Abstract
Approximately 30% of locally advanced rectal cancer patients might not benefit from chemoradiotherapy; however, the response to neoadjuvant chemoradiotherapy in these cases is difficult to predict. Pim-3 is a member of the provirus integration site for a moloney murine leukemia virus family of proteins that contributes to cell proliferation, survival, and chemotherapy resistance. Therefore, the relationship between Pim-3 expression and response to neoadjuvant chemoradiotherapy in rectal cancer patients is important to evaluate. 175 rectal cancer patients who underwent neoadjuvant treatment enrolled in this study. The relationship between Pim-3 expression on immunohistochemical analysis of rectal cancer tissue, which was obtained before treatment, the response to chemoradiotherapy and survival was investigated. The patients with no Pim-3 expression were more likely to achieve a pathologic complete response to chemoradiotherapy than patients with Pim-3 expression (P = 0.001). Cox multivariate analysis showed that the significant prognostic factors were Pim-3 expression (P = 0.003) and the number of neoadjuvant chemotherapy cycles (P = 0.005) for overall survival. Neoadjuvant chemotherapy cycles (P = 0.007), adjuvant chemotherapy cycles (P = 0.004) and pathology types (P = 0.049) were significant prognostic factors for disease-free survival. Pim-3 is a potential predictive biomarker for the response of rectal cancer to chemoradiotherapy.
Similar content being viewed by others
Introduction
Colorectal cancer is the fifth most commonly diagnosed cancer and fifth cause of cancer death in China in both men and women1. In China, the incidence of rectal cancer is higher than that of colon cancer2. In patients with rectal cancer, especially the locally advanced type, the local recurrence rates even after radical resection are high and were reported to range from 15% to 16%3 and to be associated with poor prognosis3,4. Several randomized controlled clinical trials demonstrated that chemoradiotherapy could reduce the local recurrence rate of rectal cancer, reduce the tumor mass, and increase the tumor resection rate5,6. Based on these results, neoadjuvant chemoradiotherapy has been recommended by both the ESMO and NCCN guidelines6,7 to improve the prognosis of locally advanced or stages II and III resectable rectal cancer8. Over the past decade, the use of neoadjuvant chemoradiotherapy has dramatically increased in China.
The effects of neoadjuvant chemoradiotherapy have usually been determined by histopathologic investigation using tumor regression grading (TRG) systems, such as those by Mandard, Becker, Dworak, and Rodel9. The Ryan grading system, which is based on the degree of post-neoadjuvant treatment regressive changes, including fibrosis, and the percentage of residual tumor, was recommended by the NCCN guideline and AJCC. In most cases, a complete response or subtotal tumor regression after neoadjuvant treatment is associated with better patient outcomes10. However, the pathologic complete response (pCR) rate following neoadjuvant treatment is reported to be low at 10–20%11. Previous studies on locally advanced rectal cancer patients five years after neoadjuvant treatment have reported 65.2–76% overall survival rates, 52.2–68% disease-free survival rates, 6–10.7% local recurrence rates, and 34.4–36% distant metastasis rates6,12,13. Therefore, neoadjuvant treatment for rectal cancer remains to be optimized.
Selection of the most suitable patients for chemoradiotherapy is difficult for oncologists. Neoadjuvant treatment may delay the opportunity for radical surgical resection in approximately 30% of rectal cancer patients who would not benefit from chemoradiotherapy and may even lead to progression of the tumor or metastasis during treatment3. All selection criteria used by doctors are based on pelvic magnetic resonance imaging (MRI) and ultrasound colonoscopy findings, such as invasion of all layers of the rectal wall and metastases to regional lymph nodes and the mesorectal fascia. Establishing more effective, objective markers that identify patients who are unlikely to benefit from neoadjuvant treatment would have a great impact in clinical practice.
The Pim family of kinases, including Pim-1, Pim-2, and Pim-3, promotes the inactivation of the pro-apoptotic protein Bad by phosphorylation. Pim is an oncogene that has anti-apoptotic functions and collaborates with the proto-oncogene Myc to cause tumor growth. The Pim proteins can regulate tumor proliferation and the cell cycle as well as enhance the anti-apoptotic functions of some normal and tumor cells14,15,16,17. Previous research has indicated that Pim kinase promotes the transition of the cell cycle from the G1 phase to the S phase and accelerates cell proliferation14,18,19. Until now, very few studies on the function of Pim-3 in metastasis or the response to treatment of colorectal cancer can be found20,21,22. Our previous study indicated that Pim-3 is expressed in colorectal cancer tissue at a rate of approximately 32.6%, but it was very rare in normal colorectal tissue (0.02%)23. We also demonstrated that patients who were positive for Pim-3 had a poor prognosis and showed minimal response to chemotherapy23.
We hypothesized that Pim-3 expression in rectal cancer tissue may be associated with chemotherapy resistance; however, no prior studies have reported on the relationship between response to chemoradiotherapy and Pim-3 expression in rectal cancer. Therefore, the present study aimed to detect Pim-3 expression in rectal cancer and the response to chemoradiotherapy in such cases.
Results
General characteristics
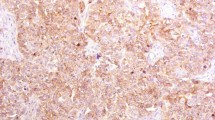
This study enrolled 175 patients with pathologically confirmed rectal cancer who received neoadjuvant chemoradiotherapy; of these, 130 patients demonstrated Pim-3 expression in the primary tumor, while 45 patients were negative for Pim-3 expression (Table 1 and Fig. 1a). According to our IHC result, Pim-3 was rarely expressed in the normal tissue (Fig. 1b). No difference was found between the Pim-3-positive and Pim-3-negative groups, except for the distribution of tumor regression grading (TRG) (P = 0.01). Of all patients, 85 patients were defined as chemoradiotherapy responders and 90 patients were defined as non-chemoradiotherapy responders. Positive lymph nodes (P = 0.008), perineural invasion (PNI) (P = 0.014) and cycles of neoadjuvant chemotherapy (P = 0.014) were significant different between two groups. Further details are presented in Table 2.
(a) Different expression levels of Pim-3 protein in the biopsy tissue before neoadjuvant chemoradiotherapy of 175 rectal cancer patients. The level of Pim-3 expression was classified as follows: negative, weak, moderate, and strong. (Immunohistochemical staining, ×100 and ×400). (b) Example of Pim-3 expression in the tumor/non-tumor tissue on the same slide. (Immunohistochemical staining, ×100 and ×200). (c) The comparison between using PBS and IgG as negative control. (d) Pim-3 expression in rectal cancer and normal tissue by western blot analysis.
Response to chemoradiotherapy
In the logistic regression analysis, Pim-3 expression showed a statistically significant relationship with the pCR [risk ratio (RR) = 5.132, 95% confidence interval (CI): 2.442–10.787; P = 0.001] and response to chemoradiotherapy (RR = 4.47, 95% CI: 1.94–10.018; P = 0.001).
Overall survival and disease-free survival
All patients were followed-up until August 15th, 2017. The 175 patients with 39 months of median follow-up were enrolled in the survival analysis. At the end of follow-up, 148 patients were alive, and 27 had died. Cox multivariate analysis showed that the significant prognostic factors were Pim-3 expression (RR = 1.991, 95% CI = 1.255–3.159; P = 0.003) and the number of neoadjuvant chemotherapy cycles (RR = 0.762, 95% CI = 0.630–0.921; P = 0.005) for overall survival. The number of neoadjuvant chemotherapy cycles (RR = 0.520, 95% CI = 0.323–0.838; P = 0.007), the number of adjuvant chemotherapy cycles (RR = 0.787, 95% CI = 0.667–0.928; P = 0.004) and pathology types (RR = 0.244, 95% CI: 0.060–0.993; P = 0.049) were significant prognostic factors for disease-free survival. More details are presented in Table 3.
At five years, the overall survival rate for all enrolled patients was 63%, and the disease-free survival rate was 62%. The five-year disease-free survival rate was 73% for the Pim-3-negative and 58% for Pim-3-positive patients (P = 0.037, Fig. 2a) The five-year overall survival rate was 92% for the Pim-3-negative patients and 55% for the Pim-3-positive patients (P = 0.028, Fig. 2b). However, the five-year overall survival rates for different level of Pim-3 expression (negative, weak, moderate, strong) showed no significant (Fig. 2c, P = 0.067).
(a) Five-year overall survival rate for 137 rectal cancer patients according to Pim-3 expression. (b) Five-year disease-free survival rate for 137 rectal cancer patients according to Pim-3 expression. (c) 5 years survival curves for 175 locally advanced rectal cancer patients underwent neoadjuvant chemoradiotherapy with different levels of Pim-3 expression. Pim-3 negative was defined as no Pim-3 expression, while weak, moderate, and strong were defined as positive Pim-3 expression. Tumor regression was classified into the following four histologic TRGs based on vital tumor tissue and the ratio of fibrosis after chemoradiotherapy: TRG 0 indicating complete regression and the absence of viable cancer cells; TRG 1 indicating the presence of only small clusters or single cancer cells; TRG 2 indicating the presence of residual cancer cells with predominant fibrosis; and TRG 3 indicating minimal or no decrease in the tumor cells or extensive residual cancer. TRG 0 and 1 patients were considered chemotherapy responders, while TRG 2 and 3 patients were chemotherapy non-responders.
Discussion
Neoadjuvant chemoradiotherapy is the standard treatment for patients with locally advanced rectal cancer at stage T3 or T4b with or without regional lymph node metastasis. However, locally advanced rectal cancer can only be diagnosed by MRI and ultrasound colonoscopy. In our previous study, downstaging of the lymph node status (cN+ to post-treatment pN0) was detected in 43 (75%) of 57 patients, and downstaging of the T status was observed in 68 (76%) of 90 patients. In total, 36 (40%) of the patients achieved grade 2 or 3 tumor regression24. Based on these results, at least 30% of patients did not benefit from the treatment. Therefore, more objective markers of the response to chemoradiotherapy in rectal cancer patients are needed.
Our previous study showed Pim-3 is expressed differently in rectal cancer tissues and that it could be an important contributor to chemoradiotherapy resistance23. Our result confirmed that Pim-3 expression (RR = 4.47, 95% CI: 1.94–10.018; P = 0.001) in rectal cancer tissue played key roles in chemoradiotherapy resistance25. Locally advanced rectal cancer patients with negative Pim-3 expression were more likely to achieve a better chemoradiotherapy response. Cancer cells have important signal transduction processes that regulate cell death caused by DNA double-strand breaks after ionizing radiation therapy26. One of the most likely causes of resistance to chemoradiotherapy is the response to DNA damage; this process is an evolutionarily conserved signaling complex that involves initiation of DNA repair, activation of cell cycle checkpoints, and extensive modulation of gene expression17,26. The ataxia-telangiectasia mutated kinase (ATM) is the major protein kinase that plays a key role in the DNA damage response complex via autophosphorylation and recruitment to DNA damage sites to phosphorylate downstream substrates that trigger DNA repair27. With irradiation, ATM expression is phosphorylated by Pim-3, which would trigger the activation of DNA damage checkpoints and the phosphorylation of their own substrates to start the DNA damage response.
Furthermore, Pim-3 contributes to chemoradiotherapy resistance by attenuating G2/M cell cycle arrest in cancer cells. ATM can activate the checkpoint kinase (Chk1) and P53 phosphorylation25. When exposed to radiation, phosphorylation of Chk1 and phosphatases initiates the G2/M checkpoint to prevent dephosphorylation of CDK1-Cyclin B, which is required for progression into mitosis. The G2/M checkpoint prevents cells with damaged DNA from entering the mitosis phase, wherein the unrepaired DNA double-strand breaks may cause mitotic catastrophe and cell death28. When Pim-3 augments the expression of phosphorylated ATM, ATM could allow cells with damaged DNA to enter mitosis and escape cell death. We hypothesized that Pim-3 overexpression induces the activation of ATM, which subsequently activates Chk1, leading to augmentation of DNA repair through cell cycle arrest and the DNA repair pathways. Therefore, a tumor that expresses a high level of Pim-3 may be more resistant to chemoradiotherapy. More research is needed to investigate the complex mechanisms of chemoradiotherapy resistance.
In the present study, Pim-3 expression, along with the number of neoadjuvant chemotherapy cycles, was demonstrated to be a predictor of prognosis in rectal cancer patients after at least 39 months of follow-up. Our previous study showed that different levels of Pim-3 expression in tumor tissue are associated with different prognoses23. This result might be explained by the findings that the patients with Pim-3 expression showed more aggressive biological behavior. The association of Pim-3 with poor prognosis and a stronger capacity for invasion and migration might be explained by the ability of Pim-3 to induce the STAT3 signaling pathway and regulate the expression of apoptosis-related genes and VEGF; these changes trigger the proliferation, differentiation, and apoptosis of cancer cells and genes inhibited the migration and proliferation29. Furthermore, Pim-3 kinases cause the phosphorylation of the pro-apoptotic molecule Bad, which promotes its inactivation and increases the expression of the anti-apoptotic family member, Bcl-2. Uncontrolled growth of the tumor would be triggered by these changes. Thirdly, previous research on solid tumors and leukemia14,15,17 indicated that Pim-3 expression may have important effects on p53 or on other members of the anti-apoptosis Bcl-2 protein family. As the most important apoptosis-inducing gene in the body, p53 critically influences the mitochondrial and death receptor pathways, which are both important for apoptosis. These are the possible reasons for the aggressive biological behaviors and poor prognosis of rectal cancer patients with high Pim-3 expression.
Some limitations of this study should be considered. First, there were a limited number of subjects in this study. A randomized, clinical trial should be conducted to provide additional data that will support our conclusions. Prior to chemoradiotherapy, more tissue samples should be obtained for detecting KRAS and BRAF mutations.
In conclusion, this study showed that Pim-3 expression in rectal cancer was associated with poor response to chemoradiotherapy and poor prognosis. The mechanism underlying these results may be related to the Pim-3 pathway, but this requires further investigation. Pim-3 is a potential predictive maker of the response to chemoradiotherapy in rectal cancer.
Methods
Patients
Patients who received neoadjuvant chemoradiotherapy for pathologically confirmed rectal cancer between May 1, 2005 and May 1, 2016 were enrolled in this study. Exclusion criteria were multiple primary colorectal cancer sites, the inability to complete standard neoadjuvant chemoradiotherapy, death within one month after surgery, not receiving radical surgical resection, and incomplete information. Prior to neoadjuvant treatment, tumor tissue obtained by colonoscopy was deposited in the tissue bank of Sun Yat-sen University Cancer Center. Adjuvant chemotherapy was recommended for all patients, including those who achieved pCR.
This study protocol was approved by the Sun Yat-sen University Cancer Center ethics committee and complied with the national legislation and the Declaration of Helsinki. Informed consent was obtained from all participants included in the study.
Of these, 114 were men and 61 were women. Tumor (T) staging and lymph node metastasis were assessed by MRI. The stages were T2 in 5 patients, T3 in 88 patients, and T4b in 82 patients. Lymph nodes were positive for metastasis in 100 patients and negative in 75 patients. All patients received standard radiotherapy, with a total dose of 50 Gy delivered to the gross tumor volume and 46 Gy delivered to the clinical target volume. High energy photons (6 or 8 MV) were used simultaneously in 25 daily fractions, from Monday to Friday, over a period of approximately five weeks. Radiotherapy was combined with chemotherapy in all patients except two.
The neoadjuvant chemotherapy regimen consisted of capecitabine in 30 patients, capecitabine and oxaliplatin (CAPOX) in 137 patients, FOLFOX in 5 patients, and 5-fluorouracil (5-FU) in 1 patient. Chemotherapy was given for one cycle in 1 patient, two cycles in 87 patients, three cycles in 34 patients, and four cycles in 51 patients. Surgical resection involved anterior resection in 118 patients, abdominal perineal resection in 52 patients, and Hartmann resection in 5 patients.
Tumor regression grading
The AJCC TRG system was used in this study to determine the effects of chemoradiotherapy. Tumor regression was classified into the following four histologic TRGs based on vital tumor tissue and the ratio of fibrosis after chemoradiotherapy: TRG 0 indicating complete regression and the absence of viable cancer cells; TRG 1 indicating the presence of only small clusters or single cancer cells; TRG 2 indicating the presence of residual cancer cells with predominant fibrosis; and TRG 3 indicating minimal or no decrease in the tumor cells or extensive residual cancer (Fig. 3). TRG 0 and 1 patients were considered chemotherapy responders, while TRG 2 and 3 patients were chemotherapy non-responders.
AJCC tumor regression grading (TRG) was classified into four histological TRGs, based on vital tumor tissue at the ratio of fibrosis: TRG 0 as complete regression and the absence of viable cancer cells; TRG 1 as the presence of only small clusters or single cancer cells; TRG 2 as the presence of residual cancer cells, but with predominant fibrosis; and TRG 3 as minimal or no decrease in tumor cells or extensive residual cancer.
Follow-up
All patients were followed up in the outpatient department every three months in the first two years, every six months for the next three years, and annually after five years. During each visit, the patients underwent physical examination; testing for CEA and CA 19-9; and abdominal and pelvic ultrasound examinations. Colonoscopy after resection was recommended at approximately one year or at three months if not performed preoperatively because of an obstructing lesion. A repeat colonoscopy was typically recommended at three years and every five years thereafter, unless follow-up colonoscopy indicated the presence of an advanced adenoma in which case the colonoscopy was repeated in one year. All patients underwent chest, abdominal, and pelvic CT scans annually until five years after surgery.
Immunohistochemical analysis and scoring
Immunohistochemistry was performed based on our previously reported standard procedure23. Paraffin-embedded specimens were serially cut into three sections with a thickness of 4 μm. One section was used for routine hematoxylin and eosin staining, and the other two sections were used for staining using the streptavidin peroxidase (SP) immunohistochemistry method. IgG was used as the negative control (when PBS was used as negative control, we got nearly the same result, Fig. 1c). The experimental procedure was performed according to the instructions of the reagent kit manufacturer. After incubation for 30 minutes at 60 °C, the tissue sections were deparaffinized with xylene, rehydrated, and immersed in distilled water for 5 minutes. After blocking, the sections were incubated with the primary antibody, secondary antibody, and enzyme-labeled SP. The sections were developed using DAB and counterstained with hematoxylin. After staining, the sections were cleared, mounted, and subjected to microscopy. The major reagent was a goat anti-human Pim-3 polyclonal antibody (Santa Cruz, CA, USA) at a concentration of 1:200. A positive Pim-3 signal was localized to the cytoplasm. Five representative high power field images of each section were selected, and the number of positive cells in 500 cells was counted.
Each slide was evaluated by applying the immunohistochemistry (IHC) scoring system that was used in our previous study30. Two pathologists reviewed all pathology results. If their conclusions were different, a third pathologist independently evaluated the case and decided on the final result. According to the intensity of positive cells, the results were classified as follows: negative, weak, moderate, and strong23. Negative was defined as no Pim-3 expression, while weak, moderate, and strong were defined as positive Pim-3 expression.
Western blotting analysis
Cells were lysed with RIPA lysis buffer (PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS), which containing 10 mg/ml aprotinin, leupeptin, and 1 mM phenylmethylsulfonyl fluoride. Samples were put on ice for 30 min incubation, and then were centrifuged at 18,000 g for 15 min to remove insoluble materials. Protein concentration in supernatant was measured by Bradford Protein assay. A total of 30 μg protein was resolved by 10% SDS polyacrylamide gel, and then transferred to a PVDF membrane. After transfer, PVDF membrane was blocked by 5% nonfat milk in Tris-buffered saline, then incubated with a mouse monoclonal antibodies against Pim-3 (1:200; Santa Cruz Biotechnology, Texas, USA) and a mouse monoclonal antibodies against GAPDH (1:5000; Proteintech, USA) overnight at 4 °C with gentle shaking. Then, proteins were detected with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature, followed by the ECL development (Bio-Rad Laboratories, Hercules, CA, USA). The western blot analysis was used to confirm the result of IHC (Fig. 1d).
Statistical analysis
The clinical and follow-up data were analyzed using SPSS v19.0. The χ2 test, continuity correction χ2 test, and Fisher’s exact test were used to assess the baseline variables of the patients. The significance of the variables was tested using multivariate Cox’s regression model and logistic regression model. Overall survival was defined as the interval between surgical resection and death or end of follow-up. Disease-free survival was defined as the interval between surgical resection and recurrence, metastasis, or end of follow-up. A P-value of <0.05 was considered statistically significant.
Data availability statement
Supporting data are available to Editorial Board Members and referees.
Ethical approval and informed consent
This was a retrospective study that had been approved by the Sun Yat-sen University Cancer Center Institutional Review Board on Medical Ethics (ethics committee approval number GZR2015-133). All patients offered written informed consent for possible future data analysis before treatment.
References
Chen, W. et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians 66, 115–132, https://doi.org/10.3322/caac.21338 (2016).
Tao, K. et al. Prognostic value of miR-221-3p, miR-342-3p and miR-491-5p expression in colon cancer. American journal of translational research 6, 391–401 (2014).
Li, Y. et al. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. International journal of biological sciences 12, 1022–1031, https://doi.org/10.7150/ijbs.15438 (2016).
van der Meij, W., Rombouts, A. J., Rutten, H., Bremers, A. J. & de Wilt, J. H. Treatment of Locally Recurrent Rectal Carcinoma in Previously (Chemo)Irradiated Patients: A Review. Diseases of the colon and rectum 59, 148–156, https://doi.org/10.1097/DCR.0000000000000547 (2016).
Sebag-Montefiore, D. et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 373, 811–820, https://doi.org/10.1016/S0140-6736(09)60484-0 (2009).
Roh, M. S. et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 27, 5124–5130, https://doi.org/10.1200/JCO.2009.22.0467 (2009).
Rodel, C. et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. The Lancet. Oncology 13, 679–687, https://doi.org/10.1016/S1470-2045(12)70187-0 (2012).
Hong, Y. S. et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. The Lancet. Oncology 15, 1245–1253, https://doi.org/10.1016/S1470-2045(14)70377-8 (2014).
Becker, K. et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 98, 1521–1530, https://doi.org/10.1002/cncr.11660 (2003).
Karamitopoulou, E. et al. Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy: comparison of 2 commonly used scoring approaches. The American journal of surgical pathology 38, 1551–1556, https://doi.org/10.1097/PAS.0000000000000255 (2014).
Hartley, A., Ho, K. F., McConkey, C. & Geh, J. I. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. The British journal of radiology 78, 934–938, https://doi.org/10.1259/bjr/86650067 (2005).
Sauer, R. et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. The New England journal of medicine 351, 1731–1740, https://doi.org/10.1056/NEJMoa040694 (2004).
Gerard, J. P. et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 24, 4620–4625, https://doi.org/10.1200/JCO.2006.06.7629 (2006).
Quan, J., Zhou, L. & Qu, J. Knockdown of Pim-3 suppresses the tumorigenicity of glioblastoma by regulating cell cycle and apoptosis. Cellular and molecular biology 61, 42–50 (2015).
Zhuang, H. et al. Aberrant expression of pim-3 promotes proliferation and migration of ovarian cancer cells. Asian Pacific journal of cancer prevention: APJCP 16, 3325–3331 (2015).
Li, Y. Y. & Mukaida, N. Pathophysiological roles of Pim-3 kinase in pancreatic cancer development and progression. World journal of gastroenterology 20, 9392–9404, https://doi.org/10.3748/wjg.v20.i28.9392 (2014).
Liu, L. H., Lai, Q. N., Chen, J. Y., Zhang, J. X. & Cheng, B. Overexpression of pim-3 and protective role in lipopolysaccharide-stimulated hepatic stellate cells. World journal of gastroenterology 21, 8858–8867, https://doi.org/10.3748/wjg.v21.i29.8858 (2015).
Lou, L. et al. Differential expression of Pim-3, c-Myc, and p-p27 proteins in adenocarcinomas of the gastric cardia and distal stomach. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 35, 5029–5036, https://doi.org/10.1007/s13277-014-1664-z (2014).
Zhang, G., Liu, Z., Cui, G., Wang, X. & Yang, Z. MicroRNA-486-5p targeting PIM-1 suppresses cell proliferation in breast cancer cells. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 35, 11137–11145, https://doi.org/10.1007/s13277-014-2412-0 (2014).
Narlik-Grassow, M., Blanco-Aparicio, C. & Carnero, A. The PIM family of serine/threonine kinases in cancer. Medicinal research reviews 34, 136–159, https://doi.org/10.1002/med.21284 (2014).
Mukaida, N., Wang, Y. Y. & Li, Y. Y. Roles of Pim-3, a novel survival kinase, in tumorigenesis. Cancer science 102, 1437–1442, https://doi.org/10.1111/j.1349-7006.2011.01966.x (2011).
Peng, Y. H. et al. Expression of pim-1 in tumors, tumor stroma and tumor-adjacent mucosa co-determines the prognosis of colon cancer patients. PloS one 8, e76693, https://doi.org/10.1371/journal.pone.0076693 (2013).
Zhou, Z. et al. Expression of Pim-3 in colorectal cancer and its relationship with prognosis. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 37, 9151–9156, https://doi.org/10.1007/s13277-016-4806-7 (2016).
Fan, W. H. et al. Surgery with versus without preoperative concurrent chemoradiotherapy for mid/low rectal cancer: an interim analysis of a prospective, randomized trial. Chinese journal of cancer 34, 394–403, https://doi.org/10.1186/s40880-015-0024-8 (2015).
Chen, X. Y., Wang, Z., Li, B., Zhang, Y. J. & Li, Y. Y. Pim-3 contributes to radioresistance through regulation of the cell cycle and DNA damage repair in pancreatic cancer cells. Biochemical and biophysical research communications 473, 296–302, https://doi.org/10.1016/j.bbrc.2016.03.099 (2016).
O’Connor, M. J. Targeting the DNA Damage Response in Cancer. Molecular cell 60, 547–560, https://doi.org/10.1016/j.molcel.2015.10.040 (2015).
Ciccia, A. & Elledge, S. J. The DNA damage response: making it safe to play with knives. Molecular cell 40, 179–204, https://doi.org/10.1016/j.molcel.2010.09.019 (2010).
Lobrich, M. & Jeggo, P. A. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nature reviews. Cancer 7, 861–869, https://doi.org/10.1038/nrc2248 (2007).
Wang, J., Lao, L., Zhao, H. & Huang, Y. Serine threonine kinase Pim-3 regulates STAT3 pathway to inhibit proliferation of human liver cancers. International journal of clinical and experimental medicine 7, 348–355 (2014).
Peng, J. H. et al. A scoring system based on artificial neural network for predicting 10-year survival in stage II A colon cancer patients after radical surgery. Oncotarget 7, 22939–22947, https://doi.org/10.18632/oncotarget.8217 (2016).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NO. 81502459 and 81602143).
Author information
Authors and Affiliations
Contributions
Rong-xin Zhang conceived and supervised this study and revised the manuscript. Zhong-guo Zhou and Shi-xun Lu made equal contributions to this article, including performing the experiments, interpreting data, statistical analyses, and drafting the manuscript. Xiao-jun Wu, De-sen Wan, Zhi-zhong Pan, Zhen-hai Lu, and Gong Chen were responsible for collecting tissue specimens and relevant clinicopathologic data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Rx., Zhou, Zg., Lu, Sx. et al. Pim-3 as a potential predictor of chemoradiotherapy resistance in locally advanced rectal cancer patients. Sci Rep 7, 16043 (2017). https://doi.org/10.1038/s41598-017-16153-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16153-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.