Abstract
The relationship of platelet-to-lymphocyte ratio (PLR) and survival in urological cancers remained inconsistent in previous studies. Therefore, we performed a meta-analysis to assess the prognostic significance of PLR in patients with urological cancers. A literature search was performed in the PubMed, Embase, and Web of Science up to July, 2017 and study quality was obtained using the Newcastle-Ottawa Scale. To estimate the association of PLR and overall survival (OS) and other survival outcomes in urological cancers, we used pooled hazard ratios (HRs). Subgroup analyses were conducted on different ethnics, sample sizes and cut-off values. 20 high quality studies involving 7562 patients with urological cancers were included in this meta-analysis. High pretreatment PLR was significantly associated with poor OS in patients with urological cancers (pooled HR = 1.58). Elevated PLR was also correlated with other survival outcomes. However, we found that PLR was significantly relevant to the OS of patients with different types of urological cancers except bladder cancer (BCa, HR = 1.16, 95%CI: 0.96–1.41). In conclusion, elevated PLR was negatively related to the OS of patients with urological cancers, except in BCa. However, more large scale prospective studies with high quality are required in the future.
Similar content being viewed by others
Introduction
Urological cancer contains major malignancies with high morbidity and mortality worldwide1. Prostate cancer (PCa) is the most prevalent cancer among western men, accounting for 19% new cancer cases and third leading cause of cancer-related death among American men in 20172. Bladder cancer (BCa) represents the ninth most common tumor with approximately 2.7 million people suffering from it on a global scale3,4. While Renal cell carcinoma (RCC) takes up for a proportion of 2–3% in overall adult cancers3. Upper urinary tract urothelial carcinoma (UTUC) is relatively rare but aggressive. Despite great progress of treatment, the prognosis and clinical outcome of urological cancers remains unsatisfactory because of local recurrence or distal metastasis. The survival rate and survival time may be increased by risk stratification and optimal treatment at early stage. Hence, it is crucial to seek useful personalized biomarkers to estimate patient prognosis.
Accumulating evidence demonstrates that inflammation plays a critical role in tumor development and progression5,6. In patients with cancer, systemic inflammation is likely to affect the tumor micro-environment and promote tumor growth, which means poor outcome7. It is well acknowledged that systemic inflammation response can be characterized by the changes of peripheral blood cell amounts. Numerous studies have revealed that blood-based biomarkers show great potential in urological cancer prognosis, such as neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP), lymphocyte-to-monocyte ratio (LMR) and platelet-to-lymphocyte ratio (PLR)8,9,10. Platelet and lymphocyte counts are easily acquired during routinely blood tests in clinical laboratories worldwide. Thus, PLR is a cheap and objective parameter to potentially help doctors assessing patient prognosis. A couple of meta-analyses have testified the prognostic impact of PLR in malignancies like breast cancer and lung cancer11,12.
During the past few years, several studies explored the prognostic significance of PLR in patients with urological cancers. However, some studies have drawn contradictory conclusions. To our knowledge, no published meta-analysis have investigated PLR and urological cancer prognosis. The aim of the current study was to quantitatively and comprehensively summarize the available evidence on the prognostic value of elevated PLR and different survival outcomes (overall survival, OS; cancer specific survival, CSS; progression free survival, PFS and disease free survival, DFS) in patients with urological cancers.
Results
Study search and characteristics
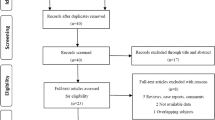
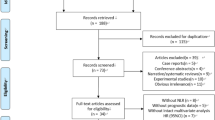
We performed literature search under the guideline of Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) statement13. The process of literature selection was shown in a flow diagram (Fig. 1). A total of 386 studies were initially identified with the keywords used to search the databases. By screening the titles and abstracts, 45 potential studies were retrieved. 25 studies were then excluded after further fully reviewed because they were insufficient of data (22 studies) or didn’t use cox model and hazard ratio (HR, 3 studies14,15,16). Finally, 20 cohort studies17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36 met the inclusion criteria for our meta-analysis.
The baseline information of the studies was shown in Table 1. The articles were published from 2013 to 2017, including 7562 patients, highlighting the recent interest of investigating this topic. Of them, 12 cohorts17,19,21,22,23,26,27,29,31,33,34,36 were from Asian countries and 8 cohorts18,20,24,25,28,30,32,35 were non-Asian. Overall, 17 studies investigated the prognostic ability of PLR and OS, while 4 studies discussed PLR and CSS19,20,28,29, 4 articles studied PLR and PFS19,20,33,36, and other 4 studies reported PLR and DFS29,30,31,32 in patients with urological cancers.
Quality assessment
While there was a small variation in the methodological quality of included studies, all of the included studies were judged relatively high quality according to the NOS assessment tool, with scores from 6 to 8.
PLR and survival in patients with urological cancers
As displayed in Fig. 2a, the forest plot showed high PLR was significantly associated with poor OS in patients with urological cancers. The pooled HR was 1.58 (95% confidence interval [CI]: 1.34–2.86, P < 0.001) from 17 studies. In addition, increased PLR was also significantly correlated with poor CSS (pooled HR = 1.78, 95%CI: 1.31–2.43, Fig. 2b). Elevated PLR was significantly associated with poor PFS (pooled HR = 1.64, 95%CI: 1.34–2.02, Fig. 2c). Furthermore, high PLR was significantly associated with poor DFS (pooled HR = 1.65, 95%CI: 1.18–2.31, Fig. 2d) in patients with urological cancers. The above pooled results were not influenced whether using univariate or multivariate HRs separately (Supplementary Table 1, Supplementary Table 2, Supplementary Figure 1).
Then we performed further analyses based on the type of urological cancers. 6 cohorts investigated PLR and OS in patients with RCC (Fig. 3a). The pooled HR was 1.69 (95%CI: 1.18–2.43, P = 0.003). While six studies provided HRs of PLR and OS in patients with PCa (Fig. 3b). Their pooled HR was 1.77 (95%CI: 1.43–2.20, P < 0.001) and no heterogeneity existed (I 2 = 0%, P = 0.631). However, other 3 cohorts reported PLR and OS in patients with BCa (Fig. 3c), whose pooled HR was 1.16 (95%CI: 0.96–1.41, P = 0.124), with low heterogeneity existed (I 2 = 30.4%, P = 0.238). Only 2 cohorts explored PLR and OS in patients with UTUC (Fig. 3d), whose pooled HR was 1.69 (95%CI: 1.16–2.48, P = 0.007). No heterogeneity was found in UTUC group (I 2 = 0%, P = 0.794).
Subgroup analysis and Meta-regression
There was slight heterogeneity (Fig. 2a, I 2 = 53.9%), so we conducted subgroup analysis to seek more information (Table 2). In epidemiological studies, ethnicity difference was usually recognized as a critical source of heterogeneity. Notably, the I 2 values of Asian and non-Asian were 62.7% and 0.0% respectively. From another aspect, there were also no heterogeneity when cut-off value of PLR > 150 (I 2 = 0.0%). When we conducted subgroup on sample size, heterogeneity existed when sample size > 254 (I 2 = 52.2%) or ≤254 (I 2 = 56.3%). The pooled HR values and their 95%CI in each subgroup analysis were demonstrated in Table 2 with corresponding forest plots shown in Fig. 4. To sum up, the pooled HRs indicated that high PLR was significantly associated with poor OS in each subgroup.
To seek possible sources of heterogeneity, we also conducted meta-regression. On account of insufficient data on other parameters such as tumor grade or stage, we choose cancer types, PLR cut-off, sample size, follow up time as covariates to estimate between-study variance. The result of this model (P = 0.86) showed no potential association between these covariates (cancer types: P = 0.367, PLR cut-off: P = 0.626, sample size: P = 0.254, follow up time: P = 0.563) and pooled HR.
Sensitivity analysis
In order to gauge the stability of the results, we conducted sensitivity analysis by removing one study in sequence to see if a single study could have significant impact on the pooled HRs for survival. The results were not significantly altered by removing anyone of the included studies (Fig. 5a: OS, Fig. 5b: CSS, Fig. 5c: PFS, Fig. 5d: DFS).
Publication bias
The funnl plots of Egger’s test were displayed in Fig. 6. Both Begg’s test and Egger’s revealed no significant publication bias in this meta-analysis about PLR and OS (Fig. 6a: Begg’s test: Z value = 1.73, <1.96; P value = 0.091, >0.05; Egger’s test: P value = 0.068, >0.05), CSS (Fig. 6b: Begg’s P value = 0.089; Egger’s P value = 0.033), PFS (Fig. 6c: Begg’s P value = 0.734; Egger’s P value = 0.557) and DFS (Fig. 6d: Begg’s P value = 0.999; Egger’s P value = 0.952).
Discussions
Currently, no standard quantitative biomarkers are perfect enough to assess the clinical outcomes in patients with urological cancers. According to the Food and Drug Administration, a clinical validating biomarker should be measured reproducible and consistently37. Blood parameters, like NLR and PLR, are convenient and easy to be acquired during routinely clinical practice.
In our current meta-analysis, we utilized the existing evidence from 14 included studies to obtain the pooled results that an elevated pretreatment PLR indicated unfavorable worse OS (HR = 1.58, 95%CI: 1.34–1.86) among patients with urological cancers. High pretreatment PLR was also correlated with poor CSS (HR = 1.78, 95%CI: 1.31–1.43), PFS (HR = 1.64, 95%CI: 1.34–2.02) and DFS (HR = 1.65, 95%CI: 1.18–2.31), with no heterogeneity.
Notably, this correlation of high PLR and poor OS was also applicable to RCC, PCa and UTUC, but not in BCa (HR = 1.16, 95%CI: 0.96–1.41). Only 3 studies available for meta-analysis on PLR and OS in patients with BCa, so it should be interpreted cautiously.
Subgroup analysis divided by ethnicity, sample sizes and PLR cut-off value did not significantly change the main results. Taken all the above results into consideration, we believed that PLR could serve as an reliable marker in urological prognostication. PLR, an parameter which is reproducible, convenient and low cost, can be available from blood routine test in daily clinical practice. Additionally, PLR changes followed by tumor changes during anticancer process are also crucial to know for its application as an indicator of treatment efficacy.
Our findings about PLR are in accordance with previous reported other cancers such as breast cancer, lung cancer and colorectal cancer38,39,40. Systemic inflammatory plays a crucial role in tumor progression at almost every single step including initiation, progression, and metastasis41. But the underlying mechanism that PLR influence the survival of patients with urological cancers remains largely unknown. Several hypotheses have been put forward to explain this phenomenon. Platelet-derived cytokines, such as platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) have been found to regulate and promote tumor angiogenesis, then further accelerate tumor aggressiveness42,43. Platelet can also release
microparticles that help to guard tumor cells from the elimination of natural killer44. On the contrary, the reduction of lymphocytes was correlated with poor prognosis, as indicated in a previous study45. Lymphocytes, like T-lymphocytes who are able to secret interleukin-4 and -5 in tumor microenvironment, have an anti-tumor activity by inducing cytotoxic cell death and inhibiting tumor proliferation46,47. In conclusion, the platelet to lymphocyte ratio could be regarded as a positive correlative marker with worse cancer prognosis theoretically.
Although this is the first meta-analysis concerning PLR and urological cancer prognostication, several limitations are still needed to be addressed. Firstly, though we didn’t restricted language, all the 20 studies included were published in English. We found no relevant studies published in Chinese qualified to the inclusion criteria, but we also failed to identify articles in other languages, which might lead to little language bias. Secondly, although sensitivity analysis supported the stability of our results and no publication bias found, the number of studies was relatively small in different cancer types (6 for RCC, 6 for PCa, 3 for BCa, 2 for UTUC) and in some subgroups (6 for non-Asians). Thus we wish to emphasize that the results should be cautiously interpreted. Thirdly, heterogeneity among studies was found, which was probably because of the relatively small sample sizes and the retrospective property of study design. Fourthly, we lack the PLR data of patients with comprehensive different urological cancer stages (localized, advanced or metastasis) at present. Large scale statistics about PLR response during different treatment strategies such as radiotherapy, chemotherapy and immunotherapy are also insufficient. So further large prospective clinical trials are urgently required to verify the prognostic value of PLR in patients with urological cancers in the future.
In summary, this meta-analysis suggested that elevated PLR was negatively related to survival of patients with urological cancers, except in BCa. However, further high quality studies with large sample size should be conducted to validate this paper’s results.
Methods
Search strategy
This meta-analysis was conducted under the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)48. A comprehensive literature search for relevant studies in the PubMed, Embase and Web of Science was performed through July 24th, 2017. The searching strategy consisted of medical subheadings and key words. The main terms were as follows: (‘urological neoplasms[MeSH]’ OR ‘prostate cancer’ OR ‘bladder cancer’ OR ‘renal cell carcinoma’ OR ‘renal cancer’ or ‘urinary tract cancer’ OR ‘upper urinary tract urothelial carcinoma’) AND (‘platelet to lymphocyte ratio’ OR ‘PLR’) AND (‘prognosis[MeSH]’ OR ‘survival’ OR ‘outcome’). The language of studies, population and sample size were not restricted. We also manually searched the reference lists for additional relevant publications.
Study Selection
Inclusion and exclusion criteria
Studies meeting the following criteria were considered eligible:1. clinical cohort evaluated the prognostic accuracy of PLR in urological cancers; 2. studies compared PLR with other prognosis models and reported survival outcomes like OS and PFS; 3. reported original hazard ratio (HR) with 95% confidence interval (95%CI) or HR could be extracted from sufficient information; 4. articles with the most complete information and the largest cohort if there were multiple studies by the same author or institute.
The exclusion criteria were: 1. repeated publications; 2. studies reporting on less than 20 patients; 3. experimental laboratory articles, animal studies, letters or review articles.
Assessment of study quality
Two investigators(DY.L. and XY.H.) independently reviewed all relevant articles and judged the methodology quality of potential studies using Newcastle–Ottawa Quality Assessment Scale (NOS) assessment tool, including selection, comparability and outcome49. A study was considered high quality if the NOS score ≥7. When disagreements occurred, the two reviewers reached consensus by involving a third author(HX.D.).
Data extraction
We extracted the following variables from each study: first author’s name; publication year; study design; country or region of the study; type of urological cancer; cut-off value of PLR; sample size; age, sex, out-come assessment and risk estimates, follow up time and HRs with 95%CI. If the HRs of both univariate and multivariate analysis for the same comparison were available, we only used the latter for analysis. We also used univariate or multivariate HRs separately to test methodological sensitivity. If the HR and 95%CI were not displayed directly, they were estimated from Kaplan–Meier curves50. If necessary, the corresponding author was contacted for further information.
Statistical analysis
HRs with 95%CI were pooled using a meta-analysis to access the strength of PLR to survival endpoints such as OS, CSS, PFS and DFS. All the PLR values from 20 included studies were categorical variables in survival analysis. The Cochrane Q test was used to determine the heterogeneity among studies. A P value < 0.10 indicated heterogeneity. Inconsistency (I 2) was also calculated to evaluate heterogeneity. AnI 2 value > 50% was considered significant heterogeneity. The fixed-effect model (inverse variance method) was used to calculate pooled results when no heterogeneity existed among included studies, otherwise, a random-effect model (DerSimonian and Laird method) was used with the weights inversely proportional to the variance of log hazard ratio of each trial51,52. To find reasons of heterogeneity among studies, we conducted subgroup analysis in ethnic difference, different cut-off value and sample size respectively. When the log-rank statistical value was maximum on receiver operating characteristic curve, the cut-off value of PLR was decided. The median value of sample size was chosen to divide subgroup. Meta-regression was also performed by using cancer type, PLR cut-off, sample size and follow up time as covariates. To test the reliability of the main outcomes in our analysis, sensitivity analysis was performed by removing one single study in turn. Egger’s and Begg’s tests with funnel plots were used to test publication bias. P value > 0.05 indicated no potential publication bias. Kaplan–Meier curves were read by Engauge Digitizer version 9.8 (http://markummitchell.github.io/engauge-digitizer/). We used Stata 12.0 software (Stata Corporation, College Station, TX, USA) to conduct all the statistical analyses. A two-sided P value less than 0.05 was considered statistically significant.
Data availability
In our current meta-analysis, all original data analyzed were derived from published articles. All data generated during this study are included in the present article.
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108 (2015).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer Statistics, 2017. CA Cancer J Clin 67, 7–30 (2017).
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136, E359–86 (2015).
Ploeg, M., Aben, K. K. & Kiemeney, L. A. The present and future burden of urinary bladder cancer in the world. World J Urol 27, 289–93 (2009).
Elinav, E. et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 13, 759–71 (2013).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–44 (2008).
Allavena, P., Sica, A., Solinas, G., Porta, C. & Mantovani, A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 66, 1–9 (2008).
Zhou, L. et al. Prognostic Role of C-Reactive Protein In Urological Cancers: A Meta-Analysis. Sci Rep 5, 12733 (2015).
Luo, Y., She, D. L., Xiong, H., Fu, S. J. & Yang, L. Pretreatment Neutrophil to Lymphocyte Ratio as a Prognostic Predictor of Urologic Tumors: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 94, e1670 (2015).
Hutterer, G. C. et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol 32, 1041–8 (2014).
Zhu, Y. et al. Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer. Oncotarget 8, 1023–1030 (2017).
Ding, N. et al. The Prognostic Value of PLR in Lung Cancer, a Meta-analysis Based on Results from a Large Consecutive Cohort. Sci Rep 6, 34823 (2016).
Panic, N., Leoncini, E., de Belvis, G., Ricciardi, W. & Boccia, S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 8, e83138 (2013).
Keskin, S. et al. Prognostic value of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios, and multiphasic renal tomography findings in histological subtypes of renal cell carcinoma. BMC Urol 14, 95 (2014).
Lee, S. M., Russell, A. & Hellawell, G. Predictive value of pretreatment inflammation-based prognostic scores (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio) for invasive bladder carcinoma. Korean J Urol 56, 749–55 (2015).
Cheng, Y. C. et al. The Prognostic Significance of Inflammation-Associated Blood Cell Markers in Patients with Upper Tract Urothelial Carcinoma. Ann Surg Oncol 23, 343–51 (2016).
Li, F., Hu, H., Gu, S., Chen, X. & Sun, Q. Platelet to lymphocyte ratio plays an important role in prostate cancer’s diagnosis and prognosis. Int J Clin Exp Med 8, 11746–51 (2015).
Lolli, C. et al. Systemic Immune-Inflammation Index Predicts the Clinical Outcome in Patients with mCRPC Treated with Abiraterone. Front Pharmacol 7, 376 (2016).
Wang, Y. et al. Platelet to lymphocyte ratio as an independent prognostic indicator for prostate cancer patients receiving androgen deprivation therapy. BMC Cancer 16, 329 (2016).
Langsenlehner, T. et al. Evaluation of the platelet-to-lymphocyte ratio as a prognostic indicator in a European cohort of patients with prostate cancer treated with radiotherapy. Urol Oncol 33, 201.e9–16 (2015).
Hu, H. et al. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol 35, 261–270 (2017).
Gu, L. et al. Prognostic value of preoperative inflammatory response biomarkers in patients with sarcomatoid renal cell carcinoma and the establishment of a nomogram. Sci Rep 6, 23846 (2016).
Park, T. J., Cho, Y. H., Chung, H. S. & Hwang, E. C. Prognostic significance of platelet-lymphocyte ratio in patients receiving first-line tyrosine kinase inhibitors for metastatic renal cell cancer. 5, 1889 (2016).
Fox, P. et al. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer 109, 147–53 (2013).
Gunduz, S. et al. Platelet to lymphocyte ratio as a new prognostic for patients with metastatic renal cell cancer. Asia Pac J Clin Oncol 11, 288–92 (2015).
Zhang, G. M. et al. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumour Biol 36, 8537–43 (2015).
Huang, J. et al. Prognostic value of preoperative plasma fibrinogen level and platelet-to-lymphocyte ratio (F-PLR) in patients with localized upper tract urothelial carcinoma. Oncotarget (2016).
Dalpiaz, O. et al. Validation of the Preoperative Platelet-to-Lymphocyte Ratio as a Prognostic Factor in a European Cohort of Patients with Upper Tract Urothelial Carcinoma. Urol Int 98, 320–327 (2017).
Kim, M. et al. Prognostic value of systemic inflammatory responses in patients with upper urinary tract urothelial carcinoma. World J Urol 33, 1439–57 (2015).
Lucca, I. et al. Comparison of the prognostic value of pretreatment measurements of systemic inflammatory response in patients undergoing curative resection of clear cell renal cell carcinoma. World journal of urology 33(12), 2045–2052 (2015).
Sun, Z., Ju, Y., Han, F., Sun, X. & Wang, F. Clinical implications of pretreatment inflammatory biomarkers as independent prognostic indicators in prostate cancer. Journal of clinical laboratory analysis, https://doi.org/10.1002/jcla.22277 (2017).
Lozano Martinez, A. J. et al. Platelet-lymphocyte and neutrophil-lymphocyte ratios are prognostic but not predictive of response to abiraterone acetate in metastatic castration-resistant prostate cancer. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico, https://doi.org/10.1007/s12094-017-1699-x (2017).
Peng, D. et al. Prognostic Value of Inflammatory and Nutritional Scores in Renal Cell Carcinoma After Nephrectomy. Clinical genitourinary cancer, https://doi.org/10.1016/j.clgc.2017.04.001 (2017).
Kang, M., Jeong, C. W., Kwak, C., Kim, H. H. & Ku, J. H. Preoperative neutrophil-lymphocyte ratio can significantly predict mortality outcomes in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Oncotarget 8, 12891–12901, https://doi.org/10.18632/oncotarget.14179 (2017).
Schulz, G. B. et al. Prognostic Value of the Preoperative Platelet-to-leukocyte Ratio for Oncologic Outcomes in Patients Undergoing Radical Cystectomy for Bladder Cancer. Clinical genitourinary cancer, https://doi.org/10.1016/j.clgc.2017.05.009 (2017).
Altan, M. & Haberal, H. B. A critical prognostic analysis of neutrophil-lymphocyte ratio for patients undergoing nephroureterectomy due to upper urinary tract urothelial carcinoma. https://doi.org/10.1007/s10147-017-1150-x (2017).
Goodsaid, F. & Frueh, F. Biomarker qualification pilot process at the US Food and Drug Administration. Aaps j 9, E105–8 (2007).
Ding, N. et al. The Prognostic Value of PLR in Lung Cancer, a Meta-analysis Based on Results from a Large Consecutive Cohort. Sci Rep 6, 34823 (2016).
Zhu, Y. et al. Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer. Oncotarget 8, 1023–1030 (2017).
Guo, Y. H. et al. The clinical use of the platelet/lymphocyte ratio and lymphocyte/monocyte ratio as prognostic predictors in colorectal cancer: a meta-analysis. Oncotarget 8, 20011–20024 (2017).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–7 (2002).
Peterson, J. E. et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis 15, 265–73 (2012).
Goubran, H. A., Burnouf, T., Radosevic, M. & El-Ekiaby, M. The platelet-cancer loop. Eur J Intern Med 24, 393–400 (2013).
Nieswandt, B., Hafner, M., Echtenacher, B. & Mannel, D. N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59, 1295–300 (1999).
Lissoni, P. et al. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers 19, 135–40 (2004).
Eerola, A. K., Soini, Y. & Paakko, P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res 6, 1875–81 (2000).
Rosenberg, S. A. Progress in human tumour immunology and immunotherapy. Nature 411, 380–4 (2001).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8, 336–41 (2010).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–5 (2010).
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S. & Sydes, M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16 (2007).
Parmar, M. K., Torri, V. & Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in medicine 17, 2815–2834 (1998).
Harris, R. J. et al. metan: fixed- and random-effects meta-analysis. Stata Journal 8(1), 3–28 (2008).
Author information
Authors and Affiliations
Contributions
Conceptualization: D.Y.L. & Y.S.S. Data curation: D.Y.L. & X.Y.H. Formal analysis: D.Y.L. & X.Y.H. Investigation: D.Y.L. & X.Y.H. Methodology: X.Y.H. & H.X.D. Project administration: T.M.M. & H.X.D. Supervision: T.M.M. & Y.S.S. Validation: Y.S.S. Visualization: D.Y.L. & T.M.M. Writing ± original draft: D.Y.L. & X.Y.H. Writing ± review & editing: D.L. & Y.S.S.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, DY., Hao, XY., Ma, TM. et al. The Prognostic Value of Platelet-to-Lymphocyte Ratio in Urological Cancers: A Meta-Analysis. Sci Rep 7, 15387 (2017). https://doi.org/10.1038/s41598-017-15673-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15673-2
This article is cited by
-
Preoperative Platelet-Lymphocyte Ratio (PLR) as a prognostic inflammation biomarker in Asian HIV-infected patients with gastric cancer: a single-center study
BMC Gastroenterology (2023)
-
The prognostic value of platelet-to-lymphocyte ratio on the long-term renal survival in patients with IgA nephropathy
International Urology and Nephrology (2021)
-
Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with abiraterone or enzalutamide: a meta-analysis
Prostate Cancer and Prostatic Diseases (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.