Abstract
Echinoderm microtubule-associated protein-like 4 gene and anaplastic lymphoma kinase gene (EML4-ALK) rearrangement is a key driver mutation in non-small cell lung cancer (NSCLC). Although Break-Apart ALK fluorescence in situ hybridization (FISH) is a reliable diagnostic method for detecting ALK gene rearrangement, it is too costly and time-consuming for use as a routine screening test. Our aim was to evaluate the clinical utility of a novel rapid FISH (RaFISH) method developed to facilitate hybridization. RaFISH takes advantage of the non-contact mixing effect of an alternating current (AC) electric field. Eighty-five specimens were used from patients diagnosed with NSCLC identified immunohistochemically as ALK 0, (1/2+) or (3+). With RaFISH, the ALK test was completed within 4.5 h, as compared to 20 h needed for the standard FISH. Although RaFISH produced results more promptly, the staining and accuracy of the ALK evaluation with RaFISH was equal to the standard. We found 97.6% agreement between FISH and RaFISH based on the status of the ALK signals. These results suggest RaFISH could be used as a clinical tool to promptly determine ALK status.
Similar content being viewed by others
Introduction
Several key genetic alterations that drive non-small cell lung cancer (NSCLC) have been detected. These include mutation of the epidermal growth factor receptor (EGFR) gene and rearrangement of the echinoderm microtubule-associated protein-like 4 gene and anaplastic lymphoma kinase gene (EML4-ALK)1,2,3,4. The ALK fusion oncogene is formed by a rearrangement occurring on the chromosome 2 short arm and involves the genes encoding for ALK (2p23.2) and EML4 (2p21) or, on rare occasions, other non-EML4 genes such as KIF5B and TFG. The protein product of this fusion gene has a constitutively active ALK because the basic domain of EML4 provides a mechanism for the dimerization of the new chimeric protein1,5. The EML4-ALK fusion gene is one of the most desirable molecular targets for patients with ALK-rearranged NSCLC because there are several specific tyrosine kinase inhibitors (TKIs) available for treatment1, including Crizotinib, Ceritinib, Alectinib, Brigatinib, and Lorlatinib6,7. Moreover, ALK TKIs are well tolerated with rapid, durable responses in ALK-positive patients, and there is potential for ongoing benefit after initial disease progression in this population6,8. However, ALK gene rearrangements are present in only ∼3–5% of patients with NSCLC1,2,9.
It is widely recognized that the detection of ALK gene rearrangements is very important for selecting effective chemotherapy for patients with advanced and/or recurrent NSCLC. Because ALK gene rearrangement involves large chromosomal inversion and translocation, fluorescence in situ hybridization (FISH) has become the method of choice for detecting all forms of ALK gene rearrangement, including the rare non-EML4 fusions, and it was the assay used to detect this genetic aberration in the first clinical trials of the ALK inhibitor therapy with Crizotinib8,10,11. As many guidelines already recommend, Break-Apart ALK FISH is a reliable standard diagnostic method in surgical pathology because it is easily applicable to formalin-fixed, paraffin-embedded (FFPE) samples, even when the exact fusion partners are not known5. However, whereas ALK immunohistochemistry (IHC) is a reliable and economical screening method, and a relatively easy diagnostic tool to apply for detection of pathologic (/overexpression) proteins in FFPE12,13,14, FISH is time-consuming and requires the use of expensive probes and a special fluorescence microscopy facility. Thus several unique obstacles to cost-effective and timely screening must be overcome before FISH is introduced as a routine test.
We recently developed a rapid-IHC method that makes use of an alternating current (AC) electric field to facilitate the antigen-antibody reaction, and reported its usefulness for detection of lung cancer metastasis, central nervous system tumors, and mammalian eggs15,16,17. The device reduces the time required for IHC as well as the amount of antibody required for these analyses. With this device, we apply a high-voltage, low-frequency AC electric field to the sections. The antibody is mixed within the microdroplet as the voltage is switched on and off at specific intervals. The resultant coulomb force stirs the diluted solution on the sections, which increases the opportunity for contact. This rapid-IHC method enables rapid detection of target cells in frozen sections and can provide a surgeon with an intraoperative diagnosis within about 30 min16. Although the rapid-IHC method still has limitations, as it has not been tested in other organs or with other detection methods, we anticipate that this technique will be applicable in multiple settings. More importantly, we demonstrated in patients with breast cancer that rapid dual in-situ hybridization performed with an AC electric field and this device could be used to detect human epidermal growth factor receptor 2 (HER2) amplification within 6 h18.
The aim of the present study was to evaluate the clinical utility, reliability and sensitivity of a novel rapid FISH (RaFISH) method that promotes ALK break-apart hybridization by taking advantage of the non-contact mixing effect in microdroplets subjected to an AC electric field.
Results
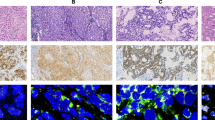
The clinical patients’ characteristics are summarized in Table 1. For all specimens from enrolled lung cancer patients, ALK was first scored using IHC. Among the 85 specimens, 63 were scored 0, 3 were scored (1+), 0 were scored (2+) and 19 were scored (3+). Thereafter, the ALK statuses of the 85 specimens were evaluated using both FISH and RaFISH. The standard FISH using an ALK break-apart probe set and the new RaFISH protocol performed equally well (Fig. 1). On the other hand, RaFISH enabled detection of ALK break apart within 4.5 h, which is much less time than the 20 h required for standard FISH (Table 2).
Detection of ALK rearrangements using standard Fluorescence in Situ Hybridization (FISH) and rapid FISH (RaFISH). Samples were classified as positive for ALK rearrangement when 15% or more of tumor nuclei showed split signals (red and green signals were separated by ≥2 signal diameters) or single red signals (3′ ALK) were observed in FISH or RaFISH. (A) Positive demonstrated using FISH. (B) Positive demonstrated using RaFISH. (C) Negative demonstrated using FISH. (D) Negative demonstrated using RaFISH.
Table 3 shows each ALK status defined as the ALK signal for the 85 specimens. Only two specimens in the ALK-IHC 0 group could not be evaluated using the RaFISH protocol because of poor ALK hybridization, which was caused by there being too much collagen tissue on the specimens. All 19 specimens scored 3+ using IHC were evaluated as ALK break apart signals when either FISH or RaFISH was used. We found 97.6% agreement between FISH and RaFISH based on the status of ALK signals (Cohen kappa coefficient = 0.904, 95% confidence interval (CI): 0.802–1.000).
Discussion
In the present study, we demonstrated that RaFISH performed using our device with an AC electric field can be used to detect break-apart ALK rearrangement within 4.5 h. In addition, specimens subjected to RaFISH stained equally well and provided the same diagnosis as specimens subjected to standard FISH, and the accuracy of the evaluation using the new RaFISH technique was equal to that obtained with standard FISH.
When using FISH for detection of ALK rearrangements, the ALK Break-Apart FISH Probe Kit (Abbott Molecular, Des Plaines, IL) has become a gold standard diagnostic for targeted therapy with an ALK inhibitor in NSCLC5. However, FISH can be costly and time-consuming, and it requires specialized fluorescence microscopy equipment. Moreover, technical challenges include FISH signal instability and scoring difficulties. On the other hand, ALK IHC is an economical and comparatively easy screening method that is familiar and available to every pathologist and diagnostician12,13,14. Our new RaFISH system has some of the same limitations as FISH because it makes use of the ALK Break-Apart Probe set. However RaFISH has clear time advantages: ALK tests using RaFISH were completed within 4.5 h, as compared to 20 h needed for conventional FISH. Moreover, our rapid system also can be used for rapid IHC15,16,17. Prompt and correct ALK test results may save time and effort later on for many advanced and/or recurrent lung cancer patients.
There are three major conventional diagnostics for ALK fusions: highly sensitive IHC using ALK1, D5F3, 5A4, and anti-ALK, FISH including chromogenic in situ hybridization, and reverse-transcriptase polymerase chain reaction (RT-PCR)12. RT-PCR is the most sensitive and rapid method and can be used to identify specific variants of the ALK rearrangements. However, RT-PCR requires ALK fusion variants to be known so that primers for those variants can be included in the reaction. Consequently, ALK fusions with unknown partners have remained undetectable19. In addition, whereas IHC and FISH can be performed on a single FFPE slide, RT-PCR requires multiple slides in order to extract sufficient RNA. For these reasons, RT-PCR is not suitable for screening patients for treatment with an ALK inhibitor. This is unfortunate, as RT-PCR enables examination of specimens that are not amenable to tissue blocks, such as bronchial lavage fluid, sputum, blood, and body cavity fluids20. The failure rate for FISH used as a screening tool were around 4–5% in recent series14,21. The main disadvantage of FISH, including RaFISH, is that interpretation requires expertise and experience22,23. Every laboratory and hospital has to plan an appropriate molecular testing service based to the skills and resources available, and the demand and expectations placed upon it.
We first tried ALK FISH using our conventional high-voltage (4.5 KV, offset 2.4 KV), low-frequency (15 Hz) AC electric field, which we described previously18. However, the quality of the hybridization signal intensity for both the 3′ (telomeric) part of the fusion breakpoint and the 5′ (centromeric) part was not sufficient to stain the slide (data not shown). We speculate that this might reflect weak fluorochrome labeling on both ALK hybridization probes that came unbound with the strong shear forces caused by the AC electric mixing. We therefore changed the condition to high frequency (90 Hz) and used a polyethylene terephthalate (PET) cover to prevent probe evaporation during mixing, instead of an oil cover. Under a high-voltage (4.5 KV, offset 2.4 KV), high-frequency (90 Hz) AC electric field, the microdroplet’s shape was not transformed as the voltage was switched on and off at specific intervals. Nonetheless, in control experiments using ferrite particles (average diameter: 50 nm) in PBS, we confirmed that the particles were mixed well within the microdroplets (Supplementary Video 1). ISH requires a warm temperature, such as 37 °C, and a longer processing time than the rapid-IHC method. Consequently, evaporation can be a problem during ISH. We have shown that PET cover cap prevented evaporation when using the RaFISH device in the present study. Further investigation will be needed to more precisely define the utility of RaFISH with other kinds of ALK probes and other types of malignant cells (e.g., anaplastic large cell lymphoma and inflammatory myofibroblastic tumors).
It is also important to consider the effect of electroporation by the high-voltage (4.5 KV, offset 2.4 KV), high frequency (90 Hz) electric field when using the RaFISH technique. Pores are created when an electric current is applied to the cell membrane, which raises the possibility of false positives and over-diagnosis. In our earlier reports, we showed that the electric current does not flow through the inside of the microdroplet during AC electric field mixing17,18. This is because the vibrated microdroplet is separated from the electrode by both glass and air, which act as insulators.
In summary, we have shown that RaFISH can be used as a clinical tool for prompt determination of ALK status in lung cancer samples. Using RaFISH with a high-voltage, high-frequency AC electric field, break-apart ALK tests can be completed within 4.5 h, as compared to 20 h needed for conventional FISH.
Methods
Patients and Specimens
All experimental protocols were approved by the institutional review board at Akita University Hospital (Permit number: 1408), and written informed consent for use of samples was obtained from all patients at each hospital. The methods in this study were carried out in accordance with the approved guidelines. Nineteen EML4-ALK-positive samples for IHC and FISH were collected at the Akita University Hospital, Akita Red Cross Hospital, Omagari Kousei Medical Center, and Nakadori General Hospital between April 2008 and December 2016. Sixty-six negative/1+(2+) samples were collected for the study between January 2015 and December 2016 at Akita University Hospital. All patients enrolled in this study had undergone radical surgery for NSCLC and were analyzed retrospectively. None of the patients received preoperative chemotherapy or radiotherapy. The patients’ characteristics are listed in Table 1. Disease stages were determined according to UICC TNM classification24.
Standard Immunohistochemistry
Anti-ALK IHC was performed using the iAEP method (ALK Detection Kit, Nichirei Bioscience, Tokyo, Japan) with an automatic staining protocol13,25. To confirm ALK positivity, IHC (and/or FISH) was also performed and evaluated by a commercial clinical laboratory (SRL, Tokyo). ALK IHC results were classified using iScore for iAEP IHC14,26.
Standard fluorescence in situ hybridization (FISH)
Standard FISH was performed on unstained 4-μm 10% formalin-fixed, paraffin-embedded tumor tissue sections using an ALK break-apart probe set (Vysis ALK Break Apart FISH Probe kit; Abbott Molecular Inc., IL, USA) with a paraffin pretreatment reagent kit (Vysis Paraffin Pretreatment IV & Post-Hybridization Wash Buffer kit; Abbott Molecular Inc., IL, USA). Assays were performed following the manufacturer’s instructions.
New rapid fluorescence in situ hybridization (RaFISH)
We used the prototype RaFISH, which we described in an earlier paper18, after mounting using a temperature control unit (Fig. 2A). The theory behind and technique for AC electric field mixing was described in detail previously15,16,17. Ten µl of Vysis LSI ALK Dual Color Break Apart FISH Probe and 40 µl of sterile distilled water were applied. Thereafter, a polyethylene terephthalate (PET) cover (Fig. 2B) was applied to the slide to prevent probe evaporation during mixing (Fig. 2C). The slide was placed between the electrodes, and a high-voltage (4.5 kV, offset 2.4 kV), high-frequency (90 Hz) AC current was applied after denaturation for 3 min at 73 °C. There was a distance of 3.8–4.0 mm between the slide and electrode plates, and the current was applied for 3 h at 37 °C. After washing the slide, 10 μL of DAPI counterstain were applied. Signals were analyzed using a Olympus BX51 fluorescence microscope (Olympus, Tokyo, Japan). A minimum of 50 nuclei from two separate areas of the tumor were independently scored by two technologists. Table 2 summarizes each procedure and the time required for conventional FISH and RaFISH.
Device used to apply a high voltage, high frequency AC electric field (4.5 kv, 90 Hz). The DNA probes are mixed within microdroplets as the voltage is switched on and off. The resultant coulomb force stirs the probe solution on the sections, which increases the opportunity for contact between the probe and gene. Note that although the microdroplets’ shape is not transformed, the solution is stirred in Supplementary Video 1. (A) The device for Rapid Fluorescence in Situ Hybridization (RaFISH). (B) Polyethylene terephthalate (PET) cover on the slide for inhibiting evaporation of the probe solution. (C) Schema of the stir within a microdroplet as the voltage is switched on and off.
Statistical analysis
Statistical analysis was performed using JMP IN 10.0.2 software (SAS Institute, Cary, NC, USA). Cohen’s kappa-coefficient was used to assess agreement of 4 × 2 contingency tables between protocols. Values of kappa < 0.4 indicated fair-to-poor agreement, 0.4-0.8 indicated moderate-to-good agreement, and > 0.8 indicated excellent agreement.
References
Soda, M. et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 448, 561–566 (2007).
Rikova, K. et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 131, 1190–1203 (2007).
Tsao, A. S. et al. Scientific Advances in Lung Cancer 2015. J Thorac Oncol. 11, 613–638 (2016).
Hiley, C. T. et al. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet. 388, 1002–1011 (2016).
Tsao, M. S., Hirsch, F. R. & Yatabe, Y. eds. IASLC atlas of ALK testing in lung cancer. Aurora, CO: IASLC Press, 2013.
Matikas, A., Kentepozidis, N., Georgoulias, V. & Kotsakis, A. Management of Resistance to Crizotinib in Anaplastic Lymphoma Kinase-Positive Non-Small-cell Lung Cancer. Clin Lung Cancer. 17, 474–482 (2016).
Hallberg, B. & Palmer, R. H. The role of the ALK receptor in cancer biology. Ann Oncol. 27(Suppl 3), iii4–iii15 (2016).
Camidge, D. R. et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 13, 1011–1019 (2012).
Blackhall, F. & Cappuzzo, F. Crizotinib: from discovery to accelerated development to front-line treatment. Ann Oncol. 27(Suppl 3), iii35–iii41 (2016).
Bang Y, et al. Clinical activity of the oral ALK inhibitor PF-02341066 in ALK-positive patients with non-small cell lung cancer (NSCLC). J Clin Oncol. 28, suppl; abstr 3 (2010).
Kwak, E. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 363, 1693–1703 (2010).
Takeuchi, K. et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 15, 3143–3149 (2009).
Jokoji, R. et al. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol. 63, 1066–1070 (2010).
Takeuchi, K. et al. Prospective and clinical validation of ALK immunohistochemistry: results from the phase I/II study of alectinib for ALK-positive lung cancer (AF-001JP study). Ann Oncol. 27, 185–192 (2016).
Toda, H. et al. A novel immunohistochemical staining method allows ultrarapid detection of lymph node micrometastases while conserving antibody. Acta Histochem Cytochem. 44, 133–139 (2011).
Tanino, M. et al. Rapid immunohistochemistry based on alternating current electric field for intraoperative diagnosis of brain tumors. Brain Tumor Pathol. 32, 12–19 (2015).
Hiromitsu, S. et al. Novel method for immunofluorescence staining of mammalian eggs using non-contact alternating-current electric-field mixing of microdroplets. Sci Rep. 5, 15371 (2015).
Saito, Y. et al. Novel method for rapid in-situ hybridization of HER2 using non-contact alternating-current electric-field mixing. Sci Rep. 6, 30034 (2016).
Kim, H. & Chung, J. H. Overview of clinicopathologic features of ALK-rearranged lung adenocarcinoma and current diagnostic testing for ALK rearrangement. Transl Lung Cancer Res. 4, 149–155 (2015).
Soda, M. et al. A prospective PCR-based screening for the EML4-ALK oncogene in non-small cell lung cancer. Clin Cancer Res. 18, 5682–5689 (2012).
Martin, V. et al. ALK testing in lung adenocarcinoma: technical aspects to improve FISH evaluation in daily practice. J Thorac Oncol. 10, 595–602 (2015).
Kerr, K. M. ALK testing in non-small cell lung carcinoma: what now? J Thorac Oncol. 9, 593–595 (2014).
Kerr, K. M. & López-Ríos, F. Precision medicine in NSCLC and pathology: how does ALK fit in the pathway? Ann Oncol. 27(Suppl 3), iii16–iii24 (2016).
Goldstraw, P. et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 11, 39–51 (2016).
Seto, T. et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol. 14, 590–598 (2013).
Takamochi, K., Takeuchi, K., Hayashi, T., Oh, S. & Suzuki, K. A rational diagnostic algorithm for the identification of ALK rearrangement in lung cancer: a comprehensive study of surgically treated Japanese patients. PLoS One. 8, e69794 (2013).
Acknowledgements
The authors are grateful to Prof. Akiteru Goto (Department of Cellular and Organ Pathology, Akita University Graduate School of Medicine) for suggesting pathological diagnoses and Prof. Katsuyuki Murata (Department of Environmental Health Sciences, Akita University Graduate School of Medicine) for suggesting statistics. And we thank Prof. Shinya Tanaka of the Department of Cancer Pathology, Hokkaido University, who provided advice on the conventional FISH.
Author information
Authors and Affiliations
Contributions
S.F. collected and analyzed the data. Y. Saito, H.S., K.T., Y. Sato, and S.M. helped to collect the data. K.I. analyzed data and wrote the paper. H.N. made pathological diagnoses and supported this research. R.N. and Y.A. contributed to the development of the device enabling application of a high-voltage, low-frequency A.C. electric field. Y.M. designed and supervised the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujishima, S., Imai, K., Nakamura, R. et al. Novel method for rapid fluorescence in-situ hybridization of ALK rearrangement using non-contact alternating current electric field mixing. Sci Rep 7, 15116 (2017). https://doi.org/10.1038/s41598-017-15515-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15515-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.