Abstract
A Bi3+ and Eu3+ ion co-doped Ba9Lu2Si6O24 single-phased phosphor was synthesized successfully via a conventional high-temperature solid-state reaction. X-ray diffraction, crystal structure analysis, diffuse reflectance and luminescent spectra, quantum efficiency measurements, and thermal stability analysis were applied to investigate the phase, structure, luminescent and thermal stability properties. From the analyses of the crystal structure and luminescent spectra, we observed four discernible Bi3+ luminescent centers with peaks at ~363.3, ~403.1, ~437.7, and ~494.5 nm. Moreover, due to the complex energy transfer processes among these Bi3+ centers, their relative emission intensity tightly depended on the incident excitation wavelength. Interestingly, the as-prepared phosphor could generate warm white light/tunable emission by changing the concentration of Eu3+ ions or adjusting the excitation wavelength. The energy transfer mechanism from Bi3+ to Eu3+ was confirmed via an electric dipole-dipole interaction, the energy transfer efficiencies \(({\eta }_{T})\) from Bi3+ to Eu3+ were 50.84% and 40.17% monitoring at 410 and 485 nm, respectively. The internal quantum efficiency of the optimized Ba9Lu2Si6O24:Bi3+, Eu3+ phosphor was calculated to be 42.6%. In addition, the configurational coordinate model was carried out to explain the energy decrease of the phonon-electron coupling effect.
Similar content being viewed by others
Introduction
Recently, phosphor materials have attained great achievement and progress in various fields, including solid-state lighting, optical temperature sensors, flat panel displays, solar cells, and optical biomarkers1,2,3,4,5,6. As next-generation lighting devices, phosphor-converted white light-emitting diodes (w-LEDs) have received much more attention since w-LEDs provide extraordinary superiorities, such as low electric consumption, high electro-optical conversion efficiency, high brightness, good stability, fast response, and environmental friendliness7,8,9,10. Until now, combining blue-emitting InGaN-based LED chips and yellow-emitting Y3Al5O12:Ce3+ phosphors to make white light emission is one of the simplest and most efficient ways for commercial application11,12. However, such a combination shows a poor color rendering index (CRI < 80) and a high correlated color temperature (CCT > 4500 K) due to the lack of red-light contribution, which limits vivid applications13,14. To overcome the above mentioned problems, another improvement method is to employ near ultraviolet (near-UV) emitting LED chips (300–410 nm) coated with trichromatic phosphors. Unfortunately, the trichromatic phosphor system produces several inevitable problems, including complex coating, fluorescence reabsorption between different components, and non-uniformity of the luminescence properties, resulting in the degradation of luminous efficiency, increased manufacturing costs and a time-dependent shift of the color point15,16. To circumvent these drawbacks, a single-phased phosphor, which is fabricated by co-doping the sensitizer and activator ions into an appropriate host, with white light emission for near-UV pumped w-LEDs would be favorable alternative17.
Silicate materials are inexpensive and readily available, and silicate-based phosphor materials serve as promising luminescent materials in the use of phosphor-converted w-LEDs because of their structural diversity, relatively easy preparation, good thermal stability, and visible light transparency18,19. Recently, a silicate-based phosphor Ba9Lu2Si6O24 having an orthosilicate structure doped with various rare-earth ions has been extensively studied due to its favorable luminescence properties. For example, Liu et al. reported the luminescence properties of Eu2+-doped Ba9Lu2Si6O24 blue phosphor20, Liu et al. investigated the high thermal and radiation stability from Ce3+ doped Ba9Lu2Si6O24 green phosphor7; Song et al. studied energy transfer in Ce3+, Mn2+ co-doped Ba9Lu2Si6O24 red phosphor19. It should be noted that the silicate-based phosphor Ba9Lu2Si6O24 possesses multiple luminescent centers, as it contains three types of Ba centers and one type of Lu center. Therefore, multicentered materials doped with appropriate rare-earth ion have greater potential than that of a single-center material using proper center engineering21.
Among various RE ion activators, bismuth is typical ns2-type luminescent center, which is sensitive to the surrounding crystal field. It can emit UV, visible and near-infrared spectral regions attributing to its 6s6p-6s2 transition22,23. Thus far, the Bi3+ ion usually acts as an efficient sensitizer for Eu3+ ions in a large number of hosts11,24. In this work, we studied the multicentered photoluminescence characteristics and site engineering in a Bi3+ and Eu2+ co-doped Ba9Lu2Si6O24 phosphor to realize white light/tunable emission for phosphor-converted w-LEDs. Due to the complex energy transfer processes, the tuning of multicentered photoluminescence can be achieved by adjusting the excitation wavelength or controlling the rare-earth ion concentration.
Results
TG-DTA and Crystal Structure Analysis of Ba9Lu2–x–y Si6O24:xBi3+, yEu3+ (abbreviated as BLSO:xBi3+, yEu3+)
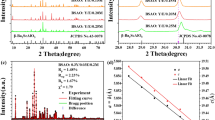
Figure 1 shows the TG-DTA curves of the as-prepared blank Ba9Lu2Si6O24 (abbreviated as BLSO) precursor sample, carried out to investigate the decomposition and thermal interaction processes among all the raw materials. It can be seen from the TG curve (blue curve) that the weight loss can be divided into two temperature regions: one is from room temperature to 630 °C with 3.14% weight loss, and the other is from 630 °C to 980 °C with 15.35% weight loss. The first weight loss is partially derived from the evaporation of residual water and ethanol contents in the powdered surface with an endothermic band at approximately 81 °C. Besides, the weight loss between 100 °C and 630 °C arises from the evaporation of remaining water molecules and decomposition of organic material. From the analysis, the second weight loss may be attributed to the decomposition of BaCO3 to CO2 gas following the equation:
The experimental value of the weight loss is 15.35% with the above reaction. Simultaneously, we calculated the theoretical value according to equation (1) and the stoichiometric ratio, which is about 15.62%. The results show that both the theoretical and the experimental values are close to each other, confirming our hypothesis for the second weight loss process. In addition, BaCO3 possesses three crystallographic structures: γ-rhombohedral, β-hexagonal and α-cubic. One can see that the phase transformations in BaCO3 can be observed in the DTA curve (red curve). The endothermic peak at 821 °C and exothermic peak at 991 °C correspond to the phase transformations of γ → β and β → α, respectively. Above 980 °C, the slope of the TG curve gradually stabilizes, forming the BLSO host lattice.
Figure 2 depicts the XRD patterns of the blank BLSO, BLSO:0.005Bi3+, BLSO:0.005Eu3+ and BLSO:0.07Bi3+, 0.025Eu3+ phosphors, the standard pattern Ba9Sc2Si6O24 (PDF#82-1119) is shown as a reference. It can be clearly seen from the XRD analysis that all the diffraction peaks in these obtained solid solutions agree well with the standard pattern of Ba9Sc2Si6O24 (PDF#82-1119), which indicates that these obtained solid solutions have a single-crystal phase, and no impurities or secondary phases were found. In addition, doping trace amounts of Bi3+ and Eu3+ ions did not cause any notable change in the crystal lattice. From the enlarged image of Fig. 2, the two main diffraction peaks at 30.40° and 31.34° (2θ) shift slightly to higher angles as Bi3+ and Eu3+ are doped into the host. This observation results from lattice shrinkage induced by the substitution of the smaller Bi3+ and Eu3+ ions for the larger Ba2+ or La3+ ions.
BLSO crystallizes in a rhombohedral structure and belongs to the \(R\bar{3}\) (148) space group20. The crystal structure of the BLSO unit cell viewed from the a-axis and the c-axis is shown in Fig. 3. It exhibits the layered distribution in which the smaller tetrahedral SiO4 units and the larger octahedral LuO6 units are corner-shared as SiO4-LuO6-SiO4-LuO6, forming a rigid three-dimensional network7. In the crystal structure, the Lu3+ ions are located at the 6c Wyckoff position with 6-fold coordination, evolving a distorted LuO6 octahedron that has two different Lu-O bond lengths20. While the Ba2+ ions are designated in three independent sites: Ba(1) at the 3a Wyckoff position with 12-fold coordination, Ba(2) at the 6c Wyckoff position with 9-fold coordination and Ba(3) at the 18 f Wyckoff position with 10-fold coordination, forming three different distorted polyhedra with different Ba-O bond lengths. For easy distinguish, we can define the three distinct types of Ba sites and one Lu site as Ba(1), Ba(2), Ba(3) and Lu, respectively, and all these sites can be substituted by Bi3+ or Eu3+ ions. For the Ba atom sites, such as the 12-fold coordination Ba(3) site, Bi3+ (r = 1.45 Å) and Eu3+ (r = 1.23 Å) ions are smaller than Ba2+ (r = 1.61 Å) ions, and therefore, the Bi3+ and Eu3+ ions are expected to randomly substitute the Ba2+ sites. This result can be extended to the other Ba2+ ions. However, for the Lu atom site with 6-fold coordination, Bi3+ (r = 1.02 Å) and Eu3+ (r = 0.947 Å) ions are bigger than Lu3+ (r = 0.861 Å) ions. According to Vegard’s law, a complete solid solution should form if the size difference of ions is in the range of ±15%25. Therefore, Bi3+ and Eu3+ ions can also randomly substitute the Lu site.
Luminescence Properties of Bi3+ Single-Doped Ba9Lu2Si6O24 Phosphor
Upon different excitation wavelengths, the Bi3+ single-doped BLSO sample shows different luminescence features. Figure 4a shows the photoluminescence and excitation spectra of the BLSO: 0.05Bi3+ sample excited at 335/370 nm and monitored at 410/485 nm, respectively. There is no luminescence from blank BLSO, and thus, the observed different luminescence features should come from the Bi3+ ions. Under excitation of 370 nm, the BLSO:0.05Bi3+ sample exhibits a symmetric broad blue emission band ranging from 380 to 500 nm with a maximum value at 410 nm. Monitoring the emission at 410 nm produces the excitation spectrum, which consists of two broad absorption bands with peaks at around 335 and 370 nm. Upon 335 nm excitation, BLSO:0.05Bi3+ presents a different emission band ranging from 350 to 650 nm with three bands peaking at about 365, 410 and 485 nm. Figure 4b shows the excitation spectrum monitored at 485 nm, which can be deconvoluted into three symmetric absorption bands centered at 3.905 eV (317.5 nm), 3.807 eV (325.7 nm), and 3.616 eV (342.9 nm). Meanwhile, Fig. 4c shows the emission spectrum excited at 355 nm, which can be deconvoluted into four symmetric absorption bands centered at 3.45 eV (359.4 nm), 3.07 eV (403.9 nm), 2.69 eV (461.0) and 2.45 eV (506.1 nm). From this spectrum, we can conclude that there are at least four kinds of Bi3+ luminescent centers in the BLSO host. To simplify the discussion below, these four deconvoluted bands are denoted as Bi(I), Bi(II), Bi(III), and Bi(IV). According to previous studies and discussions, it is deduced that the blue emission band marked as Bi(IV) at ~ 410 nm is derived from Bi3+ ions substituted at the Lu3+ sites, while the remaining three emission bands result from Bi3+ ions substituted at the three distinct types of Ba sites. However, this is still unclear and requires additional study on how to assign the emission band to a particular Ba crystallographic site.
(a) Photoluminescence and excitation spectra of BLSO:0.05Bi3+ sample (λem = 410 nm and λem = 485 nm for excitation spectra, λex = 335 nm and λex = 370 nm for emission spectra); UV-vis DRS of blank BLSO and BLSO:0.05Bi3+ samples. (b) The Gaussian deconvolution of the excitation spectrum (λem = 485 nm). (c) The Gaussian deconvolution of the emission spectrum (λex = 335 nm).
To investigate the energy transfer among the four kinds of Bi3+ ions, Fig. 5 shows the Gaussian deconvolution of the emission spectra for the BLSO:0.05Bi3+ sample under continuous excitation wavelengths from 315 to 370 nm with a step of 5 nm. From these figures, we can see that the intensity changes intuitively. As the excitation wavelength increases from 315 to 355 nm, there are always four emission bands with peaks at ~ 363.3 nm (3.413 eV), ~ 403.1 nm (3.077 eV), ~ 437.7 nm (2.833 eV), and ~ 494.5 nm (2.518 eV). However, as the excitation wavelength continues to increase from 360 to 370 nm, only one emission band of Bi(IV) remains. Based on these phenomena, it is reasonable that energy transfer occurs from Bi(I), Bi(II) and Bi(III) to Bi(IV). Furthermore, this energy transfer process is more efficient with an increasing excitation wavelength since the intensity of the Bi(IV) emission considerably increases. This may be due to the better energy match between the excitation wavelength with Bi(IV). On the other hand, it can be seen that energy transfer also occurred among the Bi(I), Bi(II) and Bi(III) ions since the three emission bands are observed under continuous excitation wavelengths from 315 to 355 nm.
The Commission International de L’Eclairage (CIE) chromaticity coordinates of the BLSO:0.05Bi3+ sample under continuous excitation wavelengths from 315 to 370 nm with a step of 5 nm are shown in Fig. S1. The inset lists the related calculated CIE chromaticity coordinates. It is clearly seen that the emission color varied from blue to bluish green by adjusting the excitation source from 315 to 340 nm with the CIE coordinates changing from (0.1721, 0.2268) to (0.1800, 0.2636). Nevertheless, the emission color shows a dramatic change as the excitation source increases from 315 to 340 nm, moving from bluish green to blue with the CIE coordinates largely varying from (0.1795, 0.2596) to (0.1950, 0.0968). Accordingly, the obtained phosphor can be used as a blue-emitting phosphor for w-LED applications. To obtain warm white light emission, we attempted to co-doped Eu3+ ions in the BLSO:Bi3+ system, as Bi3+ ions can be an efficient sensitizer for Eu3+ ions in various host lattices.
Energy Transfer from Bi3+ to Eu3+ in the Ba9Lu2Si6O24 Phosphor with White Light/Tunable Emission
Figure S2 exhibits the photoluminescence and excitation spectra of the BLSO:0.005Eu3+ sample excited at 393 nm and monitored at 611 nm. The excitation spectrum shows a charge-transfer band (CTB) at 200 to 280 nm, originating from Eu3+-O2−, and a series of sharp lines located at 300 ~ 500 nm, resulting from the 4f-4f transitions of the Eu3+ ions. Upon excitation at 393 nm, the emission spectrum shows the typical 5D0 → 7FJ (J = 0, 1, 2, 3, 4) transition of the Eu3+ ions in the range of 550–700 nm. It should be noted that 5D0 → 7F1 with ∆J = 1 and 5D0 → 7F2 with ∆J = 2 belong to the magnetic-dipole and electric-dipole transitions, respectively. The intensity of 5D0 → 7F2 is extraordinarily strong as compared to the 5D0 → 7F1 transition, which indicates that the Eu3+ ions are located in asymmetric sites in the BLSO host. It is interesting to note that the combination of these Eu3+ emission lines with the Bi3+ emission in the BLSO host is helpful to generate white light performance.
Comparing the emission spectrum of BLSO:0.05Bi3+ with the excitation spectrum of BLSO:0.005Eu3+ in Fig. 4a and Fig. S2, there is a significant spectral overlap in the range of 350–500 nm. According to this phenomenon, there may exist energy transfer from Bi3+ to Eu3+ in the BLSO host. In order to study the energy transfer from the Bi3+ to Eu3+ ions, the emission spectra of the BLSO:0.07Bi3+, yEu3+ (y = 0.0025–0.0250) phosphors excited at 344 and 370 nm are shown in Fig. 6a,b, respectively. Obviously, at either excitation wavelength, as the Eu3+ concentration increases, the emission intensity of Bi3+ rapidly reduces, while the emission intensity of Eu3+ gradually increases. In addition, there is no Eu3+ concentration quenching as y increases from 0.0025 to 0.025. Based on this, we can clearly deduce the existence of energy transfer from Bi3+ to Eu3+ in the BLSO host. The related CIE chromaticity diagram of BLSO:0.07Bi3+, yEu3+ (y = 0.0025–0.0200) samples is listed in the inset of Fig. 6. It is found that the location of the color coordinates (x, y) changes from bluish green to pink across the entire white region under 344 nm excitation and from blue to pink under 370 nm excitation. Therefore, it is possible to tune the emission color by changing the excitation wavelength and adjusting the Bi3+/Eu3+ molar ratio. Table S1 displays the calculated CIE chromaticity coordinates and correlated color temperatures (T cct ) of the BLSO:0.07Bi3+, yEu3+ (y = 0.0025–0.0200) samples excited at 344 and 370 nm. By comparison, the color coordinate point of a5 (x = 0.3523, y = 0.2973) is particularly close to the National Television Standards Committee (NTSC) white point (x = 0.330, y = 0.330) with a T cct value at 4571 K. Consequently, these results demonstrated that the combination of these Eu3+ emission lines with Bi3+ emission in the BLSO host could generate warm white emission.
In general, the energy transfer mechanism from the sensitizer to the activator takes place at a high doping concentration by an exchange interaction or a multipolar interaction. According to Dexter’s energy transfer proposal and Reisfeld’s approximation of the multipolar interaction, the following formula can be obtained26,27:
where, \({\eta }_{0}\) and \(\eta \) are the energy transfer efficiencies of the sensitizer (Bi3+) in the absence and presence of activator (Eu3+), respectively. Similarly, I 0 and I are the luminescent intensities of Bi3+ in the absence and presence of Eu3+ ions, respectively. Normally, the values (\({\eta }_{0}/\eta \)) can be estimated from the related luminescence intensity, I 0 /I. C is the total concentration of the Bi3+ and Eu3+ ions; n = 6, 8 and 10 correspond to the dipole-dipole, dipole-quadrupole and quadrupole-quadrupole interactions, respectively. In this case, the I 0 /I versus C n/3 plots with linear fittings are illustrated in Fig. S3, and the R2 values are observed from the relationships when n = 6, 8, and 10. The best fitting is R2 = 99.98% when n = 6. Therefore, the dipole-dipole interactions dominate energy transfer from Bi3+ to Eu3+ in the BLSO host.
To determine which Bi3+ centers make contributions to the emission of the Eu3+ ions, Fig. 7a exhibits the excitation spectra of the BLSO:0.07Bi3+, yEu3+ (y = 0.0025–0.0250) samples monitored at 611 nm. In addition to the Eu3+-O2− CTB at 200–280 nm and the 7F0 → 5D2 transition of the Eu3+ ions at 464 nm, the broad band at 300–400 nm originates from the Bi3+ ions. From the Gaussian deconvolution of this broad band, it is clearly to seen that there are four small Gaussian peaks fitted. By comparison of Fig. 7a with Fig. 4a, these four Gaussian peaks match well with the Bi(I), Bi(II), Bi(III), and Bi(IV) bands, which demonstrate that these four kinds of Bi3+ centers contribute to the Eu3+ emission. To more intuitively explain the contribution of the various Bi3+ centers to Eu3+ emission, the emission spectra of the BLSO:0.07Bi3+, 0.0075Eu3+ sample under continuous excitation wavelengths from 330 to 370 nm with a step of 5 nm are displayed in Fig. 7b. We attempted to excite various Bi3+ centers with the different excitation sources, and the emission spectra show a series of Bi3+ and Eu3+ emissions with different intensities and different emission colors. The emission color is tunable from white to reddish purple with the excitation wavelength increasing from 330 to 370 nm, as shown in Fig. 7c. In conclusion, we developed two simple strategies to obtained white light/tunable emission: one is to change the concentration of Eu3+ ions in the BLSO:0.07Bi3+ phosphor, and the other is to adjust the excitation wavelength.
(a) Excitation spectra of BLSO:0.07Bi3+, yEu3+ (y = 0.0025–0.0250) monitored at 611 nm. Inset shows the Gaussian deconvolution for broad band at 300–400 nm. (b) Emission spectra of BLSO:0.07Bi3+, 0.0075Eu3+ under continuous excitation wavelengths from 330 to 370 nm with a step of 5 nm. (c) The related CIE chromaticity coordinates of BLSO:0.07Bi3+, 0.0075Eu3+.
In general, energy transfer from Bi3+ to Eu3+ can also be demonstrated by an investigation of the dynamic luminescence process. The decay curves of the BLSO:0.07Bi3+, yEu3+ (y = 0.0025–0.0250) samples excited at 355 nm and monitored at 410 and 485 nm were compared and are presented in Fig. S4. According to the fitting analyses, the decay curves of the Bi3+ emission at 410 and 485 nm are non-exponential, indicating that there is more than one relaxation process. The average lifetimes τ avg of these samples can be estimated using the simple equation28:
where I(t) is the luminous intensity at time t. According to equation (3), the average lifetimes of Bi3+ were evaluated and listed in Table S2. The lifetimes of Bi3+ decrease monotonically with an increasing Eu3+ ion concentrations, strongly supporting energy transfer from Bi3+ to Eu3+.
Using these lifetimes, the energy transfer efficiencies (\({\eta }_{T}\)) from Bi3+ to Eu3+ can be approximately calculated by an equation defined by Paulose et al.29:
where τ S0 and τ S are the intrinsic lifetime of the sensitizer (Bi3+) and the lifetime of the sensitizer (Bi3+) in the presence of the activator (Eu3+), respectively. Using this equation, the \({\eta }_{T}\) values from Bi3+ to Eu3+ monitoring at 410 nm emission are calculated to be 12.35%, 21.89%, 28.39%, 32.88%, 42.09%, and 50.84%, while those monitoring at 485 nm emission are 4.26%, 8.11%, 14.70%, 20.29%, 29.25%, and 40.17%.
Figure 8a illustrates the decay curves of the BLSO:zBi3+, 0.02Eu3+ samples excited at 370 nm and monitored at 611 nm. As shown in Fig. 8a, there are two different processes: one is a rise-up process, and the other is a decay process. In the initial rise-up process, energy is trapped by the Bi(4) center and then transfers to the Eu3+ ions. Figure 8b,c show the obtained curves of the enlarged initial rise-up region from 80 to 300 μs and the corresponding simulation curves, respectively. One can see that the rise-up process shows a high-speed rising due to the increasing Bi3+ content, which indicates that energy transfer from Bi3+ to Eu3+ becomes more efficient. For the decay process, the decay curves show single-exponential decay behavior following the equation:
where I(t) and I 0 are the luminescence intensities at time t and t = 0, respectively; τ is the luminescent lifetime; A is a constant. According to equation (5), the lifetimes of Eu3+ are calculated to be 0.94, 0.96, 0.97, 0.98, 1.00, 1.02, and 1.02 ms with z = 0.001, 0.005, 0.01, 0.02, 0.03, 0.04, and 0.05, respectively. In this case, the luminescent lifetime of the activator (Eu3+) increases since the Eu3+ ion emission does not show concentration quenching.
Based on the luminescence and decay behaviors given in Figs 4, 6, 7, and S4, the energy transfer scheme of generating white light/tunable emission in the Bi3+ and Eu3+ co-doped BLSO phosphor upon UV light excitation is presented in Fig. 9. Under excitation of 344 nm, the Bi(I), Bi(II) and Bi(III) centers are excited by UV light and then give a broad blue/green emission band. Simultaneously, due to energy transfer from these three Bi3+ centers to the Eu3+ center, warm white light emission can be obtained from the Bi3+ and Eu3+ co-doped BLSO phosphor. In addition, under 370 nm excitation, energy is trapped by the Bi(IV) center, giving a strong blue emission. At the same time, by effective energy transfer from the Bi(IV) center to the Eu3+ center, a tunable emission phosphor from the blue to red region is achieved.
Thermal Analysis of the Ba9Lu1.92Si6O24:0.07Bi3+, 0.0075Eu3+ Phosphor
It is accepted that the thermal stability of a phosphor is of great significance for its practical applications, including the color rendering index and the light output of w-LEDs30. Therefore, Fig. 10a,b show the temperature-dependent luminescence properties of the BLSO: 0.07Bi3+, 0.0075Eu3+ phosphor upon 344 and 370 nm excitations, respectively, with an increasing temperature from 299 to 503 K. We can observe that both emission intensities of the BLSO:0.07Bi3+, 0.0075Eu3+ sample with an increasing temperature gradually decline. Moreover, the emission intensities at 423 K (150 °C) of the BLSO:0.07Bi3+, 0.0075Eu3+ sample under 344 and 370 nm excitations remain at approximately 66.1% and 72.8% of their initial values, respectively. To meet application requirements, the thermal stability of the obtained phosphor must be strengthened. Generally, strengthening of the thermal stability can be achieved by optimizing the preparation conditions to reduce vacancies and improve crystallinity. It is well known that the activation energy (E a) is a good indicator to assess the thermal stability of phosphors31. It can be calculated from the Arrhenius equation32:
where I 0 and I are the integrated intensity at room temperature and a particular operating temperatures, respectively; E a and T are the calculated activation energy and the operating temperature (K), respectively; A and k are a constant for a certain host and the Boltzmann constant (8.62 × 10−5 eV K−1), respectively. As depicted in Fig. 10c, through a best-fitting of the ln[I 0 /I−1] against 1/kT plot using equation (6), the calculated activation energies E a are 0.2287 and 0.2455 eV for 344 and 370 nm excitations, respectively. From the above investigation, we can see that the thermal stability of the obtained phosphor excited at 370 nm is more stable than that excited at 344 nm, which is consistent with the calculated results.
Temperature-dependent luminescence properties of BLSO: 0.07Bi3+,0.0075 Eu3+ phosphor along with temperature increase from 299 to 503 K under 344 nm (a) and 370 nm (b). (c) The plots of ln[I 0 /I−1] against 1/kT. (d) The configurational coordinate diagram of the ground and excited states of Bi3+ and Eu3+.
The configurational coordinate diagrams of the ground and excited states of Bi3+ and Eu3+ as well as the CTB of Eu3+-O2– are displayed in Fig. 10d. To simplify the structure, we divided the bismuth ions into two types: one for Bi(I), Bi(II) and Bi(III) centers excited at 344 nm; the other for Bi(IV) center excited at 370 nm. Both excitations show similar spectroscopic and energy transfer properties, and thus, the following description is focused on one of them, namely, the 344 nm excitation. Under excitation at 344 nm, the electrons of the Bi(I), Bi(II) and Bi(III) centers absorb the energy and are excited from the 1S0 ground state to the 3P1 excited state at room temperature. Then, some of the electrons undergo a non-radiative process, relaxing to the lowest state positions of 3P1 (point O), from where the electrons return to the ground state 1S0 by radiative transition. Some of the electrons cross point M’, resulting in energy transfer from the Bi3+ to Eu3+ ions and subsequent relaxation to the lowest state positions of 5D0, from where the electrons return to the ground state 7FJ (J = 0–6) by radiative transition. Furthermore, some of the electrons will donate their energy via energy transfer to the Bi(IV) ions. With an increasing temperature, the electrons at 3P1 might overcome ∆E1 with stronger phonon-electron coupling. Simultaneously, the electrons can be excited from point O to O’, from where the electrons undergo a non-radiative process relaxing to 1S0, leading to a rapid decrease of the number of electrons.
Another important indicator parameter for practical applications is the quantum efficiency (QE). The internal QE value can be calculated based on the following equation33:
where L s means the emission spectrum of the obtained sample, \({E}_{R}\) represents the spectrum of the excitation light from the empty integrated sphere (without the sample), and \({E}_{s}\) is the spectrum of the light used for exciting the sample. The internal QE of the BLSO:0.07Bi3+, yEu3+ (y = 0.0025–0.0250) phosphors excited at 344 and 370 nm were calculated and listed in Table S3. The highest QE values reach 42.6% and 32.9% upon 344 and 370 nm excitation, respectively. Since the QE closely depends on the preparation conditions, particle size, defects, and morphology, it can be further improved by optimizing the synthesis process26.
Discussion
In summary, we reported a systematic study on the Bi3+, Eu3+ co-doped BLSO single-phased phosphor with warm white light/tunable emission by manipulating energy transfer in multicentered photoluminescence. Upon different excitation wavelengths, the Bi3+ single-doped BLSO sample showed different luminescence. Four discernible Bi3+ (Bi(I)- Bi(IV)) luminescent centers were confirmed by analyzing the crystal structure and luminescent spectra. Energy transfer from Bi(I), Bi(II) and Bi(III) to Bi(IV) was manipulated by changing the excitation wavelength. For Bi3+, Eu3+ co-doped BLSO phosphor, two simple strategies were described to obtained white light/tunable emissions one was to change the concentration of Eu3+ ions in the BLSO:0.07Bi3+ phosphor, and the other was to adjust the excitation wavelength. The energy transfer mechanism from Bi3+ to Eu3+ in the BLSO host was dominated by dipole-dipole interactions, the energy transfer efficiencies (\({\eta }_{T}\)) from Bi3+ to Eu3+ were as high as 50.84% and 40.17% monitoring at 410 and 485 nm, respectively. In addition, as the temperature increased to 423 K (150 °C), the emission intensities of the BLSO:0.07Bi3+, 0.0075Eu3+ sample retained approximately 66.1% and 72.8% of their initial values under 344 and 370 nm excitations, respectively. All the results show that the BLSO: Bi3+, Eu3+ phosphor has potential application fin phosphor-converted w-LEDs.
Methods
Materials and Synthesis
These series of Ba9Lu2–x Si6O24:xBi3+ (x = 0.005, 0.01, 0.02, 0.03, 0.04, 0.05, 0.06), Ba9Lu 1.93–y Si6O24:0.07Bi3+,yEu3+ (y = 0.0025, 0.0050, 0.0075, 0.0100, 0.0150, 0.0200, 0.0250) and Ba9Lu 1.98–z Si6O24:zBi3+,0.02Eu3+ (z = 0.001, 0.005, 0.01, 0.02, 0.03, 0.04, 0.05) phosphors were prepared by the standard solid-state reaction. These phosphors were synthesized by mixing raw materials of BaCO3 (99.90%), Lu2O3 (99.99%), SiO2 (99.99%), Bi2O3 (99.99%), and Eu2O3 (99.99%) in air. All raw materials were commercially available and were used as received. Typically, all starting materials were weighed and mixed with each other, and then the mixture was ground about 40 min with proper ethanol addition in an agate mortar. After that, the mixture based precursors were pre-sintered at 850 °C for 3 h, which was followed by an intermediate grinding for 20 min to improve the homogeneity of these samples. Then, re-sintered at 1400 °C for 5 h and ground again for further characterization. This work was supplied by the Display and Lighting Phosphor Bank at Pukyong National University.
Materials Characterization
The thermogravimetric-differential thermal analysis (TG-DTA) of blank Ba9Lu2Si6O24 powder were obtained by a Material Analysis and Characterization TG-DTA 2000 system with a heating rate of 10 °C/min from room temperature to 1300 °C in air. The phase purity and the crystalline structure were checked by the powder X-ray diffraction (XRD) analysis (Bruker D8 Advance) over the angular range 10° ≤ 2θ ≤ 70° with a step size of 0.02°, with Cu Kα irradiation (λ = 1.5406 Å) operating at 40 kV voltage and 40 mA current. Ultraviolet-visible diffuse reflectance spectra (UV-vis DRS) were performed on a V-670 UV-vis spectrophotometer (JASCO Corp., Japan). Photoluminescence excitation and emission spectra were measured using Photon Technology International (PTI, USA) fluorimeter equipped with a 60 W xenon lamp and the fluorescence lifetimes of Ba9Lu 1.98–z Si6O24:zBi3+, 0.02Eu3+ (z = 0.001–0.07) samples were measured with a phosphorimeter attached to the fluorescence spectrophotometer with a 25 W xenon flash lamp. The fluorescence lifetimes of BLSO:0.07Bi3+, yEu3+ (y = 0.0025–0.0250) samples were measured under a 355 nm Nd:YAG laser excitation with a pulse duration of 5 ns. Temperature-dependent luminescence properties were obtained by a fluorescence spectrophotometer (SCINCO FS-2) with a heating apparatus as heating source. The photoluminescence quantum efficiency (QE) was carried out by a spectrofluorometer (JASCO, FP-8500, Japan) equipped with an integrating sphere attachment (ISF-834).
References
Terraschke, H. & Wickleder, C. UV, Blue, Green, Yellow, Red, and Small: Newest Developments on Eu2+-Doped Nanophosphors. Chem. Rev. 115, 11352–11378 (2015).
Wang, X., Wang, Y., Marques-Hueso, J. & Yan, X. Improving Optical Temperature Sensing Performance of Er3+ Doped Y2O3 Microtubes via Co-doping and Controlling Excitation Power. Sci. Rep. 7, 758 (2017).
Wang, X. et al. Influence of Doping and Excitation Powers on Optical Thermometry in Yb3+-Er3+ doped CaWO4. Sci. Rep. 7 (2017).
Bedyal, A., Kumar, V. & Swart, H. Charge compensated derived enhanced red emission from Sr3(VO4)2:Eu3+ nanophosphors for white light emitting diodes and flat panel displays. J. Alloy. Comp. 709, 362–372 (2017).
Guo, Y. et al. Fluorescence properties with red-shift of Eu2+ emission in novel phosphor-silicate apatite Sr3LaNa(PO4)2SiO4 phosphors. Ceram. Int. 42, 18324–18332 (2016).
Du, P., Lim, J. H., Kim, S. H. & Yu, J. S. Facile synthesis of Gd2O3:Ho3+/Yb3+ nanoparticles: an efficient upconverting material for enhanced photovoltaic performance of dye-sensitized solar cells. Opt. Mater. Express 6, 1896–1904 (2016).
Liu, Y. et al. Ba9Lu2Si6O24:Ce3+: An Efficient Green Phosphor with High Thermal and Radiation Stability for Solid-State Lighting. Adv. Opt. Mater. 3, 1096–1101 (2015).
Guo, Y. et al. A red-emitting perovskite-type SrLa(1−x)MgTaO6:xEu3+ for white LED application. J. Lumin. 167, 381–385 (2015).
Guo, Y., Moon, B. K., Choi, B. C., Jeong, J. H. & Kim, J. H. Color–tunable luminescence and energy transfer behaviors of Dy3+/Eu3+ co–doped SrLaMgTaO6 phosphors for solid state lighting applications. Mater. Res. Bull. 88, 166–173 (2017).
Du, P., Guo, Y., Lee, S. H. & Yu, J. S. Broad near-ultraviolet and blue excitation band induced dazzling red emissions in Eu3+-activated Gd2MoO6 phosphors for white light-emitting diodes. RSC Adv. 7, 3170–3178 (2017).
Huang, A. et al. Tunable and White Light Emission of a Single-Phased Ba2Y(BO3)2Cl:Bi3+, Eu3+ Phosphor by Energy Transfer for Ultraviolet Converted White LEDs. J. Phys. Chem. C 121, 5267–5276 (2017).
Gwak, S. J., Arunkumar, P. & Im, W. B. A new blue-emitting oxohalide phosphor Sr4OCl6: Eu2+ for thermally stable, efficient white-light-emitting devices under near-UV. J. Phys. Chem. C 118, 2686–2692 (2014).
Bachmann, V., Ronda, C. & Meijerink, A. Temperature quenching of yellow Ce3+ luminescence in YAG:Ce. Chem. Mater. 21, 2077–2084 (2009).
George, N. C. et al. Local environments of dilute activator ions in the solid-state lighting phosphor Y3–x Ce x Al5O12. Chem. Mater. 25, 3979–3995 (2013).
Zhang, Z., Zhong, H., Yang, S. & Chu, X. Tunable luminescence and energy transfer properties of Ca19Mg2(PO4)14:Ce3+,Tb3+,Mn2+ phosphors. J. Alloy. Comp. 708, 671–677 (2017).
Yang, Z., Xu, D., Du, J., Gao, X. & Sun, J. Tunable luminescence and energy transfer of a Eu2+/Mn2+ co-doped Sr3NaY(PO4)3F phosphor for white LEDs. RSC Adv. 6, 87493–87501 (2016).
Huang, C.-H., Liu, W.-R. & Chen, T.-M. Single-phased white-light phosphors Ca9Gd(PO4)7:Eu2+, Mn2+ under near-ultraviolet excitation. J. Phys. Chem. C 114, 18698–18701 (2010).
Lv, W. et al. Crystal structure and luminescence properties of Ca8Mg3Al2Si7O28: Eu2+ for WLEDs. Adv. Opt. Mater. 2, 183–188 (2014).
Song, K. et al. Red-emitting phosphor Ba9Lu2Si6O24: Ce3+, Mn2+ with enhanced energy transfer via self-charge compensation. J. Phys. Chem. C 119, 24558–24563 (2015).
Liu, Y. et al. Origin and Luminescence of Anomalous Red-Emitting Center in Rhombohedral Ba9Lu2Si6O24:Eu2+ Blue Phosphor. Inorg. Chem. 55, 8628–8635 (2016).
Wen, D. et al. Anomalous Orange Light-Emitting (Sr, Ba)2SiO4:Eu2+ Phosphors for Warm White LEDs. ACS Appl. Mater. Interfaces 8, 11615–11620 (2016).
Kang, F. et al. Broadly tunable emission from CaMoO4:Bi phosphor based on locally modifying the microenvironment around Bi3+ ions. Eur. J. Inorg. Chem. 2014, 1373–1380 (2014).
Kang, F., Peng, M., Zhang, Q. & Qiu, J. Abnormal Anti-Quenching and Controllable Multi-Transitions of Bi3+ Luminescence by Temperature in a Yellow-Emitting LuVO4:Bi3+ Phosphor for UV-Converted White LEDs. Chem-Eur J. 20, 11522–11530 (2014).
Zhou, H., Wang, Q. & Jin, Y. Temperature dependence of energy transfer in tunable white light-emitting phosphor BaY2Si3O10:Bi3+,Eu3+ for near UV LEDs. J. Mater. Chem. C 3, 11151–11162 (2015).
Ropp, R. Luminescence and the Solid State; (second ed.). Elsevier, (2004).
Dexter, D. & Schulman, J. H. Theory of concentration quenching in inorganic phosphors. J. Chem. Phys. 22, 1063–1070 (1954).
Reisfeld, R., Greenberg, E., Velapoldi, R. & Barnett, B. Luminescence quantum efficiency of Gd and Tb in borate glasses and the mechanism of energy transfer between them. J. Chem. Phys. 56, 1698–1705 (1972).
Dai, P.-P. et al. A single Eu2+-activated high-color-rendering oxychloride white-light phosphor for white-light-emitting diodes. Light: Sci. Appl. 5, e16024 (2016).
Paulose, P., Jose, G., Thomas, V., Unnikrishnan, N. & Warrier, M. Sensitized fluorescence of Ce3+/Mn2+ system in phosphate glass. J. Phys. Chem. Solids 64, 841–846 (2003).
Guo, Y., Moon, B. K., Choi, B. C., Jeong, J. H. & Kim, J. H. Multi-wavelength excited white-emitting K2Gd(1−x)(PO4)(WO4):xDy3+ phosphors with satisfactory thermal properties for UV-LEDs. RSC Adv. 7, 23083–23092 (2017).
Li, K., Liang, S., Shang, M., Lian, H. & Lin, J. Photoluminescence and Energy Transfer Properties with Y + SiO4 Substituting Ba + PO4 in Ba3Y(PO4)3:Ce3+/Tb3+,Tb3+/Eu3+ Phosphors for w-LEDs. Inorg. Chem. 55, 7593–7604 (2016).
Wang, C., Zhao, Z., Wu, Q., Zhu, G. & Wang, Y. Enhancing the emission intensity and decreasing the full widths at half maximum of Ba3Si6O12N2:Eu2+ by Mg2+ doping. Dalton Trans. 44, 10321–10329 (2015).
Van Deun, R. et al. Dopant and excitation wavelength dependent color-tunable white light-emitting Ln3+:Y2WO6 materials (Ln3+ = Sm, Eu, Tb, Dy). Dalton Trans. 44, 15022–15030 (2015).
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (No. 2015060315). The Bi3+ and Eu3+ ions co-doped Ba9Lu2Si6O24 phosphor was supplied by the Display and Lighting Phosphor Bank at Pukyong National University.
Author information
Authors and Affiliations
Contributions
Yue Guo conceived the idea, designed the experiment, synthesized and characterized the present samples, and completed the writing of the manuscript. Sung Heum Park and Byung Chun Choi analyzed the data and co-wrote the manuscript. All the authors discussed the results and commented on the manuscript. The whole work was carried out under the guidance of Jung Hyun Jeong and Jung Hwan Kim.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, Y., Park, S.H., Choi, B.C. et al. Dual-Mode Manipulating Multicenter Photoluminescence in a Single-Phased Ba9Lu2Si6O24:Bi3+, Eu3+ Phosphor to Realize White Light/Tunable Emissions. Sci Rep 7, 15884 (2017). https://doi.org/10.1038/s41598-017-15903-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15903-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.