Abstract
Canine Visceral Leishmaniasis (CVL) is caused by Leishmania infantum, which in the New World is transmitted by Lutzomyia longipalpis. While prospective clinical and immunological assessments of dogs experimentally challenged with L. infantum have been previously reported over a relatively short follow-up period, the long-term characterization of infected animals has not been performed to date. We evaluated dogs in a subclinical state for six years following experimental infection with L. infantum and Lu. longipalpis saliva, via an intradermal route, to characterize clinical, parasitological and immunological parameters arising from L. infantum experimental infection. We also assess these parameters in a group of naturally infected animals. The immune profiles of the experimentally and naturally infected animals exhibited increases of IFN-γ, IL-6 and IL-18, and decreases in TNF, IL-2, IL-8 and CXCL1, compared to controls. Our results indicate that over a six-year follow-up post-challenge, subclinically infected dogs presented low CVL clinical scores despite the persistence of Leishmania parasites in the lymph nodes, spleen and skin. Similarities observed among immune profiles in the context of experimental and natural infection seem to suggest that an enduring activation of the host immune response may lead to the control of parasite growth, thereby limiting disease severity.
Similar content being viewed by others
Introduction
Visceral leishmaniasis (VL) arising from L. infantum is a severe, often fatal, zoonotic disease, which represents one of the most relevant and challenging emerging diseases worldwide. The number of annual cases detected in Brazil comprises approximately 90% of the human cases occurring in Latin America1,2.
Dogs are considered the main domestic reservoir of the etiological agent of VL, L. infantum, and canine cases often precede the occurrence of human cases due the close proximity between dogs and humans2,3,4. This protozoan is mainly transmitted through the bite of infected sand flies, namely Lu. longipalpis in Brazil. The course of infection is highly variable, as not all infected dogs eventually present clinical signs of disease5.
The pathogenesis of CVL is highly associated with the immune response elicited against parasites by an infected dog6. The resistance profile is associated with the development of a specific anti-Leishmania cell-mediated response, resulting in the production of proinflammatory cytokines, such as IFN-γ and TNF, which increase the leishmanicidal activity of macrophages through the production of nitric oxide (NO) and reactive oxygen species (ROS)7,8. A susceptible profile is associated with parasite dissemination and exacerbated proliferation in combination with elevated antibody levels and a suppressed cellular immune response7,9.
CVL is a systemic disease with variable clinical signs, including lymphadenopathy, splenomegaly, weight loss, onycogryphosis and skin alterations10. Alterations in laboratory parameters, particularly hematological, often reveal normocytic anemia, thrombocytopenia, as well as mild or exacerbated leucopenia or lymphocytosis. Biochemical alterations, including hepatic and renal failure, can also be present11. Hypergammaglobulinemia is one of the most common findings, resulting in immune complex deposition and activation of the complement system, which may lead to glomerulonephritis and renal failure10,12. Typical histopathological findings in infected tissues consist of granulomatous inflammatory reactions associated with the presence of Leishmania amastigotes within macrophages13, in addition to disorganized splenic tissue14.
Our group designed an experimental challenge model of CVL that employed a combination of Leishmania and sand fly saliva. In this model, challenged animals exhibited similarities in clinical signs, parasite load and cytokine profiles when compared with naturally infected dogs15. In a previous study, only 34% (12/35) of the studied dogs remained in a subclinical state, displaying few clinical signs. Upon conclusion of the study period, the 12 subclinical dogs were maintained in the kennel for an additional four years, representing a total follow-up period of six years post-infection. These animals were clinically monitored at least twice annually throughout the evaluation period, during which time no change in clinical status was observed.
The present study details the evaluation of clinical and pathological parameters in these experimentally infected dogs for a follow-up period lasting for six years after infection. We found a persistence of parasites in the lymph nodes, spleen and skin, as well as a mild inflammatory profile that possibly allowed for infection control. Alterations in histopathological findings provide further evidence regarding the persistence of parasites, despite the fact that these dogs maintained a subclinical state. Importantly, similar results were also found in naturally infected dogs that had comparable clinical scores, suggesting that the asymptomatic state could persist for longer periods than what has been previously reported16,17.
Results
All presented parameters were obtained from canine tissue samples at the time of euthanization, six years after infection. Laboratory results of these dogs 450 days post-infection are listed in Supplementary Table S3. Tables 1, 2 and 3 delineate the clinical and clinicopathological findings of the euthanized animals. Considering all the results evaluated at regular intervals throughout the 6-year follow-up period, no significant changes were detected in the observed parameters.
Clinical scores from dogs six years after experimental infection
Thirty-five dogs were infected at the outset of the study. After infection and during follow-up, only 34% (n = 12) maintained a subclinical state. Clinical assessments of these dogs were performed by evaluating the severity of clinical manifestations arising from L. infantum infection, as characterized by weight loss, focal or generalized dermatitis, mucous membrane lesions, onychogryphosis, splenomegaly, lymphadenopathy, conjunctivitis and pale mucous membranes. Six years after experimental infection, the most frequently observed clinical signs were lymphadenopathy (50%), skin pathologies and conjunctivitis (25%) (Table 1). Other clinical signals typically observed in Leishmania-infected diseased dogs, such as onychogryphosis, pale mucosal and cachexia, were absent in the studied animals, indicating the development of a mild form of disease (Table 2).
Hematological and Biochemical Evaluation
Hematological and biochemical parameters were assessed to complement clinical evaluations when dogs were euthanized six years after infection. In the erythrocyte series, the majority of subclinical dogs exhibited a normocytic normochromic anemia profile, with 6 of 12 (50%) presenting hemoglobin levels below standard values and 8 (67%) had decreased levels of erythrocytes and hematocrit. In a white cell panel, 3 of 12 (25%) presented leukopenia and four presented (33%) lymphopenia. Despite thrombocytopenia being a common finding in CVL, this was found in just two animals (17%) (Table 3 and Supplementary Table S1).
The kidney profile revealed that while all animals had normal levels of creatinine, 8 of 12 (67%) subclinical dogs showed levels of urea above standard values. Additionally, the liver profile of these animals showed altered levels of ALT (6/12–50%) and AST (4/12–33%). Although increases in total protein and globulin, as well as decreases in albumin, are important features when diagnosing CVL, none of the evaluated dogs presented significant alterations in these parameters (Supplementary Table S2).
Histopathology
For histological analysis, samples were collected six years after infection from canine skin, popliteal lymph node, liver and spleen tissue (Fig. 1). In the ears of Leishmania-infected dogs, at a location other than the inoculation site, some mild lymphoplasmacytic pleomorphic chronic inflammatory infiltrate was present in 83% (10/12) of the infected animals (Fig. 1a and b). In a single dog, it was possible to observe an ulcer with neutrophils and fibrin at the most superficial layers of the dermis (Fig. 1c). Popliteal lymph nodes presented atrophic lymphoid follicles in 42% (5/12) of the animals (Fig. 1d), while 58% (7/12) had normal reactive lymphoid follicles (Data not shown). Plasmacytosis in 83% (10/12) and erythrophagocytosis in 67% (8/12) were also frequently observed in the popliteal lymph nodes of Leishmania-infected animals (Fig. 1e). Splenic white pulp disorganization was seen in 25% (3/12) (Fig. 1f). The liver presented periportal inflammatory infiltrate (Fig. 1g) and granulomas in portal spaces and the parenchyma in 67% (8/12) (Fig. 1h and i) of the Leishmania-infected dogs.
Histopatological aspects in, skin, lymph node, spleen and liver of experimentally infected dogs, six years after challenge. Dog were intradermally infected with 107 parasites plus five pairs of Lutzomyia longipalpis salivary glands according the parameters described in Material and Methods section. Six years after infection, dogs were euthanized and different organ samples were collected, fixed and stained with H&E for histological evaluation. (a,b) Mononuclear inflammatory infiltrate in the skin; (c) skin ulcer displaying neutrophils and fibrin; (d) atrophic lymphoid follicles in popliteal lymph nodes; (e) plasmocytosis and erythrophagocytosis in popliteal lymph node; (f) disorganized splenic white pulp in spleen; (g) periportal inflammatory infiltrate; (h,i) granuloma in liver sections from experimentally infected dogs.
Clinical, Serological and Parasitological Parameters
All dogs underwent clinical evaluations prior to and after challenge with L. infantum in the presence of sand fly saliva. In these evaluations, clinical assessments were performed via the evaluation of CVL signs. We observed that 83% (10/12) of the animals presented a clinical score <2 (Table 4), while only 17% (2/12) displayed clinical scores of 4 or 5, which reinforces their subclinical status. No animals showed any differences in clinical scoring throughout the 6-year follow-up period (data not shown).
Our analysis of anti-k28 antibodies showed that 58% (7/12) of the dogs had detectable levels of IgG anti-Leishmania (Table 4). All dogs presented converted IgG anti-SLA levels 90 days after the challenge (data not shown).
Spleen, popliteal lymph node and skin tissue biopsies were performed to quantify parasite load by qPCR using primers specific to L. infantum. Parasites were detected in 100% (12/12) of the lymph nodes and 58.3% (7/12) of the spleens and skin. Additionally, a single dog presented parasites in all evaluated organs. All animals had already presented parasites in the lymph nodes at 450 days after challenge, which persisted until the time of euthanization (Table 4). As shown in Table 4, naturally infected dogs had similar clinical scores (0–3), and all presented IgG anti-k28 in addition to parasites in the spleen, whereas 75% (9/12) had Leishmania in the skin. Parasite loads in this group of animals were higher than those in experimentally infected dogs, which could be explained by differences in infection duration, as well as exposure to different living conditions, e.g. malnutrition or co-infections (Babesia and Erlichia), as was previously described in the literature as aggravating factors of CVL18.
Cytokine and Chemokine evaluation
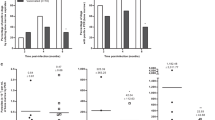
Inflammatory cytokines and chemokines were evaluated in the sera of non-infected naïve, naturally and experimentally subclinically infected dogs. To compare differences in these parameters, we performed hierarchical cluster analysis with bootstrapping to depict the overall expression profile of the indicated serum markers among the different study groups. We found that both groups of infected dogs showed similar signatures, presenting lower concentrations of ALT, albumin, TNF, creatinine, CXCL1, IL-2 and IL-8, as well as higher levels of, IL-10, IL-18, IL-6 and IFN-γ in comparison to naïve dogs. On the other hand, lower concentrations of IL-15, IL-7 and globulin were seen in naïve and experimentally infected dogs compared to naturally infected dogs. Naïve and naturally infected dogs showed similar signatures concerning CCL2 and GM-CSF expression (Fig. 2A).
Cytokines and chemokines detected in sera of experimentally infected dogs, six years after challenge. Dogs were intradermally infected with 107 parasites plus five pairs of Lutzomyia longipalpis salivary glands according the parameters described in Material and Methods section. Six years after infection, dogs were euthanized and sera were collected for Luminex evaluation. (A) Hierarchical cluster analysis and Spearman correlations was performed to depict the overall expression profile of the indicated serum biomarkers in the different study groups. (B) Serum biomarkers presented by naiive, naturally and experimentally infected dogs IFN-γ, GM-CSF, IL-6, IL-8, IL-18, CXCL1, IL-2, TNF.
As shown in Fig. 2B, both naturally and experimentally infected groups exhibited significantly higher levels of IFN-γ, IL-6 and IL-18 compared to the naïve group (p < 0.001). However, the infected (experimentally and naturally) groups presented lower IL-8, CXCL1, IL-2 and TNF levels in comparison to naïve animals (p < 0.001). Experimentally infected dogs presented a significant decrease in GM-CSF compared to controls (p < 0.001). Although other cytokines and chemokines were also evaluated, i.e. IL-7, IL-15 and CXCL-10, no significant differences were detected among the naïve and infected dogs (Data not shown).
Discussion
In long-term prospective studies conducted in areas endemic for CLV, a limited percentage of naturally infected dogs are observed to become very ill, while the vast majority of animals seem to remain healthy for many years19,20,21. In areas endemic for CVL in Brazil, the prevalence of symptomatic dogs varies from 30 to 50%, i.e. a high proportion of dogs remain in asymptomatic state. In nature, dogs are commonly exposed to a variety of adversities, and in a given endemic area it is possible that all have similar chances to develop symptomatic disease following infection with L. infantum. There is a consensus in the literature that the immune response elicited against Leishmania is essential to the outcome of infection3,22,23. Different routes have been employed to experimentally infect dogs, and limitations associated with intradermal, endovenous or subcutaneous routes of injection have been discussed in the literature24,25,26. An ideal route would be to employ infected sand flies, as reported by the Shaden Kamhawi research group24. Unfortunately, this type of experimentation requires a robust insect colony, which few laboratories possess. In an attempt to mimic the conditions surrounding natural infection, we chose to infect the presently studied dogs intradermally using Leishmania and Lu. longipalpis saliva15,22,25. In addition, sand fly saliva possesses potentially immunomodulatory properties that favor the establishment of infection27,28. A previous study conducted by our group employing an intradermal route of infection found the presence of saliva throughout a 450-day follow-up period15.
We infected 35 dogs and 23 were euthanized after 450 days upon the presentation of severe disease, with clinical scores >8. Twelve dogs remained asymptomatic, and these animals were followed-up throughout the course of the present study. These dogs showed few clinical signs related to CVL. The most frequent were lymphadenomegaly (50%), followed by skin lesions and conjunctivitis (25%). These clinical signs have been extensively described in the literature in naturally infected dogs29,30,31,32, notably lymph node enlargement (mostly the popliteal and pharynx). Interestingly, all variable numbers of parasites were found in the lymph nodes of the experimentally infected dogs, which could contribute to enlargement. In addition, skin abnormalities are often relevantly characterized by dry seborrheic dermatitis, desquamation and associated alopecia, since diseased dogs presenting these skin alterations seem to be associated with the ability to transmit L. infantum to the sand fly population31,33. On the other hand, Solano-Gallego et al. (2004) found similar parasite loads in affected skin as well as in the normal, healthy skin of infected dogs34. Indeed, the present study found just three dogs presenting alopecia, while qPCR detected the presence of parasites in the healthy skin of five dogs (42%). Moreover, parasites could also be present in different areas of healthy skin, suggesting the role of subclinical dogs as transmitters of parasites to uninfected sand flies24.
Despite alterations in hematological parameters and the biochemical profiles of dogs naturally infected by L. infantum, these characteristics are of limited use in disease diagnosis. Still, they may represent important biomarkers for evaluating the clinical progress of infected animals, thereby contributing to a better understanding of CVL pathogenesis35. In dogs with active CVL disease, anemia has been associated with a disorder in the erythroid bone marrow compartment36. This could also be related to increased hemolysis, due the trapping of erythrocytes in the enlarged spleen and liver in association with an inflammatory response to L. infantum infection37.
In a white cell panel, 25% of the evaluated dogs presented leukopenia and 33% presented lymphopenia, yet only 17% had thrombocytopenia. Similar results were observed by Reis et al.36 and Nicolato et al.17 in naturally infected dogs. The low mean platelet counts detected herein is a very common laboratorial sign in CVL, and could be associated to vasculitis or platelet destruction following renal and/or hepatic failure, or anti-platelet antibodies38,39. However, in our study, we found thrombocytopenia in just two dogs, which reinforces the notion that dogs evaluated over the long-term present similar pathological clinical profiles as those naturally exposed to Leishmania parasites that course with a subclinical status.
Biochemical parameter evaluation allowed us to evaluate liver and kidney profiles by quantifying AST, ALT, urea, creatinine and total proteins and fractions. We observed that 67% of the asymptomatic animals showed higher levels of urea with no alterations in creatinine, while only 50% presented slightly higher levels of ALT versus 33% of AST, which denotes mild dysfunction of the liver and kidney. We can hypothesize that these alterations may reflect the onset of renal injury, since changes in creatinine only occur in response to severe renal dysfunction. According to Baneth et al.32, azotemia becomes evident only when the majority of nephrons are dysfunctional, which occurs rather late in disease progression.
Histopathological analysis detected an inflammatory profile characterized by intrasinusoidal leukocytosis in all infected dogs, with 67% of the animals presenting granulomas in the liver. According to Tafuri et al.40, granulomas are the most significant lesion type observed in the livers of L. infantum-infected dogs, which are characterized by intralobular hepatic granulomatous formations with varying numbers of macrophages. Moreover, these liver granulomas could be indicative of an active cell-mediated immune response, as was observed in resistant dogs naturally infected in endemic areas41. These findings could suggest that the experimentally infected dogs developed similar hepatic alterations as those presented by naturally resistant infected dogs.
To better understand the immune response presented by the studied dogs six years after infection, hierarchical cluster analysis was performed to obtain biosignatures regarding the serum parameters evaluated. In the heatmap shown in Fig. 2A, both experimentally and naturally infected dogs exhibited similar profiles regarding lower levels of TNF, CXCL1, IL-2 and IL-8 when compared to naïve dogs. Moreover, in Fig. 2B, statistical differences among the naturally and experimentally infected dogs were see with respect to levels of IL-2 and TNF, which indicates that these cytokines present distinct behavior in accordance with different courses of infection. It is important to note that these features could also be attributed to co-infections, such as babesiosis or erlichiosis, which have been very commonly reported in our area of study18,42.
A similar profile was observed with regard to GM-CSF levels, in which experimentally infected groups showed a significant decrease in this cytokine compared to the control group.
A recent report from our group evaluating serum biomarkers characteristic of CVL severity found similar results to those shown herein, i.e. decreases in these mediators in subclinical naturally infected animals, which serves to validate our animal infection model42. Infected groups had higher expression of IL-18, IL-6 and IL-10 compared to naïve animals, which suggests a mixture of inflammatory and anti-inflammatory responses. Serum levels of IL-7 and IL15 were higher in naturally infected dogs compared to naïve and experimentally infected dogs, yet this was not statistically significant. By contrast, IFN-γ levels among the experimentally and naturally infected dogs were statistically higher in comparison to naïve dogs, which denotes an immune response capable of controlling parasite proliferation and dissemination.
Concerning CXCL-1, a chemokine responsible for the recruitment of neutrophils43, we noted that subclinically infected animals had decreased levels in comparison to the naïve group. In a study previously performed by our group, we found a significant increase in this chemokine in diseased naturally infected dogs42. Accordingly, we can infer that low parasite loads could regulate the production of CXCL1, which may lead to a milder inflammatory cellular immune response.
Our analysis of lymph node parasite load and the humoral immune response against a Leishmania antigen (rK28) found that although all dogs presented parasites in this organ, only 58% presented detectable levels of IgG anti-Leishmania. This seems to suggest that this serological test is not appropriate for CVL diagnosis among subclinical infected dogs44, while PCR for the detection of parasites is more accurate. Our collaborators on a study performed in an endemic area described similar findings. Interestingly, 100% of those dogs tested positive in at least one of the tissue samples analyzed using qPCR45. Indeed, there seems to be a strong correlation between lymph node immune response and the control of clinical status during ongoing CVL. Giunchetti et al.46 found an intense hypertrophy/hyperplasia in the lymph nodes of subclinically infected dogs, suggesting that lymphocyte activation in the lymph nodes may favor cell migration and control of parasite burden in parasitized organs, yet they could not find any relationship to clinical CVL status46.
Considering the varying degrees of infection and clinical manifestations observed in the skin and lymphoid tissues of the animals followed for six years after being intradermally challenged with L. infantum in the presence of L. longipalpis saliva, we can infer that these dogs exhibited similar clinical, immunological and pathological profiles as those that were naturally infected and maintained a subclinical status. There is a debate in the literature regarding the role of subclinically infected dogs with respect to the maintenance of infection. Our results demonstrated the presence of parasites in the skin, even in areas where no lesions were apparent, in at least in 41% of the animals evaluated. This feature calls attention to the potential ability of these animals to transmit parasites to uninfected sand flies.
In sum, the present study showed that experimentally infected dogs that were well-nourished, free of ectoparasites and worms, and without any co-infections, nonetheless exhibited an immune response characteristic of CVL, together with clinical and pathological findings resembling those observed in naturally infected animals. It is our hope that these findings may further the understanding of the subclinical status of this disease, which could play a role in controlling Leishmania growth in addition to the spread of parasites to the visceral organs.
Methods
Animals
Thirty-five healthy, 3-month-old beagle dogs of both genders were purchased from a local breeder in a non-endemic area from Brazil (Tad’s Henriques Kennel, Colombo, Paraná State, Brazil). After quarantine, all dogs received routine vaccinations (rabies, distemper, hepatitis/adenovirus type2, leptospirosis and parvovirus) and were dewormed against helminthes. Prior to challenge, blood samples were collected for serological evaluations and no dogs showed any detectable levels of anti-Leishmania or anti-saliva (Lu. longipalpis) antibodies. Throughout the study, the animals were housed at an experimentation kennel located in the municipality of Monte Gordo, Bahia State, Brazil facility that possessed an infrastructure suited to safely handle infected animals. Only 34% (12/35) of these dogs presented a subclinical state after infection and maintained this clinical profile during the 6-year follow-up period. The data presented in this manuscript refers to these 12 dogs.
Additionally, eight subclinical naturally infected mongrel dogs of both genders and different ages were obtained from a cross-sectional study conducted in the municipality of Camaçari, an area highly endemic for CVL. These dogs presented anti-Leishmania antibodies (DPP® CVL, Bio-Manguinhos Unit, Rio de Janeiro, Brazil) and positivity for Leishmania on qPCR. For the Luminex assays, we included sera from eight adult, non-infected (naïve) beagle dogs of both genders from a non-endemic CVL area, which tested negative for anti-Leishmania and anti-saliva antibodies (non-exposed dogs).
Ethical Statement
All procedures performed herein were conducted in accordance with the guidelines for animal research established by the Brazilian College of Animal experimentation (Colégio Brasileiro de Experimentação Animal) and the National Council for Animal Control Experimentation (Conselho Nacional de Controle de Experimentação Animal). The IGM - FIOCRUZ Institutional Review Board for Animal Experimentation approved all procedures involving animals (CEUA – Instituto Gonçalo Muniz - IGM/FIOCRUZ - 010/2009).
Sand Flies and SGH preparation
L. longipalpis, Cavunge strain (Cavunge, Bahia), were reared at the Laboratory of Imunoparasitology (LIP-IGM) as previously described47. Salivary glands were dissected from 5–7-day old females and stored in saline at −70 °C. Before use, salivary glands were sonicated and centrifuged at 8.000xg for 5 min. The supernatant was collected and used immediately.
Leishmania parasites and intradermal experimental infection
For experimental infection, we employed the a previously described protocol15. Briefly, L. infantum (MCAN/BR/00/BA262) promastigotes, originally isolated from a naturally infected dog (Bahia State, Brazil), were obtained from an existing collection and cultured in Schneider’s medium (LGC, Brazil) supplemented with 10% heat inactivated FBS (fetal bovine serum), 2 mM L-glutamine, 100 IU/ml penicillin and 1% streptomycin. Dogs were intradermally inoculated in the ear with 107 stationary-phase promastigotes in the presence of SGH equivalent to five pairs of glands using a 29-gauge needle at a volume of 200 µl. After infection, all dogs were housed in kennels covered with anti-insect netting, received a balanced diet and water ad-libitum. Once a year all animals were dewormed and received routine vaccinations.
Clinical evaluation
During the first two years of study, all dogs were clinically evaluated on a monthly basis for CVL signs. In subsequent years, the animals were examined at 6-month intervals. The severity of CVL was determined by the presence or absence of the following clinical signs: nutritional status as represented by weight loss, mucosal color, skin involvement (alopecia), lymphadenopathy, splenomegaly, conjunctivitis, nail size (onychogryphosis) and the presence of mucosal lesions graded from 0 to 2 at each time point, as adapted from Manna et al.48. After each evaluation, as these points were summed and the corresponding value was considered as the clinical score of each dog (minimum score = 0; maximum score = 16).
Euthanasia
Six years after infection, all dogs were euthanized by intravenous injection of an acepromazin/ketamin anesthetic prior to the administration of a supersaturated solution of potassium chloride. Death was confirmed by the presence of cardiac respiratory arrest.
Hematological and Biochemical Parameters
Hematological and biochemical parameters were evaluated during the course of infection (each two months) until 450 days and in the day of the necropsy 6 years later. Total red blood cell and white blood cell counts were determined using an automated cell counter (Pentra 80 counter, ABX Diagnostics, Montpellier, France). Micro-hematocrit tubes containing blood samples were centrifuged at 12,000 rpm for 5 min, after which hematocrit levels were estimated. Serum was collected by centrifuging blood samples, then submitted to biochemical testing using an enzymatic colorimetric method with an A15 auto-analyzer (BioSystems, Barcelona, Spain) to evaluate total protein, globulin, albumin, AST (aspartine aminotransferase), ALT (alanine aminotransferase), urea and creatinine.
Humoral immune response
Anti-Leishmania antibody levels were determined by the DPP CVL (Dual-Path Platform, Bio-Manguinhos) rapid test, which detects anti rk28-antibodies, as previously described49. All procedures were performed in accordance with manufacturer instructions.
DNA extraction and parasite burden quantification by real-time PCR
Lymph node, skin and spleen fragment DNA was extracted using a DNeasy® Blood & Tissue Kit (Qiagen, Hilden, Germany) by following manufacturer protocols. After extraction, DNA quality and concentration was determined using a digital spectrophotometer (NanoDrop® ND-1000, Thermo Scientific, Wilmington, USA) and DNA integrity was evaluated on a 0.8% agarose gel. DNA samples were then adjusted to a concentration of 30 ng/µl and stored at −20 °C until the time of cPCR and qPCR assaying. To quantify Leishmania DNA in canine spleen fragments, qPCR assays were performed using a technique as previously described by Solcà et al.45. As a positive control, splenic aspirate samples from two dogs of an endemic area that tested positive for Leishmania infection were employed. Negative controls consisted of splenic aspirates from two healthy dogs from the municipality of Pelotas, Rio Grande do Sul, Brazil, a non-endemic area.
Luminex Assay
Sera samples were profiled using a pre-defined Luminex-based multiparametric bead array kit (Milliplex Map Kit - canine cytokine magnetic bead panel, Life Technologies, Carlsbad, CA, USA) to measure the following canine inflammatory cytokines and chemokines: IFN-γ, IL-10, TNF, IL-1β, IL-2, IL-6, IL-7, IL-15, IL-8, MCP-1, CXCL-1 and GM-CSF. All procedures were performed in accordance with the manufacturer’s protocol. Briefly, a 96-well filter plate was blocked with washing buffer under agitation on a plate shaker at room temperature. Assay Buffer was then added with the appropriate matrix solution into the background, standard and control wells, followed by the addition of sera and premixed beads to each well. After incubation, the detection antibody was added followed by the conjugate (Streptavidine). After washing, results were read using a Luminex 200TM and data were obtained using software by Luminex Corporation. Results are expressed in pg/mL and mean fluorescence intensity (MFI). These assays were performed at the Laboratório de Imunofarmacologia of the Oswaldo Cruz Institute, Fiocruz – RJ.
Histological analysis
Slices 4-mm in thickness of liver, popliteal lymph node, spleen and skin tissue were fixed in formalin and embedded in paraffin. Hematoxylin-and eosin-stained sections with a thickness of 4 to 5 µm were examined by optical microscopy. Spleen samples were classified according to the degree of splenic white pulp organization using previously described criteria13.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, USA). Cytokine evaluations were performed using the non-parametric Mann Whitney U-test. Hierarchical cluster analysis (Ward’s method) with bootstrapping was performed to depict the overall expression profile of serum biomarkers in negative and naturally or experimentally infected dogs. Significant statistical differences among three or more groups were evaluated using the Kruskal-Wallis test with Dunn’s multiple comparisons. All analyses were two-tailed and pre-specified. Differences were considered significant when P values ≤ 0.05 after adjustment for multiple comparisons using the Holm-Bonferroni method.
References
Handman, E. Leishmaniasis: current status of vaccine development. Clin Microbiol Rev 14, 229–243 (2001).
Alvar, J. et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7, e35671 (2012).
Moreno, J. & Alvar, J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 18, 399–405 (2002).
Dantas-Torres, F. & Brandão-Filho, S. P. Visceral leishmaniasis in Brazil: revisiting paradigms of epidemiology and control. Rev. Inst. Med. Trop. Sao Paulo 48, 151–6 (2006).
Solano-Gallego, L. et al. Leishmania infantum-specific IgG, IgG1 and IgG2 antibody responses in healthy and ill dogs from endemic areas. Evolution in the course of infection and after treatment. Vet. Parasitol. 96, 265–76 (2001).
Reis, A. B., Giunchetti, R. C., Carrillo, E., Martins-Filho, O. A. & Moreno, J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends Parasitol. 26, 341–9 (2010).
Carrillo, E. & Moreno, J. Cytokine profiles in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 128, 67–70 (2009).
Zafra, R. et al. High iNOS expression in macrophages in canine leishmaniasis is associated with low intracellular parasite burden. Vet. Immunol. Immunopathol. 123, 353–9 (2008).
Gradoni, L. Canine Leishmania vaccines: still a long way to go. Vet. Parasitol. 208, 94–100 (2015).
Freitas, V. C., Parreiras, K. P., Duarte, A. P. M., Secundino, N. F. C. & Pimenta, P. F. P. Development of Leishmania (Leishmania) infantum chagasi in its natural sandfly vector Lutzomyia longipalpis. Am. J. Trop. Med. Hyg. 86, 606–12 (2012).
de Freitas, J. C. C. et al. Clinical and laboratory alterations in dogs naturally infected by Leishmania chagasi. Rev. Soc. Bras. Med. Trop. 45, 24–9 (2012).
Almeida, M. A. O. et al. Antileishmanial antibody profile in dogs naturally infected with Leishmania chagasi. Vet. Immunol. Immunopathol. 106, 151–8 (2005).
Giunchetti, R. C. et al. Histopathological and immunohistochemical investigations of the hepatic compartment associated with parasitism and serum biochemical changes in canine visceral leishmaniasis. Res. Vet. Sci. 84, 269–77 (2008).
Silva, J. S. et al. Low CXCL13 Expression, Splenic Lymphoid Tissue Atrophy and Germinal Center Disruption in Severe Canine Visceral Leishmaniasis. PLoS One 7, e29103 (2012).
Costa, D. J. D. J. et al. Experimental Infection of Dogs with Leishmania and Saliva as a Model to Study Canine Visceral Leishmaniasis. PLoS One 8 (2013).
de Almeida Leal, G. G. et al. Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. Vet. Parasitol. 205, 472–82 (2014).
Nicolato Rde, C. et al. Clinical forms of canine visceral Leishmaniasis in naturally Leishmania infantum-infected dogs and related myelogram and hemogram changes. PLoS One 8, e82947 (2013).
Krawczak, F. da S. et al. Leishmania, Babesia and Ehrlichia in urban pet dogs: co-infection or cross-reaction in serological methods? Rev. Soc. Bras. Med. Trop. 48, 64–8 (2015).
Chappuis, F. et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 5, 873–82 (2007).
Courtenay, O., Quinnell, R. J., Garcez, L. M., Shaw, J. J. & Dye, C. Infectiousness in a cohort of brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J. Infect. Dis. 186, 1314–20 (2002).
Foglia Manzillo, V. et al. Prospective study on the incidence and progression of clinical signs in naïve dogs naturally infected by Leishmania infantum. PLoS Negl. Trop. Dis. 7, e2225 (2013).
Afrin, F., Anam, K. & Ali, N. Induction of partial protection against Leishmania donovani by promastigote antigens in negatively charged liposomes. J. Parasitol. 86, 730–5 (2000).
Belkaid, Y. et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188, 1941–1953 (1998).
Aslan, H. et al. New Insights Into the Transmissibility of Leishmania infantum From Dogs to Sand Flies: Experimental Vector-Transmission Reveals Persistent Parasite Depots at Bite Sites. J. Infect. Dis. 213, 1752–61 (2016).
Resende, L. A. et al. Impact of LbSapSal Vaccine in Canine Immunological and Parasitological Features before and after Leishmania chagasi-Challenge. PLoS One 11, e0161169 (2016).
Fernandes, A. P. et al. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine 26, 5888–95 (2008).
Andrade, B. B., de Oliveira, C. I., Brodskyn, C. I., Barral, A. & Barral-Netto, M. Role of sand fly saliva in human and experimental leishmaniasis: current insights. Scand. J. Immunol. 66, 122–7 (2007).
Abdeladhim, M., Kamhawi, S. & Valenzuela, J. G. What’s behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infect. Genet. Evol. 28, 691–703 (2014).
Ciaramella, P. et al. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet. Rec. 141, 539–43 (1997).
Solano-Gallego, L. et al. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 165, 1–18 (2009).
Solano-Gallego, L. et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors 4, 86 (2011).
Baneth, G., Koutinas, A. F., Solano-Gallego, L., Bourdeau, P. & Ferrer, L. Canine leishmaniosis - new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 24, 324–30 (2008).
Travi, B. L. et al. Clinical, parasitologic, and immunologic evolution in dogs experimentally infected with sand fly-derived Leishmania chagasi promastigotes. Am. J. Trop. Med. Hyg. 81, 994–1003 (2009).
Solano-Gallego, L. et al. Histological and immunohistochemical study of clinically normal skin of Leishmania infantum-infected dogs. J. Comp. Pathol. 130, 7–12 (2004).
Reis, A. B. et al. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 128, 87–95 (2009).
Reis, A. B. et al. Parasite density and impaired biochemical/hematological status are associated with severe clinical aspects of canine visceral leishmaniasis. Res. Vet. Sci. 81, 68–75 (2006).
Alvar, J., Cañavate, C., Molina, R., Moreno, J. & Nieto, J. Canine leishmaniasis. Adv. Parasitol. 57, 1–88 (2004).
Terrazzano, G. et al. Presence of anti-platelet IgM and IgG antibodies in dogs naturally infected by Leishmania infantum. Vet. Immunol. Immunopathol. 110, 331–7 (2006).
Ciaramella, P. et al. Altered platelet aggregation and coagulation disorders related to clinical findings in 30 dogs naturally infected by Leishmania infantum. Vet. J. 169, 465–7 (2005).
Tafuri, W. L. et al. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J. Immunol. Methods 292, 17–23 (2004).
Sanchez, M. A. et al. Organ-specific immunity in canine visceral leishmaniasis: analysis of symptomatic and asymptomatic dogs naturally infected with Leishmania chagasi. Am. J. Trop. Med. Hyg. 70, 618–24 (2004).
Solcà, M. S. M. S. et al. Circulating Biomarkers of Immune Activation, Oxidative Stress and Inflammation Characterize Severe Canine Visceral Leishmaniasis. Sci. Rep. 6, 32619 (2016).
Bozic, C. R. et al. Expression and biologic characterization of the murine chemokine KC. J. Immunol. 154, 6048–57 (1995).
Grimaldi, G. et al. Clinical and Parasitological Protection in a Leishmania infantum-Macaque Model Vaccinated with Adenovirus and the Recombinant A2 Antigen. PLoS Negl. Trop. Dis. 8 (2014).
Solcà, MdaS. et al. Evaluating the accuracy of molecular diagnostic testing for canine visceral leishmaniasis using latent class analysis. PLoS One 9, e103635 (2014).
Giunchetti, R. C. et al. Histopathology, parasite density and cell phenotypes of the popliteal lymph node in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 121, 23–33 (2008).
Gomes, R. et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci USA 105, 7845–7850 (2008).
Manna, L., Reale, S., Vitale, F. & Gravino, A. E. Evidence for a relationship between Leishmania load and clinical manifestations. Res. Vet. Sci. 87, 76–8 (2009).
Fraga, D. B. M. et al. The Rapid Test Based on Leishmania infantum Chimeric rK28 Protein Improves the Diagnosis of Canine Visceral Leishmaniasis by Reducing the Detection of False-Positive Dogs. PLoS Negl. Trop. Dis. 10, e0004333 (2016).
Acknowledgements
The authors would like to thank Andris K. Walter for English language revision and manuscript copyediting assistance. The authors also would like to thank Andrezza Souza for technical and logistics support. This work was supported by grants from Fundação de Amparo à Pesquisa do Estado da Bahia-FAPESB (N°SUS0036/2013 and PET0024/2013 to C.I.B.); grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (402670/2012-4 to C.I.B.); and Instituto Nacional de Ciência e Tecnologia de Investigação em Imunologia (III-INCT) (to C. I. B.). C.I.B., P.S.T.V, P.T.B., are senior investigators of CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and Designed the experiments: M.M.C.A., V.A.A., L.S.P., C.I.B., W.L.C., D.B.M.F. and P.S.T.V. Performed the Experiments: M.M.C.A, V.A.A., L.S.P., M.S.S., V.A.A. and D.B.M.F. Analysed the Data: M.M.C.A., B.B.A., L.S. and C.I.B. Contributed reagents/materials/analysis tools: D.J.C., B.B.A., C.I.B. and P.T.B. Wrote the paper: M.M.C.A., C.I.B.,V.A., P.S.T.V., W.L.C. and B.B.A.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbehusen, M.M.C., Almeida, V.d.A., Solcà, M.d.S. et al. Clinical and immunopathological findings during long term follow-up in Leishmania infantum experimentally infected dogs. Sci Rep 7, 15914 (2017). https://doi.org/10.1038/s41598-017-15651-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15651-8
This article is cited by
-
Interleukin 6 and interferon gamma haplotypes are related to cytokine serum levels in dogs in an endemic Leishmania infantum region
Infectious Diseases of Poverty (2023)
-
Immunological and genomic characterization of Ibizan Hound dogs in an endemic Leishmania infantum region
Parasites & Vectors (2022)
-
An experimental challenge model for Leishmania donovani in beagle dogs, showing a similar pattern of parasite burden in the peripheral blood and liver
Parasitology Research (2022)
-
Exploring the relationship between susceptibility to canine leishmaniosis and anti-Phlebotomus perniciosus saliva antibodies in Ibizan hounds and dogs of other breeds in Mallorca, Spain
Parasites & Vectors (2020)
-
Assessment of Leishmania infantum infection in equine populations in a canine visceral leishmaniosis transmission area
BMC Veterinary Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.