movement time46.
Typical pharmacological interventions for controlling motor symptoms in parkinsonism include levodopa, dopamine agonists and monoamine oxidase type B inhibitors40. Rochester et al.71 interestingly mentioned the limitations of dopaminergic medications on gait dysfunctions associated with degeneration of non-dopaminergic pathways88. The medications allow only symptomatic relief and offer no relief from the underlying pathology146. Moreover, their benefits in terms of enhancement of gait performance is still debatable. Benefits in turn time147, stride length, gait speed148, have been reported in some studies. While some studies report no effects on gait speed149, cadence150, stride time variability151, double limb support duration152, and reduction in postural stability147. The current meta-analysis observed beneficial effects of concurrent application of medications and rhythmic auditory cueing. The analyses reported marginally larger effect sizes for stride length (g: 0.96) and gait velocity (g: 0.55) during the “on” phase of medications, in comparison to the “off” medication group for stride length (g: 0.86) and gait velocity (0.43). However, such differences were not found for cadence, where small negative effect sizes were observed in both “on” (−0.13) and “off” (−0.10) conditions. It is important to note that this analysis shows the compensatory role of rhythmic auditory cueing for counteracting motor deficits in the absence of medications. Although, studies have reported the benefits of the medications in short-term40, a long term cost concerning motor dysfunction has also been reported146. Long-term consumption of medications i.e. both levodopa and levodopa sparring therapy has been associated with severe consequences on health and quality of life such as dyskinesis, loss of drug efficacy and toxicity40,146. This is possibly due to levodopa associated decline in dopamine transported integrity located in nigrostriatal nerve terminals153. Likewise, the progression of disease has shown to reduce the effectiveness of medications154, especially on gait characteristics148. Therefore, the findings in the present review strongly suggest the use of rhythmic auditory cueing as an adjunct therapy with medications to curb the motor deficits in Parkinson’s disease. Moreover, we suggest future studies to analyse the long-term effects of rhythmic auditory cueing with withdrawal of parkinsonian medications, to observe whether the enhancements obtained are resilient and are retained, or not.
Another crucial factor in rhythmic auditory cueing that might significantly influence the rehabilitation progress of a parkinsonian patient is “change in tempo”. For instance, change in tempo has been associated with various neurophysiological changes such as, increased neuronal activation in fronto-occipital networks155, excitability of the spinal motor neurons by reticulospinal pathways, which might possibly reduce the response time for a motor task. Likewise, variation in tempo during training is suggested to be beneficial for maintaining a healthy gait pattern, as constant rhythmic pattern for longer durations have shown to decrease fractal scaling of stride times from healthy 1/f structure, possibly because of organization of stride time variability around a single frequency156,157,158. Additionally, Buchecker et al.159 demonstrated beneficial effects of enhanced variability within training on posture and electromyographic activity (see more from “dynamic system theory”160). This might serve to be beneficial for parkinsonian patients to learn how to regulate gait, when passing through fall-prone environments. Moreover, the induction of variability can also be subjected subliminally (for instance changes in tempo, frequency, timbre, interstimulus interval, see also161). This might maintain variability in the rehabilitation protocol and simultaneously prevent any conscious stress to excessively speed up, or slow down the gait. Future studies can elucidate these effects by evaluating variability in both the auditory and environmental components within training paradigms. In the current analyses, our aim was to determine the extent of tempo shift which might be beneficial in a rehabilitation protocol. Previous studies have shown that healthy participants can easily modulate gait parameters to changes such as ±20%162,163, however parkinsonian patients have failed to demonstrate such effects86. Supposedly, an exceedingly fast tempo might surpass patient’s physiological capabilities and could possibly promote the patient in a high-stress situation20. Further this increased tempo associated enhancement in gait velocity, cadence, and double limbs support parameters can lead to a speed-accuracy trade off86,164. On the contrary, too slow tempo, for instance might allow the participant more time than required to execute a movement, possibly promoting movement specific re-investment1,24. Therefore, the extent of tempo shift should be tailor made according to the patients’ capabilities.
Fast pace stimuli i.e. ≤ +10% has been suggested to effectively counteract reduction in gait velocity, cadence, stride length109. We observed enhancement in gait velocity (g: 0.7), cadence (1.0), and stride length (0.30). Likewise, use of fast paced tempo is to be encouraged during the early phase of disease. Willems et al.86 suggested an association between tempo reduction and enhanced stride length, but also with reduced cadence and gait velocity. This could possibly be attributed to a speed-accuracy trade-off mechanism, where reduction in gait velocity but enhancement in stride length offers slow, but stable gait performance165. The present meta-analysis with the application of slow-paced tempo i.e. ≥+ 10% reported benefits in stride length (g: 0.69), reduction in cadence (−1.25), and gait velocity (−0.24). Thereby, suggesting an efficient manoeuvre to counteract the shuffling gait characteristic in parkinsonian patient i.e. short stride length with faster cadence164,166, especially during the advanced stages of disease where rehabilitation aims should focus more on mobility with stability. Gait training with rhythmic auditory cueing at preferred cadence also has shown to allow benefits in gait velocity (g: 0.43), stride length (0.6), cadence (0.46), reduction in turn time (−2.2), and double limb support phase (−0.56). However, a regular use of the same tempo at preferred cadence might impact recovery in terms of fractal scaling. Therefore, in terms of practical application of different tempo in rehabilitation protocols we suggest utilization of preferred, slow and fast tempi (±10% of preferred cadence), to maintain variability in gait during training.
As per the training dosage that should possess most beneficial effects, we observed fourteen studies analysing the effects of rhythmic auditory training with 30 minutes duration, two studies each analysed the training for 20, 45 minutes and 1 hour. Beneficial enhancements in gait parameters were observed in all the studies analysing the effects during a 30 minutes gait training session. These effects were also evident during a 45 minutes session, and for 20 minutes sessions. However, one study analysing the effects of training for long sessions (1- hour) revealed beneficial effects with a fast paced stimuli112, while the other revealed no effects112. We believe, both mental and physical fatigue could have played a crucial aspect for affecting the gait parameters167 during the long sessions. Nevertheless, more evidence from training studies is required to ascertain the negative effects of long training sessions. Based on the current evidence we strongly suggest limiting the treatment duration between 25–40 minutes/ session. Likewise, a minimum of at least 3–5 sessions of rehabilitation are suggested per week, because highest enhancement in stride length (g: 0.39), cadence (0.65) and gait velocity (0.73) were observed during this period. However, this analysis of training dosage must be carefully interpreted as substantial heterogeneity was observed within studies, due to difference in severity and training regimes. These suggestions are in line with the findings of Nascimento et al.168 where the authors reported application of rhythmic auditory cueing for 30 minutes and for 4 times a week for stroke patients.
It is important to note that the retention of enhancements in gait parameters relies not only on the training received in the clinic but also depends largely on how much the patient follows the treatment protocol at home. The patient usually spends limited amount of time in a rehabilitation setting. Therefore, performing and re-executing the tasks effectively and regularly at home is vital for enhancements in motor performance and quality of life112. Lim et al.11 for instance, reported enhancement in walking activity to 35 minutes per day (qualifying the 30 minutes criteria by centres for disease control and prevention169) post home-based gait training with rhythmic auditory cueing. In addition, a home-based training device allowed a 4.2% increase in posture and gait score, 5.5% reduction in freezing instances, 4 cm increase in step length and a 5 cm increase in walking speed120. This type of home-based intervention could possibly be beneficial for people lacking proper exposure to medical interventions in developing countries125. For instance, parkinsonian patients lacking effective treatment can utilize smartphone devices with dynamic metronome apps such as Walkmate157, Listenmee91, which with proper medical guidance might allow curbing the motor deficits associated with Parkinson’s disease170. In addition, combining the use of external rhythmic entrainment process with different treatment strategies might be a useful tool in rehabilitation as Post et al.171 suggested the most effective rehabilitation protocol for parkinsonian patients to be multidisciplinary. We included studies analysing the beneficial effects of co-joint application of treadmill and rhythmic auditory cueing. Combining treadmill in gait training sessions offered additional benefits as compared to conventional over ground sessions38. For instance, Bello et al.172, reported improvements in stride length, gait speed, time up and go performance and static postural stability, with retention evitable one-month post training. The current meta-analysis revealed beneficial effects of treadmill training in gait velocity (g: 1.0).
Additionally, using the rhythmic entrainment factor with music, could possibly provoke benefits in both psycho-physiological domains173,174,175,176,177. For instance, regulating stress levels, mediating arousal, emotions, internal motivation, memory, attention, executive functions178,179, muscle power180, and endurance178. Modifications in the types of auditory cueing can also impart differential effects on psycho-physiological aspects of performance. For instance, timbre of an auditory input at a higher intensity merged in a broad ascending melody and a rich harmony can possibly motivate a patient to exert more power182,183. Also, parkinsonian and associated ageing changes in patients often characterize a higher threshold for action relevant acoustic input, therefore using ecologically valid action related sounds convening spatio-temporal information can possibly enhance saliency of sensory information, transferring spatio-temporal information effectively and therefore providing more benefits94,95,184,185. This was also demonstrated by Dotov et al.94, and95,185. These authors demonstrated beneficial effects on spatio-temporal gait parameters with biologically variable rhythmic auditory cueing as compared to isosynchronous cueing. Thereby, suggesting potential for modification of auditory signal characteristics for enhancing motor performance in parkinsonian patients. Further methods providing real-time auditory information could possess considerable benefits for enhancing gait performance. One of these methods is movement sonification73: here movement parameters are transformed in real-time to sound with an aim to enhance motor perception and performance by targeting areas associated with biological motion perception66,186,187. Although few research has been carried out to analyse its effects on parkinsonian gait performance95,188, yet, several studies highlight its impact on motor performance and its potential for motor rehabilitation189,190. Schmitz and Effenberg190 have shown that the synchronization of cyclic movement patterns with movement sonification reduces variability and increases constancy of movements as compared to discrete auditory stimuli. Furthermore, listening to sonified human movements in contrast to or in addition to non-human auditory stimuli seems to influence movement timing and strengthen entrainment effects191, possibly by activating mechanisms of biological motion processing in the human brain66,192. Moreover, listening to sonification might allow parkinsonian patients to identify their own movement amplitudes and compare their sound with the sound of an auditory movement model, thereby creating a new auditory reference frame. This reference framework might allow a comparison between instructed and intended movement, possibly amplifying the internal representation of movements193. This might then induce effects on motor behaviour beyond rhythmic adjustments58.
Moreover, counteracting alleviation in conscious attention towards autonomic control, in parkinsonian patients is very critical. Several studies have tried to co-jointly analyze the effects of dual-tasks and rhythmic auditory cueing i.e. to analyze the robustness of auditory-motor coupling with higher information processing constraints109,116. Dual-tasks are expected to protect the automaticity of the motor tasks, by possibly engaging information processing resources necessary for conscious control (see also constrained action hypothesis, [27]). This present analysis observed small effects on gait parameters with dual task application i.e. gait velocity (g: 0.38), stride length (0.31), and cadence (0.11). Beneficial effects on age related controls have been reported during similar interventions109. Nevertheless, rhythmic auditory cueing both with and without training reduced the constraining effects of a manual dual task over gait88,121. Interpretations from our results however suggest that rhythmic auditory cueing counteracts cognitive constraints imposed by cognitively demanding dual-tasks such as carrying a tray, and that this external cueing might be useful in counteracting fall prone situations such as escalators, traffic signals (see more cross-modal overload substitution194). Moreover, dual-task training has been suggested to impart beneficial impacts on stability, as the training phase might allow smoothing of cognitive abilities195. Possibly, including dual-task training regimes with different complexities with rhythmic auditory cueing might enhance functional rehabilitation progress, self-dependence for instance while carrying out activities of daily living. Lastly, patients with Parkinson’s have been shown to demonstrate considerable rigidity in trunk motions196, possibly leading to asymmetry, reduction in arm swing amplitude197, and trunk rotation during gait198. Son and Kim199 reported beneficial effects of rhythmic auditory cueing for increasing arm swing amplitude (36.4° ± 3° vs 25.2° ± 2.8°) and trunk rotation (7° ± 1.3° vs 6.6° ± 0.9°). Thereby, suggesting the beneficial effects of rhythmic auditory cueing beyond the spatiotemporal parameters of gait for enhancing stability.
Our results are consistent with the findings of previous meta-analysis by Spaulding et al.43, stride length (g: 0.49) and gait velocity (0.54), and cadence (g: 0.55). However, the review did not analyse the quality of included studies, and abstained from performing a heterogeneity analysis. Moreover, Rocha et al.42 included only seven studies and reported moderate-to-substantial heterogeneity in between studies and abstained from performing sub-group analysis to evaluate the reason for heterogeneity. Therefore, this present literature review for the first time bridges the gap in parkinsonian literature concerning the effects of presence/absence of medications, tempo variations, dual-task settings, and training dosage for improving gait performance with rhythmic auditory cueing.
In conclusion, this review strongly suggests the early incorporation of rhythmic auditory cueing for enhancing gait performance in patients affected from parkinsonism. The results based on meta-analysis suggests training with rhythmic auditory cueing should include tempo variations of ±10% with respect to the preferred cadence, for a minimal period of 20–45 minutes per day, for at least 3–5 days per week. However, in the absence of such facilities as in developing countries, smartphone based apps should be promoted by medical practitioners for home based therapy.
References
Masters, R. S. W. & Maxwell, J. The theory of reinvestment. Int. Rev. Sport. Exer. Psychol. 1, 160–183 (2008).
Tinetti, M. E., Speechley, M. & Ginter, S. F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med 319, 1701–1707 (1988).
Boudarham, J. et al. Variations in Kinematics during Clinical Gait Analysis in Stroke Patients. PLoS. ONE. 8, e66421, https://doi.org/10.1371/journal.pone.0066421 (2013).
Ghai, S., Ghai, I. & Effenberg, A. O. Effects of dual tasks and dual-task training on postural stability: a systematic review and metaanalysis. Clin. Interv. Aging. 12, 557–577, https://doi.org/10.2147/cia.s125201 (2017).
Ghai, S., Driller, M. & Ghai, I. Effects of joint stabilizers on proprioception and stability: A systematic review and meta-analysis. Phys. Ther. Sport. 25, 65–75, https://doi.org/10.1016/j.ptsp.2016.05.006 (2017).
Schlicht, J., Camaione, D. N. & Owen, S. V. Effect of intense strength training on standing balance, walking speed, and sit-to-stand performance in older adults. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 56, M281–M286 (2001).
Onder, G. et al. Change in physical performance over time in older women The Women’s Health and Aging Study. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 57, M289–M293 (2002).
Cromwell, R. L. & Newton, R. A. Relationship between balance and gait stability in healthy older adults. J. Aging. Phys. Act. 12, 90–100 (2004).
Bhatt, T., Espy, D., Yang, F. & Pai, Y.-C. Dynamic gait stability, clinical correlates, and prognosis of falls among community-dwelling older adults. Arch. Phys. Med. Rehabil. 92, 799–805 (2011).
Herman, T., Giladi, N., Gruendlinger, L. & Hausdorff, J. M. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson’s disease: a pilot study. Arch. Phys. Med. Rehabil. 88, 1154–1158 (2007).
Lim, I. et al. Does cueing training improve physical activity in patients with Parkinson’s disease? Neurorehabil. Neural. Repair. 24, 469–477 (2010).
Gelb, D. J., Oliver, E. & Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 56, 33–39 (1999).
Morris, M. E. Gait Disorders in Parkinson’s Disease: A Framework for Physical Therapy Practice. J. Neurol. Phys. Ther. 21, 125–131 (1997).
Jankovic, J. & Tolosa, E. Parkinson’s disease and movement disorders. (Lippincott Williams & Wilkins, 2007).
Asmus, F., Huber, H., Gasser, T. & Schöls, L. Kick and rush: paradoxical kinesia in Parkinson disease. Neurology. 71, 695 (2008).
Lexell, J. Evidence for nervous system degeneration with advancing age. J. Nutr. 127, 1011S–1013S (1997).
Cameron, M. H. & Wagner, J. M. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr. Neurol. Neurosci. Rep. 11, 507–515 (2011).
Cromwell, R. L., Newton, R. A. & Forrest, G. Influence of vision on head stabilization strategies in older adults during walking. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 57, M442–M448 (2002).
Medeiros, H., Bd., O., Araújo, D. S. M. Sd & Araújo, C. G. Sd Age-related mobility loss is joint-specific: an analysis from 6,000 Flexitest results. Age. 35, 2399–2407, https://doi.org/10.1007/s11357-013-9525-z (2013).
Ghai, S., Ghai, I., & Effenberg, A. O. Effect of Rhythmic Auditory Cueing on Aging Gait: A Systematic Review and Meta-Analysis. Aging. Dis. 131–200, https://doi.org/10.14336/ad.2017.1031 (2017).
Hausdorff, J. M. Gait Dynamics, Fractals and Falls: Finding Meaning in the Stride-to-Stride Fluctuations of Human Walking. Hum. Mov. Sci. 26, 555–589, https://doi.org/10.1016/j.humov.2007.05.003 (2007).
Callisaya, M. L. et al. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age. Ageing. 40, 481–487 (2011).
Schaefer, S., Schellenbach, M., Lindenberger, U. & Woollacott, M. Walking in high-risk settings: Do older adults still prioritize gait when distracted by a cognitive task? Exp. Brain. Res. 233, 79–88 (2015).
Ghai, S., Driller, M. & Masters, R. The influence of below-knee compression garments on knee-joint proprioception. Gait. Posture. in press, https://doi.org/10.1016/j.gaitpost.2016.08.008 (2016).
Masters, R. S. W. Knowledge, knerves and know‐how: The role of explicit versus implicit knowledge in the breakdown of a complex motor skill under pressure. Brit J Psychol 83, 343–358 (1992).
Ghai, S. Proprioception and Performance: The role of below-knee compression garments and secondary tasks (Master’s dissertation, University of Waikato) (2016).
Talelli, P., Ewas, A., Waddingham, W., Rothwell, J. & Ward, N. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage. 40, 1772–1781 (2008).
Miller, R. A., Thaut, M. H., McIntosh, G. C. & Rice, R. R. Components of EMG symmetry and variability in parkinsonian and healthy elderly gait. Electroencephalogr. Clin. Neurophysiol. 101, 1–7 (1996).
Hong, M., Perlmutter, J. S. & Earhart, G. M. A kinematic and electromyographic analysis of turning in people with Parkinson disease. Neurorehabil. Neural. Repair. 23, 166–176 (2009).
Nieuwboer, A. et al. The short-term effects of different cueing modalities on turn speed in people with Parkinson’s disease. Neurorehabil. Neural. Repair. 23, 831–836, doi:10.1177/1545968309337136 (2009).
Arias, P. & Cudeiro, J. Effect of rhythmic auditory stimulation on gait in Parkinsonian patients with and without freezing of gait. PLoS. ONE. 5, e9675 (2010).
Lawrence, B. J., Gasson, N., Kane, R., Bucks, R. S. & Loftus, A. M. Activities of Daily Living, Depression, and Quality of Life in Parkinson’s Disease. PLoS. ONE. 9, e102294, https://doi.org/10.1371/journal.pone.0102294 (2014).
de Rooij, I. J., van de Port, I. G. & Meijer, J. G. Effect of Virtual Reality Training on Balance and Gait Ability in Patients With Stroke: Systematic Review and Meta-Analysis. Phys. Ther. 96, 1905–1918, https://doi.org/10.2522/ptj.20160054 (2016).
Pizzolato, C. et al. Biofeedback for gait retraining based on real-time estimation of tibiofemoral joint contact forces. IEEE. Trans. Neural. Syst. Rehabil. Eng., https://doi.org/10.1109/tnsre.2017.2683488 (2017).
Eng, J. J. & Tang, P. F. Gait training strategies to optimize walking ability in people with stroke: A synthesis of the evidence. Expert. Rev. Neurother. 7, 1417–1436, https://doi.org/10.1586/14737175.7.10.1417 (2007).
Bastian, A. & Keller, J. L. A Home Balance Exercise Program Improves Walking in People with Cerebellar Ataxia. Neurorehabil. Neural. Repair. 28, 770–778, https://doi.org/10.1177/1545968314522350 (2014).
Hackney, M. E. & Earhart, G. M. Effects of Dance on Movement Control in Parkinson’s Disease: A Comparison of Argentine Tango and American Ballroom. J. Rehabil. Med.: Off. J. UEMS Euro. Board Phys. Rehabil. Med. 41, 475–481, https://doi.org/10.2340/16501977-0362 (2009).
Mehrholz, J. et al. Treadmill training for patients with Parkinson’s disease. Cochrane. Database. Syst. Rev., Cd007830, https://doi.org/10.1002/14651858.CD007830.pub3 (2015).
Thaut, M. H. & Abiru, M. Rhythmic auditory stimulation in rehabilitation of movement disorders: a review of current research. Music. Percept. 27, 263–269 (2010).
Group, P. M. C. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 384, 1196–1205 (2014).
Tedeschi, G., Sasso, E., Marshall, R. W. & Bonavita, V. Tremor in Parkinson disease: acute response to oral levodopa. Ital. J. Neurol. Sci. 11, 259–263 (1990).
Rocha, P. A., Porfírio, G. M., Ferraz, H. B. & Trevisani, V. F. Effects of external cues on gait parameters of Parkinson’s disease patients: a systematic review. Clin. Neurol. Neurosurg. 124, 127–134 (2014).
Spaulding, S. J. et al. Cueing and gait improvement among people with Parkinson’s disease: a meta-analysis. Arch. Phys. Med. Rehabil. 94, 562–570 (2013).
Maculewicz, J., Erkut, C. & Serafin, S. An investigation on the impact of auditory and haptic feedback on rhythmic walking interactions. Int. J. Hum. Comput. Stud. 85, 40–46 (2016).
Nombela, C., Hughes, L. E., Owen, A. M. & Grahn, J. A. Into the groove: can rhythm influence Parkinson’s disease? Neurosci. Biobehav. Rev. 37, 2564–2570 (2013).
Thaut, M. H. Rhythm, music, and the brain: Scientific foundations and clinical applications. Vol. 7 (Routledge, 2005).
van den Heuvel, M. R., Daffertshofer, A., Beek, P. J., Kwakkel, G. & van Wegen, E. E. The effects of visual feedback during a rhythmic weight-shifting task in patients with Parkinson’s disease. Gait. Posture. 48, 140–145, https://doi.org/10.1016/j.gaitpost.2016.03.020 (2016).
Raglio, A. Music therapy interventions in Parkinson’s disease: the state-of-the-art. Front. Neurol. 6 (2015).
Shelton, J. & Kumar, G. P. Comparison between auditory and visual simple reaction times. Neurosci. Med. 1, 30 (2010).
Thaut, M., Kenyon, G., Schauer, M. & McIntosh, G. The connection between rhythmicity and brain function. IEEE Eng. Med. Biol. Magazine 18, 101–108 (1999).
Ermolaeva, V. Y. & Borgest, A. Intercortical connections of the auditory areas with the motor area. Neurosci. Behav. Physiol. 10, 210–215 (1980).
Felix, R. A., Fridberger, A., Leijon, S., Berrebi, A. S. & Magnusson, A. K. Sound rhythms are encoded by postinhibitory rebound spiking in the superior paraolivary nucleus. J. Neurosci. 31, 12566–12578 (2011).
Thaut, M. H., McIntosh, G. C. & Hoemberg, V. Neurobiological foundations of neurologic music therapy: rhythmic entrainment and the motor system. Front. Psychol. 5, 1185, https://doi.org/10.3389/fpsyg.2014.01185 (2014).
Butler, J. S., Foxe, J. J., Fiebelkorn, I. C., Mercier, M. R. & Molholm, S. Multisensory representation of frequency across audition and touch: high density electrical mapping reveals early sensory-perceptual coupling. J. Neurosci. 32, 15338–15344 (2012).
Lega, C., Vecchi, T., D’Angelo, E. & Cattaneo, Z. A. TMS investigation on the role of the cerebellum in pitch and timbre discrimination. Cerebellum. Ataxia. 3, 6, https://doi.org/10.1186/s40673-016-0044-4 (2016).
Bukowska, A. A., Krężałek, P., Mirek, E., Bujas, P. & Marchewka, A. Neurologic music therapy training for mobility and stability rehabilitation with Parkinson’s disease–A pilot study. Front. Hum. Neurosci. 9 (2015).
Effenberg, A. O., Fehse, U., Schmitz, G., Krueger, B. & Mechling, H. Movement sonification: Effects on motor learning beyond rhythmic adjustments. Front. Neurosci. 10, 219 (2016).
Yoo, G. E. & Kim, S. J. Rhythmic auditory cueing in motor rehabilitation for stroke patients: systematic review and meta-analysis. J. Music. Ther. 53, 149–177 (2016).
Kim, S. J. et al. Changes in gait patterns with rhythmic auditory stimulation in adults with cerebral palsy. Neuro. Rehabil. 29, 233–241 (2011).
Ghai, S., Ghai, I. & Effenberg, A. O. Effect of rhythmic auditory cueing on gait in Cerebral palsy: A systematic review and metaanalysis. Neuropsychiatr. Dis. Treat., 1–17 (2017) Accepted, In press.
Thaut, M. H., Miltner, R., Lange, H. W., Hurt, C. P. & Hoemberg, V. Velocity modulation and rhythmic synchronization of gait in Huntington’s disease. Mov. Disord. 14, 808–819 (1999).
Baram, Y. & Miller, A. Auditory feedback control for improvement of gait in patients with Multiple Sclerosis. J. Neurol. Sci. 254, 90–94 (2007).
Shannon, K. The effect of rhythmic auditory stimulation on the gait parameters of patients with incomplete spinal cord injury: an exploratory pilot study. Int. J. Rehabil. Res. 31, 155–157 (2008).
Thaut, M. H. Neural basis of rhythmic timing networks in the human brain. Ann. N. Y. Acad. Sci. 999, 364–373 (2003).
Fujioka, T., Trainor, L. J., Large, E. W. & Ross, B. Internalized timing of isochronous sounds is represented in neuromagnetic beta oscillations. J. Neurosci. 32, 1791–1802 (2012).
Schmitz, G. et al. Observation of sonified movements engages a basal ganglia frontocortical network. BMC. Neurosci. 14, 1 (2013).
Heremans, E. et al. Cued motor imagery in patients with multiple sclerosis. Neuroscience. 206, 115–121 (2012).
Heremans, E. et al. External cueing improves motor imagery quality in patients with Parkinson disease. Neurorehabil. Neural. Repair. 26, 27–35 (2012).
Miller, R. A., Thaut, M. H., McIntosh, G. C. & Rice, R. R. Components of EMG symmetry and variability in parkinsonian and healthy elderly gait. Electroencephalogr. Clin. Neurophysiol. 101, 1–7 (1996).
Luft, A. R. et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 292, 1853–1861 (2004).
Rochester, L., Baker, K., Nieuwboer, A. & Burn, D. Targeting dopa‐sensitive and dopa‐resistant gait dysfunction in Parkinson’s disease: Selective responses to internal and external cues. Mov. Disord. 26, 430–435 (2011).
Raglio, A. Music Therapy Interventions in Parkinson’s Disease: The State-of-the-Art. Front. Neurol. 6, 185, https://doi.org/10.3389/fneur.2015.00185 (2015).
Ghai, S., Schmitz, G., Hwang, T.-H. & Effenberg, A. O. In 22nd Annunal Congress of European College of Sports Science. (edsFerrauti, A. et al.) 36.
Lim, I. et al. Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: a systematic review. Clin. Rehabil. 19, 695–713 (2005).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 151, W-65–W-94 (2009).
de Morton, N. A. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust. J. Physiother. 55, 129–133 (2009).
Maher, C. G., Sherrington, C., Herbert, R. D., Moseley, A. M. & Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 83, 713–721 (2003).
Teasell, Robert, N. Bayona, & J. Bitensky. Background concepts in stroke rehabilitation. EBRSR: Evidence-Based Review of Stroke Rehabilitation (2008).
Ramsey, L., Winder, R. J. & McVeigh, J. G. The effectiveness of working wrist splints in adults with rheumatoid arthritis: A mixed methods systematic review. J. Rehabil. Med. 46, 481–492 (2014).
Borenstein, M., Hedges, L. V., Higgins, J. & Rothstein, H. R. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res. Synthesis Met. 1, 97–111 (2010).
Higgins, J. P. & Green, S. Cochrane handbook for systematic reviews of interventions. Vol. 4 (John Wiley & Sons, 2011).
Cumming, G. Understanding the new statistics: Effect sizes, confidence intervals, and meta-analysis. (Routledge, 2013).
Cohen, J. Statistical power analysis for the behavioral sciences. 2nd edn, (L, Erlbaum Associates, 1988).
Rochester, L., Burn, D. J., Woods, G., Godwin, J. & Nieuwboer, A. Does auditory rhythmical cueing improve gait in people with Parkinson’s disease and cognitive impairment? A feasibility study. Movement. Disorders. 24, 839–845 (2009).
Chaiwanichsiri, D., Wangno, W., Kitisomprayoonkul, W. & Bhiyadayasiri, R. Treadmill training with music cueing: a new approach for Parkinson’s gait facilitation. Asian. Biomed. 5(5), 649–654, https://doi.org/10.5372/1905-7415.0505.086 (2011).
Willems, A.-M. et al. The use of rhythmic auditory cues to influence gait in patients with Parkinson’s disease, the differential effect for freezers and non-freezers, an explorative study. Disabil. Rehabil. 28, 721–728 (2006).
Willems, A. M. et al. Turning in Parkinson’s disease patients and controls: the effect of auditory cues. Mov. Disord. 22, 1871–1878 (2007).
Rochester, L. et al. Evidence for motor learning in Parkinson’s disease: acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain. Res. 1319, 103–111 (2010).
Nieuwboer, A. et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J. Neurol. Neurosurg. Psych. 78, 134–140 (2007).
Chen, P.-H. et al. Walking Turns in Parkinson’s Disease Patients with Freezing of Gait: The Short-term Effects of Different Cueing Strategies. Int. J. Gerontol. 10, 71–75 (2016).
Lopez, W. O. et al. Listenmee and Listenmee smartphone application: synchronizing walking to rhythmic auditory cues to improve gait in Parkinson’s disease. Hum. Mov. Sci. 37, 147–156, https://doi.org/10.1016/j.humov.2014.08.001 (2014).
Espay, A. J. et al. At-home training with closed-loop augmented-reality cueing device for improving gait in patients with Parkinson disease. J. Rehabil. Res. Dev. 47, 573 (2010).
Howe, T. E., Lövgreen, B., Cody, F. W., Ashton, V. & Oldham, J. Auditory cues can modify the gait of persons with early-stage Parkinson’s disease: a method for enhancing parkinsonian walking performance? Clin. Rehabil. 17, 363–367 (2003).
Dotov, D. et al. Biologically-variable rhythmic auditory cues are superior to isochronous cues in fostering natural gait variability in Parkinson’s disease. Gait. Posture. 51, 64–69 (2017).
Young, W. R., Rodger, M. W. & Craig, C. M. Auditory observation of stepping actions can cue both spatial and temporal components of gait in Parkinson’s disease patients. Neuropsychologia. 57, 140–153 (2014).
Bella, S. D. et al. Gait improvement via rhythmic stimulation in Parkinson’s disease is linked to rhythmic skills. Sci. Rep. 7, 42005, https://doi.org/10.1038/srep42005 (2017).
Benoit, C.-E. et al. Musically cued gait-training improves both perceptual and motor timing in Parkinson’s disease. Front. Hum. Neurosci. 8, 494 (2014).
de Bruin, N. et al. Walking with music is a safe and viable tool for gait training in Parkinson’s disease: the effect of a 13-week feasibility study on single and dual task walking. Parkinsons. Dis. 2010 (2010).
Elston, J., Honan, W., Powell, R., Gormley, J. & Stein, K. Do metronomes improve the quality of life in people with Parkinson’s disease? A pragmatic, single-blind, randomized cross-over trial. Clin. Rehabil. 24, 523–532 (2010).
Ma, H.-I., Hwang, W.-J. & Lin, K.-C. The effects of two different auditory stimuli on functional arm movement in persons with Parkinson’s disease: a dual-task paradigm. Clin. Rehabil. 23, 229–237 (2009).
Jiang, Y. & Norman, K. E. Effects of visual and auditory cues on gait initiation in people with Parkinson’s disease. Clin. Rehabil. 20, 36–45, https://doi.org/10.1191/0269215506cr925oa (2006).
del Olmo, M. F., Arias, P., Furio, M., Pozo, M. & Cudeiro, J. Evaluation of the effect of training using auditory stimulation on rhythmic movement in Parkinsonian patients—a combined motor and [18 F]-FDG PET study. Parkinsonism. Relat. Disord. 12, 155–164 (2006).
Bolier, L. et al. Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC. Public. Health. 13, 119 (2013).
Arias, P. & Cudeiro, J. Effects of rhythmic sensory stimulation (auditory, visual) on gait in Parkinson’s disease patients. Exp. Brain. Res. 186, 589–601 (2008).
Picelli, A. et al. Three-dimensional motion analysis of the effects of auditory cueing on gait pattern in patients with Parkinson’s disease: a preliminary investigation. Neurol. Sci. 31, 423–430 (2010).
Frazzitta, G., Maestri, R., Uccellini, D., Bertotti, G. & Abelli, P. Rehabilitation treatment of gait in patients with Parkinson’s disease with freezing: a comparison between two physical therapy protocols using visual and auditory cues with or without treadmill training. Mov. Disord. 24, 1139–1143 (2009).
McIntosh, G. C., Brown, S. H., Rice, R. R. & Thaut, M. H. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psych. 62, 22–26 (1997).
Thaut, M. H. et al. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov. Disord. 11, 193–200, https://doi.org/10.1002/mds.870110213 (1996).
Lohnes, C. A. & Earhart, G. M. The impact of attentional, auditory, and combined cues on walking during single and cognitive dual tasks in Parkinson disease. Gait. Posture. 33, 478–483 (2011).
Chester, E. L., Turnbull, G. I. & Kozey, J. The effect of auditory cues on gait at different stages of parkinson’s disease and during “on”/“off” fluctuations: a preliminary study. Top. Geriatr. Rehabil. 22, 187–195 (2006).
Bryant, M., Rintala, D., Lai, E. & Protas, E. An evaluation of self-administration of auditory cueing to improve gait in people with Parkinson’s disease. Clin. Rehabil. 23, 1078–1085 (2009).
del Olmo, M. F. & Cudeiro, J. Temporal variability of gait in Parkinson disease: Effectsof a rehabilitation programme based on rhythmic sound cues. Parkinsonism. Relat. Disord. 11, 25–33 (2005).
Harro, C. et al. The effects of speed-dependent treadmill training and rhythmic auditory-cued overground walking on balance function, fall incidence, and quality of life in individuals with idiopathic Parkinson’s disease: A randomized controlled trial. NeuroRehabilitation. 34, 541–556 (2014).
Hausdorff, J. M. et al. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. Euro. J. Neurosci. 26, 2369–2375 (2007).
Suteerawattananon, M., Morris, G., Etnyre, B., Jankovic, J. & Protas, E. Effects of visual and auditory cues on gait in individuals with Parkinson’s disease. J. Neurol. Sci. 219, 63–69 (2004).
Baker, K., Rochester, L. & Nieuwboer, A. The immediate effect of attentional, auditory, and a combined cue strategy on gait during single and dual tasks in Parkinson’s disease. Arch. Phys. Med. Rehabil. 88, 1593–1600 (2007).
Baker, K., Rochester, L. & Nieuwboer, A. The effect of cues on gait variability—Reducing the attentional cost of walking in people with Parkinson’s disease. Parkinsonism. Relat. Disord. 14, 314–320 (2008).
Chen, J. L., Zatorre, R. J. & Penhune, V. B. Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. Neuroimage. 32, 1771–1781 (2006).
Rochester, L. et al. The effect of external rhythmic cues (auditory and visual) on walking during a functional task in homes of people with Parkinson’s disease. Arch. Phys. Med. Rehabil. 86, 999–1006 (2005).
Nieuwboer, A. et al. Impact of a therapeutic cueing program in the home on gait related mobility in Parkinson’s disease. A randomised clinical trial. J. Neurol. Neurosurg. Psychiatry. 78, 134–140 (2007).
Rochester, L. et al. The attentional cost of external rhythmical cues and their impact on gait in Parkinson’s disease: effect of cue modality and task complexity. J. Neural. Transm. 114, 1243 (2007).
Pau, M. et al. effects of Physical rehabilitation integrated with rhythmic auditory stimulation on spatio-Temporal and Kinematic Parameters of gait in Parkinson’s Disease. Front. Neurol. 7 (2016).
Song, J. et al. Rhythmic auditory stimulation with visual stimuli on motor and balance function of patients with Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 19, 2001–2007 (2015).
Song, G.-b & Ryu, H. J. Effects of gait training with rhythmic auditory stimulation on gait ability in stroke patients. J. Phys. Ther. Sci. 28, 1403–1406 (2016).
Rochester, L. et al. The effect of cueing therapy on single and dual‐task gait in a drug naïve population of people with Parkinson’s disease in northern Tanzania. Mov. Disord. 25, 906–911 (2010).
De Icco, R. et al. Acute and chronic effect of acoustic and visual cues on gait training in Parkinson’s disease: a randomized, controlled study. Parkinsons. Dis. 2015 (2015).
Morris, M. E., Martin, C. L. & Schenkman, M. L. Striding out with Parkinson disease: evidence-based physical therapy for gait disorders. Phys. Ther. 90, 280 (2010).
Morris, M. E., Iansek, R., Matyas, T. A. & Summers, J. J. The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain. 117, 1169–1181 (1994).
Wearden, J. H. et al. Stimulus timing by people with Parkinson’s disease. Brain. Cogn. 67, 264–279 (2008).
Skodda, S., Flasskamp, A. & Schlegel, U. Instability of syllable repetition as a model for impaired motor processing: is Parkinson’s disease a “rhythm disorder. J. Neural. Transm. 117, 605–612 (2010).
Thaut, M. H., McIntosh, K. W., McIntosh, G. C. & Hoemberg, V. Auditory rhythmicity enhances movement and speech motor control in patients with Parkinson’s disease. Funct. Neurol. 16, 163–172 (2001).
Kotz, S. A. & Schwartze, M. Differential input of the supplementary motor area to a dedicated temporal processing network: functional and clinical implications. Front. Integrat. Neurosci. 5 (2011).
Elsinger, C. L. et al. Neural basis for impaired time reproduction in Parkinson’s disease: an fMRI study. J. Int. Neuropsychol. Soc. 9, 1088–1098 (2003).
Rubinstein, T. C., Giladi, N. & Hausdorff, J. M. The power of cueing to circumvent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson’s disease. Mov. Disord. 17, 1148–1160 (2002).
Tierney, A. & Kraus, N. The ability to move to a beat is linked to the consistency of neural responses to sound. Journal of Neuroscience 33, 14981–14988 (2013).
Debaere, F., Wenderoth, N., Sunaert, S., Van Hecke, P. & Swinnen, S. P. Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage. 19, 764–776 (2003).
Asanuma, H. & Keller, A. Neuronal mechanisms of motor learning in mammals. Neuroreport. 2, 217–224 (1991).
Suh, J. H. et al. Effect of rhythmic auditory stimulation on gait and balance in hemiplegic stroke patients. NeuroRehabilitation. 34, 193–199 (2014).
Torres, E. B., Heilman, K. M. & Poizner, H. Impaired endogenously evoked automated reaching in Parkinson’s disease. J. Neurosci. 31, 17848–17863 (2011).
Rinehart, N. J. et al. An examination of movement kinematics in young people with high-functioning autism and Asperger’s disorder: further evidence for a motor planning deficit. J. Autism. Dev. Disord. 36, 757–767 (2006).
Hanakawa, T., Fukuyama, H., Katsumi, Y., Honda, M. & Shibasaki, H. Enhanced lateral premotor activity during paradoxical gait in Parkinson’s disease. Ann. Neurol. 45, 329–336 (1999).
Cunnington, R., Iansek, R., Bradshaw, J. L. & Phillips, J. G. Movement-related potentials in Parkinson’s disease. Brain: J. Neurol. 118, 935–950 (1995).
Whitall, J. et al. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms a singleblinded randomized controlled trial. Neurorehabil. Neural. Repair. 25, 118–129 (2011).
Ford, M. P., Malone, L. A., Nyikos, I., Yelisetty, R. & Bickel, C. S. Gait training with progressive external auditory cueing in persons with Parkinson’s disease. Arch. Phys. Med. Rehabil. 91, 1255–1261 (2010).
Thaut, M. H. et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single-blind, randomized trial. Neurorehabil. Neural. Repair. 21, 455–459, https://doi.org/10.1177/1545968307300523 (2007).
Marsden, C. D. Problems with long-term levodopa therapy for Parkinson’s disease. Clin. Neuropharmacol. 17(Suppl 2), S32–44 (1994).
Curtze, C., Nutt, J. G., Carlson-Kuhta, P., Mancini, M. & Horak, F. B. Levodopa is a Double-Edged Sword for Balance and Gait in People with Parkinson’s Disease. Mov. Disord. 30, 1361–1370, https://doi.org/10.1002/mds.26269 (2015).
Bryant, M. S., Rintala, D. H., Hou, J. G., Lai, E. C. & Protas, E. J. Effects of Levodopa on Forward and Backward Gait Patterns in Persons with Parkinson’s Disease. NeuroRehabilitation. 29, 247–252, https://doi.org/10.3233/NRE-2011-0700 (2011).
Hausdorff, J. M., Cudkowicz, M. E., Firtion, R., Wei, J. Y. & Goldberger, A. L. Gait variability and basal ganglia disorders: Stride‐tostride variations of gait cycle timing in parkinson’s disease and Huntington’s disease. Movement disorders 13, 428–437 (1998).
Schaafsma, J. D. et al. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J. Neurol. Sci. 212, 47–53 (2003).
Baltadjieva, R., Giladi, N., Gruendlinger, L., Peretz, C. & Hausdorff, J. M. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. Eur. J. Neurosci. 24, 1815–1820 (2006).
Almeida, Q. J., Frank, J. S., Roy, E. A., Patla, A. E. & Jog, M. S. Dopaminergic modulation of timing control and variability in the gait of Parkinson’s disease. Mov. Disord. 22, 1735–1742 (2007).
Fahn, S. Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J. Neurol. 252(Suppl 4), Iv37–iv42, https://doi.org/10.1007/s00415-005-4008-5 (2005).
Levy, G. The relationship of Parkinson disease with aging. Arch. Neurol. 64, 1242–1246 (2007).
Thaut, M. H. et al. Neurologic music therapy improves executive function and emotional adjustment in traumatic brain injury rehabilitation. Ann. N. Y. Acad. Sci. 1169, 406–416 (2009).
Delignières, D. & Torre, K. et al. Fractal dynamics of human gait: a reassessment of the 1996 data of Hausdorff. J. Appl. Physiol. 106, 1272–1279 (2009).
Hove, M. J., Suzuki, K., Uchitomi, H., Orimo, S. & Miyake, Y. Interactive rhythmic auditory stimulation reinstates natural 1/f timing in gait of Parkinson’s patients. PLoS. ONE. 7, e32600 (2012).
Hausdorff, J. M. et al. Fractal dynamics of human gait: stability of long-range correlations in stride interval fluctuations. J. Appl. Physiol. 80, 1448–1457 (1996).
Buchecker, M., Wegenkittl, S., Stöggl, T. & Müller, E. Unstable Footwear Affects Magnitude and Structure of Variability in Postural Control. Motor. Control. 1–35 (2017).
Clark, J. E. & Phillips, S. J. A longitudinal study of intralimb coordination in the first year of independent walking: a dynamical systems analysis. Child. Dev. 64, 1143–1157 (1993).
Tecchio, F., Salustri, C., Thaut, M. H., Pasqualetti, P. & Rossini, P. Conscious and preconscious adaptation to rhythmic auditory stimuli: a magnetoencephalographic study of human brain responses. Exp. Brain. Res. 135, 222–230 (2000).
Peper, C. L. E., Oorthuizen, J. K. & Roerdink, M. Attentional demands of cued walking in healthy young and elderly adults. Gait. Posture. 36, 378–382 (2012).
Roerdink, M., Bank, P. J., Peper, C. L. E. & Beek, P. J. Walking to the beat of different drums: Practical implications for the use of acoustic rhythms in gait rehabilitation. Gait. Posture. 33, 690–694 (2011).
Morris, M. E., Martin, C. L. & Schenkman, M. L. Striding Out With Parkinson Disease: Evidence-Based Physical Therapy for Gait Disorders. Phys. Ther. 90, 280–288, https://doi.org/10.2522/ptj.20090091 (2010).
Sheridan, M. & Flowers, K. Movement variability and bradykinesia in Parkinson’s disease. Brain: J. Neurol. 113, 1149–1161 (1990).
Williams, A. J., Peterson, D. S. & Earhart, G. M. Gait Coordination in Parkinson Disease: Effects of Step Length and Cadence Manipulations. Gait. Posture. 38, 340–344, https://doi.org/10.1016/j.gaitpost.2012.12.009 (2013).
Friedman, J. H. Fatigue in Parkinson’s disease patients. Curr. Treat. Options. Neurol. 11, 186–190 (2009).
Nascimento, L. R., de Oliveira, C. Q., Ada, L., Michaelsen, S. M. & Teixeira-Salmela, L. F. Walking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: a systematic review. J. Physiother. 61, 10–15 (2015).
Pate, R. R. et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 273, 402–407 (1995).
Poushter, J. Smartphone ownership and internet usage continues to climb in emerging economies. Pew Research Center 22 (2016).
Post, B., van der Eijk, M., Munneke, M. & Bloem, B. R. Multidisciplinary care for Parkinson’s disease: not if, but how! Pract. Neurol. 11, 58–61, https://doi.org/10.1136/jnnp.2011.241604 (2011).
Bello, O. et al. The effects of treadmill or overground walking training program on gait in Parkinson’s disease. Gait. Posture. 38, 590–595, https://doi.org/10.1016/j.gaitpost.2013.02.005 (2013).
Fang, R., Ye, S., Huangfu, J. & Calimag, D. P. Music therapy is a potential intervention for cognition of Alzheimer’s Disease: a minireview. Transl. Neurodegener. 6, 2, https://doi.org/10.1186/s40035-017-0073-9 (2017).
Hanna-Pladdy, B. & MacKay, A. The Relation Between Instrumental Musical Activity and Cognitive Aging. Neuropsychology. 25, 378–386, https://doi.org/10.1037/a0021895 (2011).
Sturman, M. T. et al. Physical activity, cognitive activity, and cognitive decline in a biracial community population. Arch. Neurol. 62, 1750–1754, https://doi.org/10.1001/archneur.62.11.1750 (2005).
Mammarella, N., Fairfield, B. & Cornoldi, C. Does music enhance cognitive performance in healthy older adults? The Vivaldi effect. Aging. Clin. Exp. Res. 19, 394–399 (2007).
Stork, M. J., Kwan, M. Y., Gibala, M. J. & Martin Ginis, K. A. Music enhances performance and perceived enjoyment of sprint interval exercise. Med. Sci. Sports. Exerc. 47, 1052–1060, https://doi.org/10.1249/mss.0000000000000494 (2015).
Cha, Y., Kim, Y., Hwang, S. & Chung, Y. Intensive gait training with rhythmic auditory stimulation in individuals with chronic hemiparetic stroke: A pilot randomized controlled study. NeuroRehabilitation. 35, 681–688 (2014).
Menon, V. & Levitin, D. J. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage. 28, 175–184 (2005).
Eliakim, M., Meckel, Y., Nemet, D. & Eliakim, A. The effect of music during warm-up on consecutive anaerobic performance in elite adolescent volleyball players. Int. J. Sports. Med. 28, 321–325 (2007).
Crust, L. Carry-over effects of music in an isometric muscular endurance task. Percept. Mot. Skills. 98, 985–991 (2004).
Millington, P. J., Myklebust, B. M. & Shambes, G. M. Biomechanical analysis of the sit-to-stand motion in elderly persons. Arch. Phys. Med. Rehabil. 73, 609–617 (1992).
Peng, Y.-C. et al. Immediate effects of therapeutic music on loaded sit-to-stand movement in children with spastic diplegia. Gait. Posture. 33, 274–278 (2011).
Gaver, W. W. How do we hear in the world? Explorations in ecological acoustics. Ecol. Psychol. 5, 285–313 (1993).
Young, W., Rodger, M. & Craig, C. M. Perceiving and reenacting spatiotemporal characteristics of walking sounds. J. Exp. Psychol. Hum. Percept. Perform. 39, 464 (2013).
Effenberg, A. O. In Encyclopedia of Sport and Exercise Psychology Vol. 2 (eds Eklund, R. C. & Tenenbaum, G.) 663–667 (SAGE Publications, 2014).
Ghai, S., Ghai, I., & Effenberg, A. O. “Low road” to rehabilitation. Neuropsychiatr. Dis. Treat. (2017): Accepted, In press.
Rodger, M. W., Young, W. R. & Craig, C. M. Synthesis of walking sounds for alleviating gait disturbances in Parkinson’s disease. IEEE. Trans. Neural. Syst. Rehabil. Eng. 22, 543–548, https://doi.org/10.1109/tnsre.2013.2285410 (2014).
Schmitz, Gerd, Daniela Kroeger & Alfred, O. Effenberg. A mobile sonification system for stroke rehabilitation. Geo. Ins. Tech. (2014).
Scholz, D. S. et al. Sonification as a possible stroke rehabilitation strategy. Front. Neurosci. 8 (2014).
Schmitz, G. & Effenberg, A. O. Schlagmann 2.0–Bewegungsakustische Dimensionen interpersonaler Koordination im Mannschaftssport. Ger. J. Exerc. Sport. Res. 1–14 (2017).
Demos, A. P., Chaffin, R., Begosh, K. T., Daniels, J. R. & Marsh, K. L. Rocking to the beat: Effects of music and partner’s movements on spontaneous interpersonal coordination. J. Exp. Psychol. Gen. 141, 49 (2012).
Tagliabue, M. & McIntyre, J. A modular theory of multisensory integration for motor control. Front. Computat. Neurosci. 8 (2014).
Hameed, S., Ferris, T., Jayaraman, S. & Sarter, N. Using informative peripheral visual and tactile cues to support task and interruption management. Hum. Factors. 51, 126–135, https://doi.org/10.1177/0018720809336434 (2009).
Hiyamizu, M., Morioka, S., Shomoto, K. & Shimada, T. Effects of dual task balance training on dual task performance in elderly people: a randomized controlled trial. Clin. Rehabil. 26, 58–67 (2012).
Schenkman, M., Morey, M. & Kuchibhatla, M. Spinal flexibility and balance control among community-dwelling adults with and without Parkinson’s disease. J. Gerontol. A. Biol. Sci. Med. Sci. 55, M441–M445 (2000).
Sterling, N. W. et al. Dopaminergic modulation of arm swing during gait among Parkinson’s disease patients. J. Parkinsons. Dis. 5, 141–150, https://doi.org/10.3233/JPD-140447 (2015).
Vaugoyeau, M. et al. Axial rotation in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 77, 815–821, https://doi.org/10.1136/jnnp.2004.050666 (2006).
Son, H. & Kim, E. Kinematic analysis of arm and trunk movements in the gait of Parkinson’s disease patients based on external signals. J. Phys. Ther. Sci. 27, 3783–3786 (2015).
Acknowledgements
The publication of this article was funded by the Open Access funds of Leibniz Universität Hannover.
Author information
Authors and Affiliations
Contributions
S.G. conceptualized the study, carried out the systematic-review, statistical analysis, and wrote the paper. I.G. assisted in statistical analysis. I.G., G.S. and A.O.E. acted as additional reviewers and assisted in the review of the final manuscript. A.O.E., G.S. conceptualized the application of real-time movement sonification approach on parkinsonian patients.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghai, S., Ghai, I., Schmitz, G. et al. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci Rep 8, 506 (2018). https://doi.org/10.1038/s41598-017-16232-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16232-5
This article is cited by
-
Comparison of the effects of rhythmic vibrotactile stimulations and rhythmic auditory stimulations on Parkinson's disease patients’ gait variability: a pilot study
Acta Neurologica Belgica (2024)
-
Investigating gait-responsive somatosensory cueing from a wearable device to improve walking in Parkinson’s disease
BioMedical Engineering OnLine (2023)
-
Good vibrations: tactile cueing for freezing of gait in Parkinson’s disease
Journal of Neurology (2023)
-
Modelling and analysis of Parkinsonian gait
Nonlinear Dynamics (2023)
-
mHealth Impact on Gait and Dynamic Balance Outcomes in Neurorehabilitation: Systematic Review and Meta-analysis
Journal of Medical Systems (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.