Abstract
The aim of this retrospective study was to analyze the morbidity, mortality, and survival rates of extended multiorgan resection (EMR) for locally advanced gastric cancer patients compared to gastrectomy alone and a palliative operation. 893 locally advanced gastric cancer patients without distant metastasis had surgery including gastrectomy alone (GA group, n = 798), EMR resection (EMR group, n = 75), and palliative operation (palliative gastrectomy or gastrojejunostomy (PO group, n = 20)). Postoperative mortality and complication rates in the EMR group were significantly higher than in the GA group (2.7% vs 0.4%, P = 0.010 and 25.3% vs 8.1%, P < 0.001, respectively), but similar in the PO group. The median survival time of the EMR group was significantly longer than in the PO group (27 months vs 11 months, P = 0.020), but significantly worse (P = 0.020) than in the GA group (44 months). Incompleteness of resection (R1) and linitis plastica were independent prognostic factors for survival in the EMR group. Three different gastric cancer surgeries led to different postoperative mortality and complication rates. EMR had a better survival rate compared with PO while GA had the longest survival time with the lowest mortality and complication rates.
Similar content being viewed by others
Introduction
Gastric cancer is one of the most common forms of cancer detected worldwide and is a leading cause of death. In general, it occurs most prevalently in Eastern Asia, particularly in Korea, Mongolia, Japan and China1. Unfortunately, gastric cancer typically presents at a locally advanced stage, even invading into adjacent organs in many of our patients in China. For these patients, extended multiorgan resection (EMR) is advocated as the operation of choice for achieving R0 resections, which has been identified as an important indicator of longer term survival in patients undergoing curative surgery for gastric cancer2,3,4 and the long-term outcomes of palliative resection or non-surgical treatments such as chemotherapy for locally advanced gastric cancer are dismal5. On the other hand, some studies concluded that EMR should be cautiously performed in strictly selected patients because of increased postoperative morbidity and mortality6,7. As far as we are aware, no studies have directly compared the outcomes of EMR and palliative operations for gastric cancer in which invasion of nearby organs was suspected. When designing treatment for patients with advanced gastric cancer the optimal surgical procedures remain unclear. In the present study, we retrospectively assessed the effectiveness of EMR compared with gastrectomy alone or palliative surgery in patients with local gastric cancer.
Patients and Methods
From November 2010 to September 2015, 893 patients diagnosed with locally advanced gastric cancer without distant metastasis underwent surgery in the Department of Gastroduodenal and Pancreatic Surgery, Affiliated Cancer Hospital of Xiangya Medical School, Central South University. The inclusion criteria were: (1) patients diagnosed with locally advanced gastric cancer who underwent gastrectomy with a curative intent or a palliative operation including gastrojejunostomy or palliative gastrectomy, (2) patients without distant metastasis, or other malignancies occurring at the same time, (3) patients who had comprehensive and complete clinical records, postoperative pathological and follow-up data. The diagnosis of the cancer stage was based on clinical data, including an enhanced computed tomography (CT) scan, endoscopic ultrasonography (US), and intraoperative assessments. Laparoscopy was performed in patients with linitis plastica in order to detect peritoneal metastasis. In patients with ascites, cytology of the intraperitoneal fluid was performed, while positive cytology was considered as M1 cancer and excluded from this study. EMR was suggested to all patients with locally advanced gastric cancer and suspected invasion into the adjacent organs to achieve R0 resection, but the final choice between EMR and PO has been made by the patients.
Our study was performed in accordance with the Declaration of Helsinki regarding the ethical principles for medical research involving human subjects. This study was approved by the Ethics Committee for Clinical Pharmacology in Central South University, and all patient data were kept strictly confidential. All participants signed informed consent forms.
Surgical procedures
All of the surgical procedures were carried out by highly qualified surgeons. The standard surgical procedures for advanced gastric cancer with curative-intent included D2 or D2 + lymphadenectomy8. The definition of EMR employed was simultaneous en-bloc resection of the stomach and the suspected invading organs. Postoperative morbidity and mortality were graded using a modified Clavien–Dindo classification of surgical complications9. Postoperative mortality was defined as deaths that occurred within 30 days after surgery. The TNM stage was defined by the 7th TNM AJCC/UICC guidelines10. Standard treatment regimens for neoadjuvant chemotherapy or adjuvant therapy after surgery were fluorouracil (such as S-1) + platinum (such as oxaliplatin) chemotherapy for 6 months11.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows (Ver. 19. IBM Corp., Armonk, NY). Demographic data that included the clinicopathological characteristics of the patients, the perioperative outcome, and overall survival rates were retrospectively collected and analyzed. All continuous variables are expressed as the mean ± s.d. and potential differences between groups were assessed using an independent-samples t-test or a Mann–Whitney U-test. Categorical variables are reported as the total number of cases and prevalence, and differences between groups were compared by Fisher exact or χ2 tests. Survival curves were constructed from the observed survival times using the Kaplan-Meier procedure. A log-rank test was used to test for any significant differences in the survival rates. A Cox regression model was used for multivariate analysis. A P-value < 0.05 was considered to be statistically significant.
Results
Basic demographic information of enrolled patients
In total, 1,594 patients were operated on for gastric cancer from November 2010 to September 2015. Of these, 893 patients who underwent surgical treatments were enrolled in our study after being diagnosed with advanced local gastric cancer without the complication of distant metastasis. Patients were allocated into 3 groups according to the surgical approaches used: radical gastrectomy alone (GA group, n = 798), extended multiorgan resection (EMR group, n = 75) and a palliative operation group including palliative gastrectomy and gastrojejunostomy (PO group, n = 20). The mean patient age was 54.6 ± 10.7 years (range, 19–83 years, 893 patients); 608 were men (68.1%) and 285 were women (31.9%). In the PO group, 11 patients (55.0%) with bleeding or pyloric obstruction underwent palliative gastrectomy and the remaining 9 patients with pyloric obstruction underwent gastrojejunostomy.
The proportion of male, American Society of Anesthesiologists score ≥ 3, any comorbidity, neoadjuvant chemotherapy, differentiation type, mean age and body mass index (BMI) were comparable among the 3 groups (Table 1 ). Preoperative albumin and hemoglobin levels were both significantly increased in the GA group patients compared with the EMR and PO groups. Upper third located cancer and linitis plastica were more common in the EMR group (38.7%) and as a result, a significantly greater number of total gastrectomy operations were performed (P < 0.001). The numbers of harvested lymph nodes were significantly greater in the EMR group when compared with the GA group. The majority of the EMR group presented with a higher T and TNM stage, although the N stage distribution was not significantly different from that found in the GA and EMR groups. Prolonged operative time and post-operative hospital stays, increased intraoperative blood loss, and perioperative blood transfusion were mainly observed in the EMR group.
Preoperative albumin and hemoglobin levels were both significantly lower in the EMR and PO patients compared with the GA group, reflecting higher TNM stages as well as more like hood to suffer from pyloric obstruction or bleedings and thus with worse nutritional status. Adjuvant chemotherapy was administered to the majority of the patients (570 cases, 63.3%). The percentages of patients receiving adjuvant chemotherapy in the GA, EMR and PO group were 63.2%, 69.3% and 70.0%, respectively, without significant difference. Neo-adjuvant chemotherapy was administered to 4.5%, 6.7% and 2% of the GA, EMR and PO cases, also without significant difference (Table 1).
Short-term results
The overall morbidity and 30-day mortality for the entire study cohort were 9.6% (86/893) and 0.7% (6/893), respectively. Morbidity occured more commonly in the EMR group with a rate of 25.3% vs 8.1% in the GA group (P < 0.001) respectively, but was comparable with the PO group (10.0%, P = 0.14). And the 30-day mortality for the GA group was 0.4% (3/798), for the EMR group 3.7% (2/75), and for the PO group 5% (1/20) (P = 0.004) (Table 1). The 90-day mortality was higher in the EMR (5.3%) than in the GA group (1.6%) (P = 0.03), but similar in the PO patients (10.0%) (P = 0.45). Peri-operative mortality occurred in 6 patients: 3 subjects in the GA group (0.4%), 2 in the EMR group (2.7%), and 1 in the PO group (5.0%). Significant higher peri-operative mortality was found in the EMR group compared with the GA group (P = 0.01). Severe complications, defined as Clavien–Dindo classification ≥ IIIa, occurred in 10 patients in the EMR group (13.3%), which was significantly higher than in the GA group (3.1%, P < 0.001) but similar to the PO group (5.0%, P = 0.30) (Table 1, Table 2).
Time to recurrence
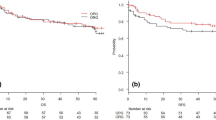
The median follow-up time for patients was 32 months (range, 1–62 months). Recurrence were seen in 36.9% (322/873) of the patients who underwent resection with curative intent (patients in the GA and EMR group). The incidence of recurrence in the EMR group (34/75, 45.3%) was significantly greater than in the GA group (288/798, 36.1%, P = 0.040, Fig. 1A). The median time to recurrence was 17 months in the EMR group compared with 41 months in the GA group (P = 0.005).
(A) Disease free survival cures based on the extent of resection. 798 patients underwent gastrectomy alone (GA group), and 75 patients underwent extended multiorgan resection (EMR group). (Kaplan–Meier procedure, log rank test, P = 0.040). (B) Overall survival cures based on the extent of resection. 798 patients underwent gastrectomy alone (GA group), 75 patients underwent extended multiorgan resection (EMR group), and 20 patients had palliative operations (11 palliative gastrectomy and 9 gastrojejunostomy, PO group). (Kaplan–Meier method, log rank test. P = 0.020 between GA and EMR group; P = 0.020 between EMR and PO group; P < 0.001 between GA and PO group.). (C) Overall survival cures of patients with non-curative resection (R1, n = 10) or curative resection (R0, n = 65) in the EMR group. (Kaplan–Meier method, log rank test, P < 0.001 between the 2 groups). (D) Overall survival cures of patients with linitis plastica (n = 11) or other type of gastric cancer (n = 64) in the EMR group. (Kaplan–Meier method, log rank test, P < 0.001 between the 2 groups).
Survival
The median disease free survival time was significantly longer in the GA compared to the EMR group (P = 0.04). The median survival time for the entire cohort of patients was 20 months. The median survival time of the EMR group (27 months) was significantly shorter than the GA group (44 months, P = 0.020), but significantly longer than the survival time of the PO group (11 months, P = 0.020) (Fig. 1B). The overall 1-year, 3-year and 5-year survival rates were 69.8%, 40.6% and 29.8% respectively in the EMR group, which were statistically worse than in the GA group (84.8%, 58.5%, 34.0%, P = 0.020), and as expected, better than the PO group (44.7%, 9.3%, 0, P = 0.020).
Focusing on the EMR group
The clinicopathological characteristics of the 75 patients in the EMR group are summarized in Table 1. The organ that was most frequently resected was the colon (n = 17), followed by the liver (n = 15) and pancreas/spleen (n = 13) (Table 3). More than 1 organ simultaneous resection occurred in 22 cases (29.3%) and the remaining 53 patients underwent only 1 organ resection. pT4b disease (directing invaded to the nearby organs) was confirmed in 53 patients (70.7%) by post-operative histopathological examination, whereas the remaining 22 patients (29.3%) were identified as pT4a (18 cases) or pT3 (4 cases). Although all EMR surgeries were performed with a curative intent, the performance of R0 resection was accomplished in 65 patients (86.7%), while microscopic (R1) residual disease was obtained in the remaining 10 patients (13.3%) (Table 1).
The median survival time was significantly greater in those patients who underwent curative resection (34 months, R0) than in patients with microscopic residual resection (11 months, R1). Therefore, resections significantly influenced survival (P < 0.001, Fig. 1C). Patients with linitis plastica had a median survival time of 11 months, which was statistically significantly shorter than the 33 months in patients with non-linitis plastica (P < 0.001, Fig. 1D).
The type of organ resected was evaluated for the time to recurrence but no differences were found between pancreatectomy and the resection of other organs. Neither was the number of organs resected a predictor of survival in the entire cohort of 75 patients.
Overwhelmingly after multivariate analysis of the data based on univariate analysis results (Table 4), positive surgical margin (R1 resection, 95% CI, 1.551–10.405, P = 0.004) and linitis plastica (95% CI, 1.293–2.502, P = 0.019) were shown to be independent predictors for survival after EMR (Table 5).
Discussion
Gastric cancer usually presents at an advanced stage in the West, China and in undeveloped countries12,13. Bleeding and pyloric obstruction were commonly associated with these patients. Unfortunately, of those patients with advanced gastric cancer, especially those who presented with pyloric obstruction, nearly half showed direct invasion of adjacent organs14,15. For gastric cancer invading adjacent organs, EMR may be warranted to achieve tumor clearance16. On the other hand, with improvement of surgical techniques and perioperative managements, the morbidity decreased. More importantly, the higher morbidity did not translate into higher perioperative mortality7. Several studies have confirmed that curative resection was beneficial to patients with advanced gastric cancer and EMR can be performed in carefully selected patients with acceptable morbidity and mortality3,7,17. More and more studies have advocated that EMR should be used to treat patients with locally advanced gastric cancer, being the only choice for achieving R0 resections, which is arguably the most significant prognostic factor for the management of gastric cancer7,18,19. As far as we are aware, there is a paucity of data that directly compared the safety and efficiency of EMR with gastrectomy alone or palliative surgery for gastric cancer. Moreover, some patients classified as TNM stage IV3,4,20, or experiencing distress from additional procedures due to ailments other than tumor invasion, such as simultaneous chlolecystectomy for gallstones3 were enrolled into the EMR group, which may affect the credibility of the conclusions. In this large-scale retrospective study, we divided locally advanced gastric cancer patients into EMR, GA and PO groups by surgery approaches for the first time and directly compared the safety and efficiency among the 3 groups.
As reported in previous studies3,6,7,19, patients undergoing EMR experienced a higher risk of postoperative complications in the present study, as did the severe complications classified as Clavien ≥ IIIa. Perioperative death was statistically more common in the EMR group (2.7%) compared with the GA group (0.4%, P = 0.01). The median survival time of the EMR group in the present study was 27 months, which was similar to previously reported values3,7,16,19,20. Though the survival was worse than that in the GA group (44 months), it was significantly better than that in the PO group (11 months). Focusing on the 65 patients undergoing R0 resection in the EMR group, the median survival reached 34 months. Thus, EMR was associated with a satisfactory long-term outcome and acceptable morbidity and mortality for locally advanced gastric cancer in carefully selected patients.
As demonstrated in the present study, a powerful prognostic parameter is R0 resection, which has been widely confirmed by previous studies. The median survival in patients with R0 resection was 34 months, which decreased to 11 months for patients with R1 resection (P < 0.001). Although the majority of previous studies advocated carrying out EMR for locally advanced gastric cancer in pursuit of negative margins, a significant proportion of patients had positive microscopic or macroscopic margins (range 11.3–61.6%)2,6,7,13,19. The present study confirmed these results with a R1 resection rate of 13.3%. Given the high risk of postoperative morbidity and poor survival, palliative EMR should be avoided as much as possible. To our knowledge, for the first time, linitis plastica has been identified as an independent predictor for survival. Given the diffuse nature and high incidence of peritoneal disease, achieving R0 resection and satisfactory prognosis seems difficult in linitis plastica patients21,22. Three patients (27.3%) were found as R1 resection and the median survival for the 11 linitis plastica patients (11 months) undergoing EMR was the same as those undergoing palliative surgery in our study. The extremely poor prognosis of linitis plastica has led others to suggest that it is not a disease requiring surgery with many oncology physicians being against surgical resection for gastric linitis plastica23. Although some strictly selected linitis plastica patients who underwent optimal resections24 and multimodality therapy25 seemed to have an improved long-term survival, the selecting criteria had not been well designated. Thus performing EMR for patients with linitis plastica should be cautiously considered regarding the increased morbidity risk and poor survival. Some studies have demonstrated that tumor size2, the depth of invasion3,19, lymph node metastasis3,16,19,20 and number or type of simultaneous resected organs7,16,20 were associated with poor prognosis. However, in the present study, we did not find that any of the above parameters were correlated with survival.
Only 53 patients (70.7%) were confirmed with pT4b disease by postoperative pathological examinations, while 29.3% of the patients had T4a or T3 disease. Our findings are consistent with prior studies that demonstrated that the percentage of patients with confirmed pathological T4 disease ranged from 13.8% to 89% in those undergoing EMR with the goal of R0 resection3,7,20,26,27,28. The precise identification of T4 disease can be difficult by preoperative CT or endoscopic US. In a research project that evaluated the role of CT in the preoperative assessment of T stage, the predictive value in identifying T4 disease was found to be ≤50%27. In a recent multi-institutional analysis, the accuracy of endoscopic ultrasound for gastric cancer was found to be only 46.2% for T stage and 66.7% for N stage29. It can also be difficult to identify correctly T4 disease by intraoperative assessment. Colen et al.27 reported that the incidence of pathologically confirmed T4 cancers was only 38.1% (8 in 21) by intraoperative assessment. Given the significantly poorer survival in patients undergoing R1 resection and not sufficiently accurate assessment, it seemed reasonable to perform EMR to achieve R0 resection.
Surprisingly, patients undergoing PO experienced a 30 day mortality rate of 5.0% and a 90 day morbidity rate of 10.0%, which was comparable with those undergoing EMR, though the burden of surgery was smaller in the PO group due to less intraoperative blood loss and shorter operation time and none of them experienced surgery-related complications. A possible explanation might be that the patients in the PO group suffered from pyloric obstruction or bleeding, which may affect the patients’ general condition thereby leading to higher risk of systemic complications. On the other hand, accidental factors may have had an impact on the result in the PO group since they comprised a relatively small number of patients.
The present study had several limitations. First, we tried our best to enroll only patients undergoing resection of the adjacent organs and gastrectomy owing to doubts on the invasion of these organs into the EMR group. We could not precisely exclude some other conditions such as iatrogenic injury, or to facilitate extensive lymphadenectomy as this study was retrospective in nature. Second, patients in the EMR and PO groups were more frequently associated with pyloric obstruction and bleeding, suggesting a systemically advanced tumor and unfortunate clinical outcomes compared to those patients without outlet obstruction even after curative resection16,30. Third, patients who underwent either gastrojejunostomy or palliative gastrectomy were enrolled into the PO group and the small sample size in a single institution requires cautious interpretation of the conclusions.
Despite the limitations, the present study provides novel evidence that non-linitis plastica patients undergoing EMR with R0 resection could achieve acceptable postoperative morbidity, mortality and improved survival. Positive residual margin and gastric linitis plastica were associated with poorer survival. These two factors should be borne in mind during the selection process of patients who will most likely benefit from extensive surgery.
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108 (2015).
Mita, K. et al. Surgical outcomes and survival after extended multiorgan resection for T4 gastric cancer. Am J Surg 203, 107–111 (2012).
Martin, R. C. II, Jaques, D. P., Brennan, M. F. & Karpeh, M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg 236, 159–165 (2002).
Xiao, L. et al. Extended multi-organ resection for cT4 gastric carcinoma: A retrospective analysis. Pak J Med Sci 29, 581–585 (2013).
Okumura, Y. et al. Palliative distal gastrectomy offers no survival benefit over gastrojejunostomy for gastric cancer with outlet obstruction: retrospective analysis of an 11-year experience. World J Surg Oncol 12, 364 (2014).
Martin, R. C. II, Jaques, D. P., Brennan, M. F. & Karpeh, M. Achieving RO resection for locally advanced gastric cancer: is it worth the risk of multiorgan resection? J Am Coll Surg 194, 568–577 (2002).
Tran, T. B. et al. Multivisceral Resection for Gastric Cancer: Results from the US Gastric Cancer Collaborative. Ann Surg Oncol 22(Suppl 3), S840–847 (2015).
Japanese Gastric Cancer, A. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14, 113–123 (2011).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240, 205–213 (2004).
Santiago, J. M., Sasako, M. & Osorio, J. TNM-7th edition 2009 (UICC/AJCC) and Japanese Classification 2010 in Gastric Cancer. Towards simplicity and standardisation in the management of gastric cancer. Cir Esp 89, 275–281 (2011).
Noh, S. H. et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 15, 1389–1396 (2014).
Xiao, H. et al. Clavien-Dindo classification and risk factors of gastrectomy-related complications: an analysis of 1049 patients. Int J Clin Exp Med 8, 8262–8268 (2015).
Carboni, F. et al. Extended multiorgan resection for T4 gastric carcinoma: 25-year experience. J Surg Oncol 90, 95–100 (2005).
Watanabe, A. et al. Gastric carcinoma with pyloric stenosis. Surgery 123, 330–334 (1998).
Lee, H. J., Park, D. J., Yang, H. K., Lee, K. U. & Choe, K. J. Outcome after emergency surgery in gastric cancer patients with free perforation or severe bleeding. Dig Surg 23, 217–223 (2006).
Min, J. S. et al. Prognosis of curatively resected pT4b gastric cancer with respect to invaded organ type. Ann Surg Oncol 19, 494–501 (2012).
Li, M. Z. et al. Surgical outcomes and prognostic factors of T4 gastric cancer patients without distant metastasis. PLoS One 9, e107061 (2014).
Brar, S. S. et al. Multivisceral resection for gastric cancer: a systematic review. Gastric Cancer 15(Suppl 1), S100–107 (2012).
Pacelli, F. et al. Multivisceral resection for locally advanced gastric cancer: an Italian multicenter observational study. JAMA Surg 148, 353–360 (2013).
Ozer, I. et al. Surgical outcomes and survival after multiorgan resection for locally advanced gastric cancer. Am J Surg 198, 25–30 (2009).
An, J. Y. et al. Borrmann type IV: an independent prognostic factor for survival in gastric cancer. J Gastrointest Surg 12, 1364–1369 (2008).
Kodera, Y. et al. The number of metastatic lymph nodes is a significant risk factor for bone metastasis and poor outcome after surgery for linitis plastica-type gastric carcinoma. World J Surg 32, 2015–2020 (2008).
Jafferbhoy, S., Shiwani, H. & Rustum, Q. Managing Gastric LinitisPlastica: Keep the scalpel sheathed. Sultan Qaboos Univ Med J 13, 451–453 (2013).
Blackham, A. U. et al. Is Linitis Plastica a Contraindication for Surgical Resection: A Multi-Institution Study of the U.S. Gastric Cancer Collaborative. Ann Surg Oncol 23, 1203–1211 (2016).
Yamashita, K. et al. Survival outcome of Borrmann type IV gastric cancer potentially improved by multimodality treatment. Anticancer Res 35, 897–906 (2015).
Shchepotin, I. B. et al. Extended surgical resection in T4 gastric cancer. Am J Surg 175, 123–126 (1998).
Colen, K. L. et al. Multiorgan resection for gastric cancer: intraoperative and computed tomography assessment of locally advanced disease is inaccurate. J Gastrointest Surg 8, 899–902 (2004).
Jeong, O., Choi, W. Y. & Park, Y. K. Appropriate selection of patients for combined organ resection in cases of gastric carcinoma invading adjacent organs. J Surg Oncol 100, 115–120 (2009).
Spolverato, G. et al. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. J Am Coll Surg 220, 48–56 (2015).
Chen, J. H. et al. Outcome of distal gastric cancer with pyloric stenosis after curative resection. Eur J Surg Oncol 33, 556–560 (2007).
Author information
Authors and Affiliations
Contributions
Hua Xiao, Min Ma, Yanping Xiao, Ming Tang and Chaohui Zuo were responsible for the conception and design of the study. Hua Xiao, Min Ma, Yanping Xiao, Yongzhong Ouyang, Ming Tang, Kunyan Zhou, Yuan Hong, Bo Tang and Chaohui Zuo were responsible for acquisition of data. Hua Xiao, Min Ma, Yanping Xiao, Yongzhong Ouyang and Chaohui Zuo performed the data analysis. Hua Xiao, Min Ma, Yuan Hong and Chaohui Zuo drafted the manuscript. All authors participated in interpretation of the findings. Hua Xiao, Min Ma, Yanping Xiao, Yongzhong Ouyang, Ming Tang, Kunyan Zhou, Yuan Hong, Bo Tang and Chaohui Zuo revised and commented the draft, and all authors read and approved the final version of the manuscript. All authors confirm that the content has not been published elsewhere and does not overlap with or duplicate their published work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiao, H., Ma, M., Xiao, Y. et al. Incomplete resection and linitis plastica are factors for poor survival after extended multiorgan resection in gastric cancer patients. Sci Rep 7, 15800 (2017). https://doi.org/10.1038/s41598-017-16078-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16078-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.