Abstract
This systematic review aims to assess the efficacy of titanium (Ti) implant surfaces with or without strontium (Sr) incorporation on osseointegration in animal experimental studies. An electronic search was conducted using databases of PubMed and EMBASE up to November 2016 to identify studies focusing on osseointegration of strontium-modified titanium implants following PRISMA criteria. The primary outcome was the percentage of bone-to-implant contact (BIC) around the implants with or without strontium-modified surface. Of the 1320 studies, 17 studies fulfilling the inclusion criteria were finally included. A random effect meta-analysis was conducted based on BIC in 17 studies, and the results demonstrated considerable heterogeneity (I² = 79%). A sensitivity analysis found that three studies using the same surface modification method were the major source of the heterogeneity. Therefore, exploratory subgroup analysis was performed. Subgroup one including 14 studies showed a standard mean differences (SMD) of 1.42 (95% CI, 1.13–1.71) with no heterogeneity (I² = 0.0%), while subgroup two including the other three studies showed a SMD of 9.49.95% CI, 7.51–11.47) with low heterogeneity (I² = 0.1%). Sr-modified implants in both subgroups showed significantly higher BIC than unmodified implants (P < 0.01). The results showed a statistically significant effect of Sr-modified titanium implant surfaces on osseointegration and bone apposition in animal models.
Similar content being viewed by others
Introduction
The long-term success of endosseous implant mainly depends on osseointegration, which is defined as a direct contact between living bone and implant in histological sections. Albrektsson et al. defined six factors as pre-requisites for the establishment of osseointegration, implant material, implant design, implant surface, status of bone, surgical technique and the implant loading condition1.
Nowadays, titanium and its alloys have been widely applied for fabricating endosseous implant devices such as artificial knees, hip prosthesis and dental implants owing to its excellent biocompatibility, bio-inertness and adequate mechanical properties2. The evolution of clinical protocols have not only shortened treatment time but also expanded indications for implant therapy with significant progress of titanium surfaces. Physical, chemical, biological and topographical modifications have been proposed to accelerate bone healing and promote bone formation attempting to reach a rapid, long-living implant anchorage3,4,5. Currently, various studies have demonstrated surface modification with inorganic metal elements such as magnesium (Mg), zinc (Zn), strontium (Sr) incorporation could achieve rapid osseointegration and promote new bone formation6,7,8.
Strontium(Sr) aroused great attention clinically since strontium ranelate (SrRan) had been proved to have significant effect on reducing the risk of fracture in osteoporosis patients9,10. Sr, an essential trace element in human body, has been reported to enhance the osseointegration in vitro and in vivo. In vitro studies have found that Sr ion stimulates osteogenic differentiation of mesenchymal stem cells (MSCs) by activating Wnt signaling11,12. Sr exerts an inhibitory effect on osteoclast activity and differentiation through the activation of RANK/RANKL pathway and expression of osteoprotegerin (OPG)13,14. Moreover, the mechanism is believed to depend on the results of angiogenesis and osteogenesis that Sr facilitates osteogenic differentiation of BMSCs as well as promotes the angiogenic growth factors secretion, which can result in blood vessel formation15,16. In vivo studies showed that Sr could promote osseointegration both in Sr-loaded implants and via oral administration of SrRan8,17. In addition, the beneficial effect on osteogenesis was also observed in Sr-enriched applications such as CaSiO3 ceramics18, bioactive glasses19 and bone cement20.
However, due to the high cost of animal studies, most of the sample sizes in previous studies were limited. Moreover, the methods of adding Sr into implant surfaces varied. Meanwhile, limited high-quality evidence for effect of Sr-modified implant on bone apposition was available. Thus, a systematic review is highly in demand for evaluating the effect of Sr-modified titanium implants surface on enhancing osseointegration.
Therefore, the aim of present review was to systematically analyze the scientific literature reporting the efficacy of treating titanium surfaces with Sr on osseointegration of implants in animal experimental models. A parameter frequently used to quantify osseointegaration is bone-implant contact rate (BIC). It is defined as the ratio between the linear measurement of the surface of implant in direct contact with bone and the total length of the implant profile. As a crucial parameter of histomorphometry, BIC was selected as the primary outcome in the present review. In addition, the null hypothesis was no significant difference of osseointegration could be found between Sr-modified implants and unmodified implants.
Results
Study selection
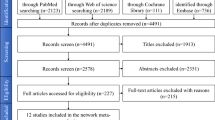
Electronic search showed a total number of 1760 titles, of which 835 titles and abstracts were retrieved for possible inclusion after automatic duplication removed. After manual searching bibliographies of the selected studies, 5 studies were added to full-text evaluation. 23 articles were selected for full text evaluation. Three studies were excluded due to no Sr-only experimental group21,22,23. Another three studies were excluded because BIC was not reported8,24,25. The final 17 studies26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 were included in this systematic review. The search pathway was showed in Fig. 1. An overview on details about experimental details per study was given in Table 1.
Risk of bias and quality assessment of included studies
The results of the risk of bias evaluation of included studies were shown in Fig. 2. For items 9 and 10, 60% of included studies reported the experiment was randomized at some level, while 29% reported blinding at any level during the study.
The ARRIVE criteria of included studies was shown in Table 2. The mean score of all studies was 17.2 (±1.98) out of a maximum of 24. All studies reported adequate information concerning title, abstract, introduction, ethical statement, species, surgical procedure, outcome evaluation, statistical analysis and results. Information regarding experimental animals housing conditions and study limitation were generally inadequate. Animals were randomly allocated to different treatment groups in nine studies (53%). Moreover, five studies reported blinding of assessors to test groups (29%). The 3Rs (in the results section) was not reported in any of studies.
Bone to implant contact
All included studies measured the effect on BIC around implants with or without Sr-enriched surface. A random effect meta-analysis was conducted based on BIC in 17 studies, and the overall results demonstrated considerable heterogeneity (I² = 79%). A sensitivity analysis found that three studies using the same surface modification method were the major source of the heterogeneity. Therefore, exploratory subgroup analysis was performed. The subgroup1 including 14 studies showed a standard mean differences (SMD) of 1.42 (95% CI, 1.13–1.71) with no heterogeneity (I² = 0.0%), while subgroup2 including the other three studies showed a SMD of 9.49 (95% CI, 7.51–11.47) with low heterogeneity (I² = 0.1%) (Fig. 3). The Sr-modified implants in both subgroups showed significantly higher BIC than unmodified implants (P < 0.01). However, high publication bias was found in the present study. (Begg, p = 0.039; Egger, p = 0.000) (Fig. 4.).
Bone area ratio
14 of 17 studies26,27,28,30,31,32,33,34,37,38,39,40,41,42 calculated the percentage of new bone area (BA) of peri-implants. No meta-analysis could be conducted due to the considerable heterogeneity. 10 studies reported that significant higher bone area could be observed around strontium modified implants than those implants without strontium incorporation. However, no significant difference in bone area was reported in other four studies28,30,37,42.
Micro-CT evaluation and biomechanical test
Micro-computed tomography (micro-CT) evaluation was performed in seven of included studies29,31,32,34,35,36. In quantitative assessment, four studies31,32,34,38 showed Sr-enhanced implants demonstrated stronger effect on all micro-CT parameters including bone volume per total volume (BV/TV), 3D bone distribution such as trabecular number (Tb.N), thickness (Tb.Th), and/or spacing (Tb.Sp) and/or the connective density (Conn.D) than control implants, while other three studies found no statistically significant changes in some parameters.
Nine of included studies28,29,31,32,34,35,36,37,38 reported the results of biomechanical test including pull-out test or removal torque testing. All studies revealed a significant increase in implant fixation, with removal torque value, the maximal push-out force and/or the ultimate shear strength markedly raised compared to control groups.
Discussion
The primary finding of this meta-analysis was that Sr-modified implant surfaces significantly increased the percentage of BIC. Therefore, the null hypothesis should be rejected.
To our knowledge, this is the first systematic review to assess the effect of Sr-modified implants on enhancing osseointegration and bone apposition in animal experimental studies. To quantify the potential effect of Sr-containing surfaces on peri-implant bone apposition, a meta-analysis of BIC was performed. Overall, the results of subgroup meta-analysis revealed that titanium implants with strontium incorporation demonstrated significantly better BIC than unmodified implants in small (rats, rabbits) and large (dogs) animals. The result verified the expectation in vitro studies that the Sr-containing titanium surface is expected to shorten bone healing period and enhance implant osseointegration43,44. Similarly, this result was in agreement with studies for appraising the efficiency of Sr-modified magnesium (Mg) based implant45,46. In these studies, Sr-enriched implant showed significantly higher percentage of BIC than that of pure Mg or Mg alloy based implant.
It is worth mentioning that subgroup two reported Sr-modified surfaces had significant effect on BIC and the difference between implants with and without strontium incorporation was more significant than that in subgroup one. The identical electrochemical deposition process was applied to incorporate strontium-substituted hydroxyapatite (Sr-HA) into surface in these three studies indicating the heterogeneity may be caused by method of surface modification. It has been reported that an electrochemical process can produce a homogeneous 2- to approximately 3 μm HA coating and nano-hydroxyapatite (nano-HA) on the metallic substrate surface47. Yang et al.48 reported that significant superiority of osseointegration and bone apposition was found when electrochemical deposition method was used. However, it is difficult to determine the best surface modification methods due to the limited information.
New bone area plays an important role in evaluating the osteoconductive property of biomaterials. The new mineralized bone tissue area inside all the implant threads was measured to evaluate the percentage of bone area. Several studies have demonstrated that Sr-containing biomaterials could increase new bone apposition. Studies for evaluating Sr-incorporated bioactive glass scaffolds and bone cement in impaired bone found that materials were covered by more new bone than unmodified groups49,50. In this review, ten studies using rat animal model reported significantly higher BA in Sr-modified implants than unmodified implants, while the other four studies used rabbit animal model reporting no significant difference in BA. The difference may be attributed to the different animal model, which implies different dynamics of bone formation especially in early healing intervals51. Therefore, additional preclinical and clinical studies should be performed to assess the effect of Sr-modified implant on bone apposition and osseointegration.
The 3-D micro-CT image clearly provides the information of bone–implant interface and trabecular microstructure of peri-implant bone tissue from both qualitative and quantitative perspectives. Seven included studies29,31,32,34,35,36,38 performed CT evaluation and four of them showed significantly improved BV/TV, Tb.N, Tb.Th, Tb.Sp and/or Conn.D. Other three studies found the parameters of CT evaluation were partially improved by Sr-modified titanium surface. Possible reasons that cause the disparity could be the different surface topography and Sr concentration of titanium implant surfaces. Furthermore, biomechanical testing further demonstrated a significant increase in implant fixation. It was apparent to be verified by the improved trabecular bone microarchitecture together with increased BIC and BA around the implant.
All the included studies reported that the improved implant osseointegration and bone apposition was attributed to the released Sr ions and modified surface topography. Actually, the exact mechanism regarding bone remodeling effect of Sr has not been clearly understood. Recently, it has been suggested that the possible mechanism of Sr relies on the calcium-sensing receptor (CaR) which is expressed in several types of pre-osteoblastic cells and bone marrow stromal cells52. Through a CaR-mediated mechanism, Sr is reported to increase bone apposition by promoting pre-osteoblastic cell proliferation and differentiation, reducing osteoclast differentiation, enhancing matrix mineralization52,53. In addition, the surface topography changed by strontium incorporation process also contributed to bone-implant integration. Three of included studies revealed that micro/nanoscale topography enhanced new bone apposition and osseointegration of Sr-modified implants or had a synergistic effect with released Sr ions28,29,35. Hence, in-depth investigations are required to isolate the pure and independent effect of strontium or surface topography on improving bone-implant integration.
The present systematic review had several limitations. Firstly, the follow-up of included studies ranged from 1 week to 12 weeks, so it remained unclear whether the osseointegration of Sr-incorporated Ti implants would be a stable anchorage, which could contribute to their long-term survival. Secondly, high publication bias (Begg, p = 0.039; Egger, p = 0.000) was found in Begg’s and Egger’s test. Therefore, the results need to be interpreted with caution. Thirdly, none of the implants included in the present study were loaded. Thus, future studies should evaluate the effect of Sr-incorporated Ti implants under loading conditions.
Conclusion
Based on available evidence so far, it can be concluded that Sr-modified titanium implants could enhance osseointegration and new bone formation of peri-implant area in animal models. Nonetheless, future clinical investigations are needed to verify the safety and effectiveness of Sr-modified implants.
Methods
PICO
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines54, a specific question was identified based on the Participants, Interventions, Control, Outcomes (PICO) principle. The focused question was, “Does incorporating strontium into titanium implant surfaces influence the osseointegration?”
(P) Participants: Subjects received endosseous implantation.
(I) Interventions: Implants with strontium incorporation.
(C) Control: Implants without strontium incorporation.
(O) Outcome: BIC, BA and results from biomechanical test and micro-CT evaluation.
Search strategy and study selection
Databases of PubMed and EMBASE were searched up to November 2016 for relevant articles published, using search terms “strontium”, in combination with “osseointegration”, “bone apposition”, “osteogenic”, “osteogenesis”, “new bone formation”, “bone to implant contact” and “bone regeneration”. Additionally, bibliographies of the selected studies and relevant review articles were also scrutinized for cross-references. Titles and abstracts of searches were initially screened by two authors (SJY, LY). Uncertainty in the determination of eligibility was resolved by discussion. Two authors reviewed full-text articles independently and final inclusion was based on the inclusion criteria.
Inclusion and exclusion criteria
The inclusion criteria for the study selection were:
-
1.
Studies regarding titanium implants modified with strontium;
-
2.
Studies reporting the percentage of bone-to-implant contact of Sr-modified and unmodified implants;
-
3.
Studies with a minimum of 3 implants/group;
The exclusion criteria for the study selection were:
-
1.
In vitro studies;
-
2.
Studies assessing the combined effect of Sr and other inorganic elements (e.g. Ag, phosphate) modified surface without strontium-only test group.
Risk of bias and quality assessment
The risk of bias (RoB) of included studies was assessed using the SYRCLE RoB tool for animal studies55. The tool, which aims to assess methodological quality, was adapted to appraise bias in animal studies. RoB was evaluated by providing a response of “high”, “low” or “unclear” in each of the 10 items. As reported in a previous review, a modified RoB tool was used in which items 9 and 10 were adjusted to include information on whether the experiment was randomized or blinded at any level (Fig. 2.).
Reporting quality of the included studies was assessed based on a modified ARRIVE guidelines in which a checklist of 24 items was included56. Each item was judged as “0” (not reported) or “1” (reported). The total score of each of included studies was also recorded (Table 2).
Data extraction
Two independent reviewers (SJY, LY) extracted data from the full-texts of selected articles. General information, animal parameters (total number, species), methods of strontium incorporation, evaluation time points, analysis methods and outcomes and implant parameters (total number, material, length, diameter, shape, location and surface characteristics of test and control implants) were retrieved. The primary outcome and secondary outcomes were extracted (Tables 3 and 4). If data were only expressed graphically, numerical values were requested from the authors, and if a response was not received, digital ruler software was used to measure graphical data (ImageJ, National Institutes of Health, Bethesda, MD).
Statistical analysis
The primary and secondary outcomes were present in descriptive statistics. The standardized mean differences, together with 95% confidence intervals, were analyzed using random-effect model. Heterogeneity was tested using the I 2 statistic to describe the proportion of total variation. Values of 25, 50 and 75% were regarded as low, moderate and considerable heterogeneity, respectively57. When the value was >50%, qualitative analysis was conducted. A forest plot was generated, and heterogeneity was calculated by use of the statistical software package STATA (v11.0; StataCorp, College Station, TX). A p value < 0.05 was considered to indicate statistical significance, unless specified otherwise.
Potential publication bias was assessed using Begg’s funnel plots and Egger’s test at the p < 0.10 level of significance.
References
Albrektsson, T., Branemark, P. I., Hansson, H. A. & Lindstrom, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta orthopaedica Scandinavica 52, 155–170 (1981).
Annunziata, M. & Guida, L. The Effect of Titanium Surface Modifications on Dental Implant Osseointegration. Frontiers of oral biology 17, 62–77, https://doi.org/10.1159/000381694 (2015).
Kloss, F. R. et al. The role of oxygen termination of nanocrystalline diamond on immobilisation of BMP-2 and subsequent bone formation. Biomaterials 29, 2433–2442, https://doi.org/10.1016/j.biomaterials.2008.01.036 (2008).
Schwarz, F. et al. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants. Journal of biomedical materials research. Part B, Applied biomaterials 88, 544–557, https://doi.org/10.1002/jbm.b.31233 (2009).
Yazici, H. et al. Biological response on a titanium implant-grade surface functionalized with modular peptides. Acta biomaterialia 9, 5341–5352, https://doi.org/10.1016/j.actbio.2012.11.004 (2013).
Park, J. W., An, C. H., Jeong, S. H. & Suh, J. Y. Osseointegration of commercial microstructured titanium implants incorporating magnesium: a histomorphometric study in rabbit cancellous bone. Clinical oral implants research 23, 294–300, https://doi.org/10.1111/j.1600-0501.2010.02144.x (2012).
Li, X. et al. Effect of zinc ions on improving implant fixation in osteoporotic bone. Connective tissue research 54, 290–296, https://doi.org/10.3109/03008207.2013.813495 (2013).
Liang, Y. et al. Strontium coating by electrochemical deposition improves implant osseointegration in osteopenic models. Experimental and therapeutic medicine 9, 172–176, https://doi.org/10.3892/etm.2014.2038 (2015).
Deeks, E. D. & Dhillon, S. Strontium ranelate: a review of its use in the treatment of postmenopausal osteoporosis. Drugs 70, 733 (2010).
Reginster, J. Y., Deroisy, R. & Jupsin, I. Strontium ranelate: a new paradigm in the treatment of osteoporosis. Drugs of Today 39, 89 (2004).
Yang, F. et al. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem cells (Dayton, Ohio) 29, 981–991, https://doi.org/10.1002/stem.646 (2011).
Saidak, Z. & Marie, P. J. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacology & therapeutics 136, 216–226, https://doi.org/10.1016/j.pharmthera.2012.07.009 (2012).
Peng, S. et al. The cross-talk between osteoclasts and osteoblasts in response to strontium treatment: involvement of osteoprotegerin. Bone 49, 1290–1298, https://doi.org/10.1016/j.bone.2011.08.031 (2011).
Yamaguchi, M. & Weitzmann, M. N. The intact strontium ranelate complex stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-kappaB activation. Molecular and cellular biochemistry 359, 399–407, https://doi.org/10.1007/s11010-011-1034-8 (2012).
Lin, K. et al. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 34, 10028–10042, https://doi.org/10.1016/j.biomaterials.2013.09.056 (2013).
Bose, S., Fielding, G., Tarafder, S. & Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends in biotechnology 31, 594–605, https://doi.org/10.1016/j.tibtech.2013.06.005 (2013).
Li, Y. et al. Strontium ranelate treatment enhances hydroxyapatite-coated titanium screws fixation in osteoporotic rats. Journal of Orthopaedic Research Official Publication of the Orthopaedic Research Society 28, 578–582 (2010).
Wu, C., Ramaswamy, Y., Kwik, D. & Zreiqat, H. The effect of strontium incorporation into CaSiO3 ceramics on their physical and biological properties. Biomaterials 28, 3171–3181, https://doi.org/10.1016/j.biomaterials.2007.04.002 (2007).
Santocildes-Romero, M. E. et al. The osteogenic response of mesenchymal stromal cells to strontium-substituted bioactive glasses. Journal of tissue engineering and regenerative medicine 9, 619–631, https://doi.org/10.1002/term.2003 (2015).
Schumacher, M., Lode, A., Helth, A. & Gelinsky, M. A novel strontium(II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta biomaterialia 9, 9547–9557 (2013).
Cheng, H. et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta biomaterialia 31, 388–400, https://doi.org/10.1016/j.actbio.2015.11.046 (2016).
Park, J. W. Increased bone apposition on a titanium oxide surface incorporating phosphate and strontium. Clinical oral implants research 22, 230–234, https://doi.org/10.1111/j.1600-0501.2010.01974.x (2011).
Newman, S. D. et al. Enhanced osseous implant fixation with strontium-substituted bioactive glass coating. Tissue engineering. Part A 20, 1850–1857, https://doi.org/10.1089/ten.TEA.2013.0304 (2014).
Lovati, A. B. et al. In vivoevaluation of bone deposition in macroporous titanium implants loaded with mesenchymal stem cells and strontium-enriched hydrogel. Journal of Biomedical Materials Research Part B: Applied Biomaterials 103, 448–456, https://doi.org/10.1002/jbm.b.33228 (2015).
Liu, P. et al. Entangled titanium fibre balls combined with nano strontium hydroxyapatite in repairing bone defects. Medical principles and practice: international journal of the Kuwait University, Health Science Centre 23, 264–270, https://doi.org/10.1159/000359951 (2014).
Offermanns, V. et al. Bone regenerating effect of surface-functionalized titanium implants with sustained-release characteristics of strontium in ovariectomized rats. International journal of nanomedicine 11, 2431–2442, https://doi.org/10.2147/ijn.s101673 (2016).
Zhang, W. et al. A strontium-incorporated nanoporous titanium implant surface for rapid osseointegration. Nanoscale 8, 5291–5301, https://doi.org/10.1039/c5nr08580b (2016).
Fan, Y. P. et al. Positive effect of strontium-oxide layer on the osseointegration of moderately rough titanium surface in non-osteoporotic rabbits. Clinical oral implants research 28, 911–919, https://doi.org/10.1111/clr.12897 (2017).
Dang, Y. et al. In vivo osseointegration of Ti implants with a strontium-containing nanotubular coating. International journal of nanomedicine 11, 1003–1011, https://doi.org/10.2147/ijn.s102552 (2016).
Park, J. W., Kwon, T. G. & Suh, J. Y. The relative effect of surface strontium chemistry and super-hydrophilicity on the early osseointegration of moderately rough titanium surface in the rabbit femur. Clinical oral implants research 24, 706–709, https://doi.org/10.1111/j.1600-0501.2012.02444.x (2013).
Li, Y. et al. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials 31, 9006–9014, https://doi.org/10.1016/j.biomaterials.2010.07.112 (2010).
Tao, Z. S. et al. A comparative study of zinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Materials science & engineering. C, Materials for biological applications 62, 226–232, https://doi.org/10.1016/j.msec. 2016.01.034 (2016).
Andersen, O. Z. et al. Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials 34, 5883–5890, https://doi.org/10.1016/j.biomaterials.2013.04.031 (2013).
Tao, Z. S. et al. A comparative study of strontium-substituted hydroxyapatite coating on implant’s osseointegration for osteopenic rats. Medical & biological engineering & computing 54, 1959–1968, https://doi.org/10.1007/s11517-016-1494-9 (2016).
Li, Y. et al. Effects of a micro/nano rough strontium-loaded surface on osseointegration. International journal of nanomedicine 10, 4549–4563, https://doi.org/10.2147/ijn.s84398 (2015).
Yan, J., Sun, J. F., Chu, P. K., Han, Y. & Zhang, Y. M. Bone integration capability of a series of strontium-containing hydroxyapatite coatings formed by micro-arc oxidation. Journal of biomedical materials research. Part A 101, 2465–2480, https://doi.org/10.1002/jbm.a.34548 (2013).
Park, J. W. et al. Osteoblast response and osseointegration of a Ti-6Al-4V alloy implant incorporating strontium. Acta biomaterialia 6, 2843–2851, https://doi.org/10.1016/j.actbio.2010.01.017 (2010).
Zhang, W. et al. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration. Biomaterials 34, 3184–3195, https://doi.org/10.1016/j.biomaterials.2013.01.008 (2013).
Zhang, J., Liu, L., Zhao, S., Wang, H. & Yang, G. Characterization and In Vivo Evaluation of Trace Element-Loaded Implant Surfaces in Ovariectomized Rats. The International journal of oral & maxillofacial implants, doi:https://doi.org/10.11607/jomi.3906 (2015).
Offermanns, V. et al. Enhanced osseointegration of endosseous implants by predictable sustained release properties of strontium. Journal of biomedical materials research. Part B, Applied biomaterials 103, 1099–1106, https://doi.org/10.1002/jbm.b.33279 (2015).
Ballo, A. M. et al. Bone tissue reactions to biomimetic ion-substituted apatite surfaces on titanium implants. Journal of the Royal Society, Interface 9, 1615–1624, https://doi.org/10.1098/rsif.2011.0808 (2012).
Fu, D. L., Jiang, Q. H., He, F. M., Yang, G. L. & Liu, L. Fluorescence microscopic analysis of bone osseointegration of strontium-substituted hydroxyapatite implants. Journal of Zhejiang University. Science. B 13, 364–371, https://doi.org/10.1631/jzus.B1100381 (2012).
Yang, H. W. Osteogenesis of bone marrow mesenchymal stem cells on strontium-substituted nano-hydroxyapatite coated roughened titanium surfaces. Journal of Tongji University 8, 257–264 (2015).
Ni, G. X., Yao, Z. P., Huang, G. T., Liu, W. G. & Lu, W. W. The effect of strontium incorporation in hydroxyapatite on osteoblasts in vitro. Journal of Materials Science: Materials in Medicine 22, 961–967 (2011).
Mushahary, D. et al. Zirconium, calcium, and strontium contents in magnesium based biodegradable alloys modulate the efficiency of implant-induced osseointegration. International journal of nanomedicine 8, 2887–2902, https://doi.org/10.2147/IJN.S47378 (2013).
Tie, D. et al. An in vivo study on the metabolism and osteogenic activity of bioabsorbable Mg-1Sr alloy. Acta biomaterialia 29, 455–467, https://doi.org/10.1016/j.actbio.2015.11.014 (2016).
Ye, W. & Wang, X. X. Morphologies of Hydroxyapatite Crystal Deposited on Titanium Surface with Electrochemical Technique. Key Engineering Materials 330-332, 601–604 (2007).
Yang, G. L., He, F. M., Hu, J. A., Wang, X. X. & Zhao, S. F. Effects of biomimetically and electrochemically deposited nano-hydroxyapatite coatings on osseointegration of porous titanium implants. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 107, 782–789, https://doi.org/10.1016/j.tripleo.2008.12.023 (2009).
Wu, C. C., Kuo, C. L., Fan, F. Y. & Yang, K. C. Strontium-impregnated bioabsorbable composite for osteoporotic fracture fixation. Journal of biomedical materials research. Part A 103, 3355–3363, https://doi.org/10.1002/jbm.a.35471 (2015).
Wei, L. et al. A comparative study of Sr-incorporated mesoporous bioactive glass scaffolds for regeneration of osteopenic bone defects. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 25, 2089–2096, https://doi.org/10.1007/s00198-014-2735-0 (2014).
Pearce, A. I., Richards, R. G., Milz, S., Schneider, E. & Pearce, S. G. Animal models for implant biomaterial research in bone: a review. European cells & materials 13, 1–10 (2007).
Chattopadhyay, N., Quinn, S. J., Kifor, O., Ye, C. & Brown, E. M. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem Pharmacol 74, 438–447, https://doi.org/10.1016/j.bcp.2007.04.020 (2007).
Brown, E. M. Is the calcium receptor a molecular target for the actions of strontium on bone? Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 14(Suppl 3), S25–34, https://doi.org/10.1007/s00198-002-1343-6 (2003).
Vrabel, M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Oncology nursing forum 42, 552–554, https://doi.org/10.1188/15.onf.552-554 (2015).
Hooijmans, C. R. et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14, 43, https://doi.org/10.1186/1471-2288-14-43 (2014).
Stadlinger, B., Pourmand, P., Locher, M. C. & Schulz, M. C. Systematic review of animal models for the study of implant integration, assessing the influence of material, surface and design. Journal of clinical periodontology 39(Suppl 12), 28–36, https://doi.org/10.1111/j.1600-051X.2011.01835.x (2012).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 327, 557-560, doi:10.1136/bmj.327.7414.557 (2003).
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (81500892).
Author information
Authors and Affiliations
Contributions
H.C.L. contributed the concept and study design. J.Y.S. and Y.L. screened the literature, selected studies for exclusion and inclusion, performed data extraction and meta-analysis and drafted the manuscript. Y.X.G. contributed part of figures of this manuscript and S.C.Q. contributed the statistical consultation. In addition, X.M.Z. critically reviewed the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, J., Li, Y., Gu, Y. et al. Effect of titanium implants with strontium incorporation on bone apposition in animal models: A systematic review and meta-analysis. Sci Rep 7, 15563 (2017). https://doi.org/10.1038/s41598-017-15488-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15488-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.