Abstract
A conventional anaerobic baffled reactors (ABRs) treating high strength sweet potato starch wastewater at ambient temperatures resulted in acidification and bad performances. After modification, the acidification was remitted and COD removal efficiencies reached 92.73% at high temperatures and were maintained at 71.19% at low temperatures. Moreover, as much as 1.014 ± 0.056 L CH4/L/d were collected at Stage III. The q-PCR results revealed that the largest methanogen populations emerged at Stage III as well, which was 5.29 × 108 mcrA copies per milliliter sludge. A comparable shift in the archaeal community structure at different stages and acetoclastic methanogens Methanosaeta predominated the archaeal community in every compartment in Stages I (63.73%) and II (48.63%). Finally, the net energy gains analysis at mesophilic, thermophilic, and ambient temperature revealed that modified ABR at ambient temperature was not only economical but also profitable and could generated 3.68 KJ energy per gram COD removed.
Similar content being viewed by others
Introduction
China is the world’s largest sweet potato (Ipomoea batatas Lam) producer with the output exceeding 100 million tons in 2011, accounting for ~90% of global production1. Approximately 1.5 million tons of sweet potato are used to produce sweet potato starch in southern China. Pre-processing of one ton of sweet potato root followed by starch extraction, separation, and drying generates ~12 m3 sweet potato starch wastewater (SPSW). Thus, a huge amount of SPSW is produced from the sweet potato starch industry and causes serious environmental problems in local rural areas. SPSW is highly organic in nature with a chemical oxygen demand (COD) of up to 10000 mg/L and a biochemical oxygen demand (BOD) of up to 6000 mg/L2. Generally, anaerobic digestion is a suitable technology for SPSW treatment3. Of all the anaerobic digestion bioreactors, the anaerobic baffled reactor (ABR) has gained popularity in recent decades and has been successfully used to treat many kinds of high- and medium-strength wastewaters4,5. A conventional ABR comprises several equal compartments, with vertical baffles hanging between them, and wastewater flow alternates upward and downward between the partitions6. The wide application is because of its various advantages, e.g., simple construction, good performance, low sludge yields, energy production, and low cost5,7,8. Another important advantage of the ABR is its ability to separate acidogenesis and methanogenesis longitudinally along the course of the reactor and achieve greater overall COD removal efficiencies than many other anaerobic reactors under the same conditions9,10. Laboratory, pilot, and full-scale studies have shown that the ABR is capable of treating a variety of organic wastewaters of varying strength (1000–10000 mg/l) in the mesophilic temperature range (30–35 °C) with high overall COD removal efficiencies (85–98%)4,7,11.
However, when treating high strength SPSW using ABRs in south China, there are some limitations reported by other researchers. First, ABR’s plug-flow structure caused a much higher load than average in the front compartments and incomplete degradation of large organic matter into volatile fatty acids (VFAs). An increase in VFAs concentration could lead to a simultaneous reduction in pH and exceed the optimal acid-base range (6.6–7.7) for microorganisms, such as methanogens, which could contribute to the decline in COD removal efficiency and methane production12. Second, a non-homogeneous influent flow rate caused by the simple baffled distribution structure and high particulate matter concentrations in SPSW could contribute to increased dead space and therefore, decrease the reactor’s volume utilization13,14. Finally, most studies resorted to above-ambient temperatures to maintain good ABR performance and maximize CH4 yield, overlooking the energy input to the process and hence, loss of net energy gain. A large amount of energy is usually needed to heat wastewater to mesophilic or thermophilic fermentation temperatures, particularly in subtropical and temperate zones. For instance, the wastewater usually heated to 35 °C, which could increase treatment costs by ~0.39 US Dollars per ton compared with 25 °C. The relatively high costs of anaerobic SPSW treatment has limited its application, while operated at low temperatures (10–15 °C), a fall in overall COD removal efficiency is usually observed in ABR performance, even when fed low strength wastewater (4000 mg/LCOD)11.
SPSW (containing saccharides, proteins, and fats, etc.) degradation in an ABR is a synergistic and complex bioprocess that involves different anaerobic microbial groups. These functional microorganisms separate spatially along the course of the reactor and degrade various organic compounds in a concerted effort into CH4, CO2, and H2O through hydrolysis, acetogenesis, and methanogenesis15. Generally, hydrolytic and acidogenic bacteria and methanogens are the two major groups involved in anaerobic digestion, with methanogenesis being a rate-limiting step and requiring effective control to achieve good treatment performance16. Therefore, it is important to elucidate the composition and function of the methanogen communities and their shifts in response to environmental changes in ABRs, which can be used to optimize ABR operation. However, few studies have focused on the methanogen community, its shifts in response to environmental changes, and its relationship with ABR performance when run at ambient temperatures. Among all the culture-independent molecular techniques, real-time PCR and pyrosequencing are the most powerful methods that can provide significant insights into the composition and evolution of microbial community structure in the ABR system.
In this study, a conventional ABR was constructed to treat SPSW and its performance was investigated in the first period. Limitations mentioned above were observed during the conventional ABR operation. To solve these problems, the conventional ABR was then reconstructed in the second period. First, we rearranged the reaction compartments in a decline to save the problem of acidulation caused by a high hydraulic loading rate in the front ABR compartments. In doing this, the volume load in the front part of the ABR decreased properly. Furthermore, to avoid a channeling effect, we changed the baffle structure by perforating 1–2 cm diameter holes on the slanted edge. We then hung plastic membranes in each reaction compartment to increase the attachable area for bacterial and therefore, enhanced the reaction rate. After these improvements, the performance of the modified ABR treating high strength SPSW was investigated at ambient temperatures. Meanwhile we evaluated the benefit of a modified ABR operated at ambient temperatures in a subtropical zone compared with those operated at mesophilic or thermophilic fermentation temperatures, in the context of net energy gain. Additionally, quantitative methanogen community shifts and changes in archaeal community structure in relation to operation conditions at ambient temperatures was monitored by real-time PCR and high-throughput 16 S rRNA gene sequencing, respectively.
Results and Discussion
ABR Performance at ambient temperature
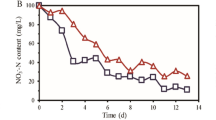
Performance of the conventional and modified ABRs during the entire study period are shown in Tables 1 and 2. The pH increased gradually as the SPSW advanced from compartment 1 to compartment 5 during all five stages in both ABRs. In the conventional ABR, average pH values (5.34 ± 0.75 and 5.69 ± 0.75, respectively) in the first and second compartments were significantly lower than that in the last three compartments (6.32 ± 0.38, 6.64 ± 0.33, and 6.81 ± 0.31 on average) at the p = 0.01 level (Table 1). Additionally, pH in all five compartments dropped continuously from Stages II to IV. This tendency was particularly obvious in the first two compartments, in which the pH decreased from 5.80 ± 0.41 and 6.03 ± 0.40, respectively, in Stage I to 4.62 ± 0.14 and 4.90 ± 0.1, respectively, in Stage IV. Similarly, the total COD removal efficiency fell by more than 20% in Stage IV compared with Stage II. The decline in COD removal efficiency might have been caused by decrease of pH in the first two compartments in the conventional ABR and therefore influence the utility of organic matters in SPSW. pH optima for hydrolytic and acidogenic bacteria is between 5.5 and 6.517,18,19 and methanogenic microorganisms is around pH 720,21,22. Afterwards, excessive acidification led to the collapse of the conventional ABR.
Similar transformation rules of pH were observed in the improved ABR, in which the pH was significantly lower in the first two compartments compared with the last three (Table 2). However, after reconstruction, the COD removal efficiency did not decrease so rapidly in the first two compartments. The overall COD removal efficiencies in Stages III and IV were remained at 78.30 ± 6.01% and 71.19 ± 6.65%, respectively, which were much higher than the results reported by Alette A.M. et al.23. In their research, COD removal efficiencies dropped from 80% to 70% and then to 60% when the operation temperature decreased from 35 °C to 20 °C and then to 10 °C, however, what should be mentioned was that the HRT was 10 h. Moreover, the improved ABR did not collapse because of excessive acidulation at any point in the experiment. And the COD removal performance of the improved ABR was more stable than the conventional ABR with the standard deviation being 11 versus 15. Furthermore, COD removal in the improved ABR was less affected by temperature than in the conventional ABR. The correlation coefficient was 0.840, p < 0.01 for the conventional ABR and 0.419, p < 0.01 for the improved ABR.
Because of the poor construction design and treatment performance, very little biogas was collected and detected in the conventional ABR (data not shown). However, quite a large quantity of biogas was collected from the improved ABR. As shown in Table 2, the methane production rate (MPR) first increased, then decreased over the course of the experiment and reached a maximum in Stage III. The max MPR was 1.014 ± 0.056 L CH4/L/d, much larger than the results reported by Yu, H. et al.24 and R. Grover et al.25, which were 0.30 and 0.35 L CH4/L/d, when treating municipal wastewater and pulp and paper mill black liquors, respectively. Moreover, this observations were not in accordance with previous reports that longer HRT and higher temperature could promote methane production26,27. The drop of temperature and increase of HRT did not cause decline of COD removal of improved ABR. This might be because of enhanced reaction rate by adding carriers in compartments of improved ABR. The highest MPR compartment moved forward from compartment 3 in Stages I and II to compartment 2 in Stages III and IV, which indicated that the microorganism community structure was tending towards stability from Stages I to IV.
Net energy gains of the modified ABR under different operating temperatures
Net energy gains (NEG) was adopted to assess the benefits of the modified ABR operated at ambient temperature compared with those at mesophilic or thermophilic temperatures. Energy costs and gains of anaerobic digestion under different operating temperatures are summarized in Fig. 1. When the temperature was maintained at 35 °C, most investigated temperature in anaerobic digestion researches, the NEG was usually negative. For instance, the NEG was −1.34 KJ/g COD based on data reported by Ban et al.28. If the reaction temperature was raised to 50 °C, the NEG decreased to −1.94 KJ/g COD according to Hahn and Figueroa’s report29. If the temperature was maintained at 24 °C, NEG decreased dramatically to −0.72 KJ/g COD, a 46.3% reduction compared with that at 35 °C30. However, if the modified ABR was run at ambient temperature (2.7–30.8 °C), the NEG were 0.86, 3.67, 3.68 and 2.30 KJ/g COD, respectively, from Stage IV to Stage I. Hence, operating the modified ABR at ambient temperature was not only economical but also profitable.
Changes in total methanogenic archaea population
The methanogen populations of modified ABR were determined by quantifying the gene encoding the alpha subunit of methyl-coenzyme M reductase (mcrA). As shown in Fig. 2, the average number of copies of the mcrA gene in the bioreactor increased from Stages I to III and decreased in Stage IV, which were 1.2 × 108 ± 2.1 × 107, 2.12 × 108 ± 1.3 × 107, 5.29 × 108 ± 1.3 × 107, and 1.60 × 108 ± 1.4 × 108 copies per milliliter sludge, respectively. In Stage I, the methanogenic population in the first two compartments was smaller than that in the seed sludge. The mcrA gene were 1.99 × 107 ± 1.2 × 106 and 2.03 × 107 ± 1.8 × 106 copies/mL in compartment 1 and compartment 2, versus 8.25 × 107 ± 8.4 × 107 copies/mL in seed sludge. The decrease in the population might have been the result of disadvantageous pH conditions for methanogens, pH optima for which was reported to be around 7. In the last three compartments, the mcrA gene copy numbers increased by 36, 46, and 45%, respectively. Although the mcrA gene copy numbers were different in each compartment, the methane production rates were similar. This phenomenon suggested that the surviving microorganisms in the set-up period could not function efficiently. The methanogen numbers kept increasing in Stage II, with the third compartment being the largest, which was consistent with the methane production rate. In Stage III, the mcrA gene copies did not decrease as a result of the temperature drop. In contrast, methanogen abundance increased 2.8-, 4.6-, 0.9-, 1.5-, and 0.1-fold in each compartment compared with Stage II and reached their peak during the experimental period. In Stage IV, the mcrA gene copy numbers were still greater than that in the seed sludge, which meant that the refitting of the conventional ABR provided suitable conditions for methanogen growth, even at low temperatures.
Analysis of the high-throughput sequencing profiles of the biomass samples taken from the improved ABR on days 1 (seed sludge), 43 (Stage I), 139 (Stage II), 178 (Stage III), and 275 (Stage IV) revealed a distinct shift in the archaeal community structure. The changes in the archaeal community in different treatments at the genus level is showed in Fig. 3. In the seed sludge, the archaeal genus Methanobacterium, hydrogenotrophic methanogens that typically dominate in livestock manure fermentation, was initially predominant31,32. After the improved ABR had been inoculated, their abundance decreased significantly from 46.68% to 4.94–15.40% on average on the following stages with the lowest abundance recorded in Stage III. The genus was then replaced as the major archaeal group by Methanosaeta in every compartment in Stages I and II, with an average abundance of 63.73 and 48.63%, respectively. Methanosaeta are acetoclastic methanogens and are the most dominant archaea genera in the anaerobic digestion of food waste27,33. On days 43 and 139 the predominant archaeal species in the five compartments along the ABR did not change, which suggested that a partial spatial separation of archaea along the ABR had not taken place. In Stages III and IV, the number of Methanosaeta dropped significantly in the front compartments. Take compartments 1 and 2 for instance, Methanosaeta abundance decreased by 84.59 and 91.53%, respectively, from Stages II to IV. Meanwhile, the genus Methanobrevibacter became the dominant methanogen in the first four compartments in Stage III and the first three compartments in Stage IV. A proliferation of Methanobrevibacter and a parallel decrease in Methanosaeta in Stage II to Stage IV might have resulted from a decline in pH in the front compartments. Acidic conditions have a negative effect on Methanosaeta growth; thus, Methanobacteriales was more tolerant to acidulation compared to Methanosaeta 34. An increase in pH occurred along the reactor in Stages III and IV, which was caused by a significant increase in the Methanosaeta populations, 83.04 and 83.92%, respectively. On day 178, the dominant archaea in each compartment started to differentiate, with Methanobrevibacter being the most abundant methanogen in the first four compartments and Methanosaeta in the fifth compartment. The situation was similar on day 275, when Methanobrevibacter was the dominant archaea in the first three compartments and Methanosaeta in the last two compartments. Performance improved in Stage III and no partial spatial separation of archaea took place with the changes in predominant methanogens in the initial compartments and final compartments. The partial spatial separation of archaea in the improved ABR, with hydrogenotrophic methanogens (Methanobrevibacter) in the front compartments and acetoclastic methanogens (Methanosaeta) in the final compartments, is beneficial to COD degradation and methane production.
Conclusion
The performance and archaeal community shifts in a modified anaerobic baffled reactor treating sweet potato starch wastewater at ambient temperatures were investigated in this study. The following conclusions can be drawn based on our results:
-
After modification, the acidification was remitted in the front compartments and the pH was maintained at 5.61 ± 0.17 in the first compartments compared with 4.62 ± 0.14 in the conventional ABR.
-
COD removal efficiencies in the improved ABR reach 92.73% at high temperatures (~30 °C) and remained at 71.19% at low temperatures (~10 °C), increasing by 12.37 and 17.67% compared with the conventional ABR.
-
As much as 1.014 ± 0.056 L CH4/L/d was collected from the improved ABR in Stage III, however, methane could not be collected from the conventional ABR.
-
The modified ABR provided suitable conditions for methanogen growth at both low and high temperatures. The largest methanogen populations emerged at Stage III, which was 5.29 × 108 ± 1.3 × 107 mcrA copies per milliliter sludge on average. Methanogen quantities remained at high levels when temperature decreased.
-
A distinct shift in the archaeal community structure took place during the experimental period in the modified ABR. Acetoclastic methanogens, i.e., Methanosaeta, predominated the archaeal community in Stages I and II and the genera Methanosaeta and Methanobrevibacter predominates in different compartments in Stages III and IV.
-
Partial spatial separation of archaea along the ABR occurred in Stages III and IV, with the genus Methanobrevibacter becoming the dominant methanogen in the front compartments and Methanosaeta dominating subsequent compartments.
-
Net energy gains (NEG) revealed that operation of the modified ABR at ambient temperature was not only economical but also profitable and could generated 0.86,3.67,3.68 and 2.30 KJ energy per gram COD removed, respectively, from Stage I to Stage IV.
Methods
Conventional ABR structure
During the first period of the investigation, from June 2011 to December 2011, a pilot-scale conventional structure ABR was configured to treat SPWS at ambient temperatures, as shown in Fig. 4. The bioreactor with a working volume of 480 L (L × W × H = 150 cm × 40 cm × 80 cm) was divided into five equal compartments by baffles. At the top of each compartment, a biogas outlet was installed to collect and measure the biogas generated inside. The characteristics of the conventional ABR used in this experiment are listed in Table 3.
Improved ABR structure
During the second period of the experiment, the conventional ABR structure was improved to solve the abovementioned problems, as shown in Fig. 5. The improved laboratory scale ABR unit was made of transparent Plexiglas (100 cm long, 15 cm wide and 60 cm high) with an available capacity of 60 L. The bioreactor was then divided into five reaction compartments by vertical slanted edge baffles. Each reaction compartment was further divided into two parts, the down- and up-comer regions, which encouraged mixing within each compartment. To avoid acidulation caused by a much higher load than average in the first two compartments, we arranged the five reaction compartments in a decline, with the volume ratio of 12:10.8:9.7:8.7:7.9. In doing this, the volume load of the front part of the ABR decreased properly. Furthermore, to avoid a channeling effect and decrease dead space, we perforated nine circular holes on slanted edge of baffles in each reaction compartment. The diameter of those holes was 2, 1.5, and 1 cm, respectively, with the largest holes on the top and the smallest on the bottom. Moreover, we also added porous double circle plastic ring carriers (see Fig. 5) in each reaction compartment to increase the attachable area for bacterial and therefore, enhanced the reaction rate. The main characteristics of the carriers were: diameter, 80 mm; pitch, 30 mm; surface area, 800 m2/m3; and porosity 90%. The characteristics of the improved ABR used in this experiment are listed in Table 4.
Experimental plans and operating conditions
The experiment was carried out in Changsha, Hunan Province from June 2011 to April 2013 at ambient temperature ranging from 5 to 30 °C in a subtropical hilly region. The whole experiment was divided into two periods according to the ABR used: the operation of conventional ABR (Period I, 2011.7–2011.12) and the operation of improved ABR (Period II, 2012.04–2013.03). The experimental plans and operating conditions for both periods are described in the following sections.
Conventional ABR operation
Characterization of the sweet potato wastewater and digested sludge: The sweet potato wastewater used in this research was obtained from a sweet potato wastewater treatment plant adjusting tank in a company extracting starch from sweet potato for glass noodle production in Changsha, China. The characteristics of the wastewater were as follows: COD (mg/L) = 8000–12000; BOD (mg/L) = 6000–9000; TN (mg/L) = 400–600; TP (mg/L) = 70–120; pH = 4.5–5.5. The SPSW was diluted and pH was adjusted to 11.5 with NaOH to neutralize acid produced during reactor operation. The ABR was inoculated with the anaerobic stabilized sludge from a pig farm digester at Baisha Town in Changsha, Hunan, China. The amount of sludge used for the ABR inoculation was 288 L (60% of the working volume). The characteristics of the inoculated sludge were: 21.3 ± 0.4 g/L total suspended solid (TSS), 15.2 ± 0.7 g/L volatile suspended solid (VSS), and pH = 7.8.
Experimental design and conventional ABR operating conditions: The conventional ABR operation was divided into four phases with respect to the influent COD level, operating temperature, and COD removal performance: Phase I (Set-up phase, days 1–24); Phase II (Operation phase at high temperature, days 25–58); Phase III (Operation phase at moderate temperature, days 59–137); Phase IV (Operation phase at low temperature, days 138–172). More conventional ABR operating information is listed in Table 5. The reactor was continuously fed with sweet potato wastewater at room temperature from the feed tank.
Improved ABR operation
The origin and characteristics of SPSW and seed sludge used in the improved ABR were the same as those used in the conventional ABR. The SPSW was diluted and pH was adjusted to 7.5 with NaOH before used. Because the reactor was improved and in order to save NaOH dosage, the pH value of SPSW used in improved ABR was not adjusted to 11.5, the same value as conventional ABR. We inoculated 36 L (60% of the working volume) seed sludge into the improved ABR. Similar to the conventional ABR operation, the improved ABR was divided into four phases: Phase I (Set-up phase, days 1–76); Phase II (Operation phase at high temperature, days 77–139); Phase III (Operation phase at moderate temperature, days 140–205); Phase IV (Operation phase at low temperature, day 206-303). More improved ABR operating information is listed in Table 6. The reactor was continuously fed with sweet potato wastewater at room temperature from the feed tank.
Analytical methods
To investigate each chamber’s performance in both conventional and improved ABRs, wastewater samples were taken with three duplicates at the influent and the upper part of each internal chamber, and COD was measured. Sludge was taken from sample ports with three duplicates at the bottom of every compartment for total DNA extraction, qPCR and archaea community analysis. The amount of biogas generated in every compartment was measured by wet gas flow meter and the biogas CH4 concentrations were measured by gas chromatography.
DNA extraction, quantification, and 16S rRNA gene sequences
Total genomic DNA used for qPCR and pyrosequencing was extracted from sludge on days 1 (seed sludge), 43 (Stage I), 139 (Stage II), 178 (Stage III), and 275 (Stage IV) with triplicates using the FastDNA™ SPIN Kit for Soil (MP Biomedical, OH, USA) and stored at −20 °C. To investigate the relationship among methanogenic archaea quantity, biogas yield and operation condition, methanogen mcrA genes were quantified. Real-time PCR was performed using forward, 5ʹ-GGTGGTGTMGGATTCACACARTAYGCWACAGC-3ʹ and reverse 5ʹ-TTCATTGCRTAGTTWGGRTAGTT-3ʹ primers35 on a CFX96TM Real-Time PCR system (Bio-Rad Laboratories, USA) with SYBR® Premix Ex TaqTM II (Takara, Japan). Each reaction was performed in a total reaction mixture of 25 μl containing 9.5 μl nuclease-free water, 12.5 μl SYBR Premix, 0.5 μl each primer and 2 μl of DNA. The PCR amplification and detection program contained an initial denaturation at 95 °C for 2 min, followed by 40 cycles of denaturing at 95 °C for 15 s, annealing at 55 °C for 45 s, and an extension at 72 °C for 30 s. Tenfold serial dilutions of a known copy number of the plasmid DNA were subjected to real-time PCR in triplicate to generate an external standard curve. The results with efficiency and correlation coefficients above 95% and 0.98, respectively, were employed.
PCR amplification of the archaeal V3-V4 region of 16 S rRNA gene was performed with the Archaea-specific forward primer 344 F (3ʹ-ACGGGGYGCAGCAGGCGCGA-5ʹ) and the universal reverse primer Univ806R (5ʹ-GGACTACHVGGGTWTCTAAT-3ʹ)36. The PCR reaction mixture (50 μl) contained 30 ng template DNA, 2 μl forward and reverse primers, 4 μl dNTPs, 5 μl 10 × Pyrobest Buffer and 0.3 μl Pyrobest DNA polymerase (Takara, Kyoto, Japan). Amplification was performed under the following conditions: 95 °C for 5 min; 30 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 40 s; and then a final extension at 72 °C for 10 min. The 16 S rRNA gene was sequenced using the MiSeq System (Illumina Inc., CA, USA).
Statistical analysis
Phylogenetic analysis
Raw reads obtained through high-throughput sequencing process were further treated for the purpose of an ecological analysis of archaeal populations. Data quality control and analyses were mostly performed using the QIIME (v1.8.0)37. Single-end reads were quality filtered with Trimmomatic tool using the following options: TRAILING:20, MINLEN:200 and CROP:200, to remove trailing sequences below a phred quality score of 2038. Overlapping pair-end reads were connected with COPE (V1.2.3.3), followed by detection of Chimeric sequences by USEARCH39,40. Operational taxonomic units (OTUs) were picked de novo from quality-filtered reads using a 97% similarity cut off and assigned to a taxonomic lineage using QIIME (v1.8.0).
Improved ABR net energy gain analysis
To assess the benefit of the improved ABR operated at ambient temperatures, the net energy gain (NEG) was adopted and calculated based on data from this study and fermentative biogas production researches at mesophilic or thermophilic temperatures41. The approach for assessing net energy gain introduced by Perera et al. is extended here to replace energy produced by hydrogen to methane generated in reactors. Thus, the theoretical net energy gain, EN [kJ/g COD in feedstock] in this study is defined as the total energy produced equivalent to methane volume generated in reactors, \({E}_{C{H}_{4}}\) [kJ], minus any heat energy input, \({E}_{IN}\) [kJ] is energy needed to raise the reactor contents from ambient temperature [Ta] to the set fermentation temperature [Ts]. The following equations form the basis of our analysis in batch reactors:
where, \({V}_{C{H}_{4}}\)is the volume of methane generated [L]; \({\rho }_{C{H}_{4}}\)is the density of gaseous methane [7.2 × 10−4 kg/L]; \(LH{V}_{C{H}_{4}}\) are the lower heating value of methane [50,000 kJ/kg]; V is the liquid volume in the reactor [L]; C is the COD concentration of feedstock [g COD/L]; \({\rho }_{w}\) is the density of water [1 kg/L]; \({c}_{p}\) is the specific heat of water [4.2 kJ/kg-°C]; TS and Ta are ambient temperature and set fermentation temperature [°C]; HRT is hydraulic retention time [h].
References
Abegunde, O. K., Mu, T.-H., Chen, J.-W. & Deng, F.-M. Physicochemical characterization of sweet potato starches popularly used in Chinese starch industry. Food Hydrocolloids 33, 169–177 (2013).
Xu, S., Bai, Z., Jin, B., Xiao, R. & Zhuang, G. Bioconversion of wastewater from sweet potato starch production to Paenibacillus polymyxa biofertilizer for tea plants. Scientific Reports 4, https://doi.org/10.1038/srep04131 (2014).
Rajbhandari, B. & Annachhatre, A. Anaerobic ponds treatment of starch wastewater: case study in Thailand. Bioresource technology 95, 135–143 (2004).
Ahamed, A. et al. Multi-phased anaerobic baffled reactor treating food waste. Bioresour Technol 182, 239–244, https://doi.org/10.1016/j.biortech.2015.01.117 (2015).
Barber, W. P. & Stuckey, D. C. The use of the anaerobic baffled reactor (ABR) for wastewater treatment: a review. Water Research 33, 1559–1578 (1999).
Reynaud, N. & Buckley, C. A. The anaerobic baffled reactor (ABR) treating communal wastewater under mesophilic conditions: a review. Water Sci Technol 73, 463–478, https://doi.org/10.2166/wst.2015.539 (2016).
Wang, J., Huang, Y. & Zhao, X. Performance and characteristics of an anaerobic baffled reactor. Bioresour Technol 93, 205–208, https://doi.org/10.1016/j.biortech.2003.06.004 (2004).
Bachmann, A., Beard, V. L. & McCarty, P. L. Performance characteristics of the anaerobic baffled reactor. Water Research 19, 99–106 (1985).
Uyanik, S., Sallis, P. & Anderson, G. The effect of polymer addition on granulation in an anaerobic baffled reactor (ABR). Part II:: compartmentalization of bacterial populations. Water Research 36, 944–955 (2002).
Uyanik, S., Sallis, P. & Anderson, G. The effect of polymer addition on granulation in an anaerobic baffled reactor (ABR). Part I: process performance. Water Research 36, 933–943 (2002).
Nachaiyasit, S. & Stuckey, D. C. Effect of low temperatures on the performance of an anaerobic baffled reactor (ABR). Journal of Chemical Technology and Biotechnology 69, 276–284 (1997).
Zhu, G. et al. Recent Developments and Future Perspectives of Anaerobic Baffled Bioreactor for Wastewater Treatment and Energy Recovery. Critical Reviews in Environmental Science and Technology 45, 1243–1276, https://doi.org/10.1080/10643389.2014.924182 (2015).
Li, S.-N., Nan, J., Li, H.-Y. & Yao, M. Comparative analyses of hydraulic characteristics between the different structures of two anaerobic baffled reactors (ABRs). Ecological Engineering 82, 138–144 (2015).
Sarathai, Y., Koottatep, T. & Morel, A. Hydraulic characteristics of an anaerobic baffled reactor as onsite wastewater treatment system. Journal of Environmental Sciences 22, 1319–1326 (2010).
Zamanzadeh, M., Hagen, L. H., Svensson, K., Linjordet, R. & Horn, S. J. Anaerobic digestion of food waste - Effect of recirculation and temperature on performance and microbiology. Water Res 96, 246–254, https://doi.org/10.1016/j.watres.2016.03.058 (2016).
De Vrieze, J. & Verstraete, W. Perspectives for microbial community composition in anaerobic digestion: From abundance and activity to connectivity. Environ Microbiol, https://doi.org/10.1111/1462-2920.13437 (2016).
Ariesyady, H. D., Ito, T. & Okabe, S. Functional bacterial and archaeal community structures of major trophic groups in a full-scale anaerobic sludge digester. Water Research 41, 1554–1568, https://doi.org/10.1016/j.watres.2006.12.036 (2007).
Chouari, R. et al. Novel predominant archaeal and bacterial groups revealed by molecular analysis of an anaerobic sludge digester. Environmental Microbiology 7, 1104–1115, https://doi.org/10.1111/j.1462-2920.2005.00795.x (2005).
Lee, S. et al. Distribution and abundance of Spirochaetes in full-scale anaerobic digesters. Bioresource Technology 145, 25–32, https://doi.org/10.1016/j.biortech.2013.02.070 (2013).
De La Rubia, M. A., Raposo, F., Rincón, B. & Borja, R. Evaluation of the hydrolytic–acidogenic step of a two-stage mesophilic anaerobic digestion process of sunflower oil cake. Bioresource Technology 100, 4133–4138, https://doi.org/10.1016/j.biortech.2009.04.001 (2009).
Lee, C., Kim, J., Hwang, K., O’Flaherty, V. & Hwang, S. Quantitative analysis of methanogenic community dynamics in three anaerobic batch digesters treating different wastewaters. Water Research 43, 157–165, https://doi.org/10.1016/j.watres.2008.09.032 (2009).
Zahedi, S., Solera, R., Micolucci, F., Cavinato, C. & Bolzonella, D. Changes in microbial community during hydrogen and methane production in two-stage thermophilic anaerobic co-digestion process from biowaste. Waste Manag 49, 40–46, https://doi.org/10.1016/j.wasman.2016.01.016 (2016).
Langenhoff, A. A. M. & Stuckey, D. C. Treatment of dilute wastewater using an anaerobic baffled reactor: effect of low temperature. Water Research 34, 3867–3875, https://doi.org/10.1016/S0043-1354(00)00136-6 (2000).
Yu, H. & Anderson, G. K. Performance of a combined anaerobic reactor for municipal wastewater treatment at ambient temperature. Resources, Conservation and Recycling 17, 259–271, https://doi.org/10.1016/0921-3449(96)01101-9 (1996).
Grover, R., Marwaha, S. S. & Kennedy, J. F. Studies on the use of an anaerobic baffled reactor for the continuous anaerobic digestion of pulp and paper mill black liquors. Process Biochemistry 34, 653–657, https://doi.org/10.1016/s0032-9592(98)00138-1 (1999).
Farghaly, A. & Tawfik, A. Simultaneous Hydrogen and Methane Production Through Multi-Phase Anaerobic Digestion of Paperboard Mill Wastewater Under Different Operating Conditions. Applied Biochemistry and Biotechnology 181, 142–156, https://doi.org/10.1007/s12010-016-2204-7 (2017).
Jang, H. M. et al. Effect of increased load of high-strength food wastewater in thermophilic and mesophilic anaerobic co-digestion of waste activated sludge on bacterial community structure. Water Res 99, 140–148, https://doi.org/10.1016/j.watres.2016.04.051 (2016).
Ban, Q., Li, J., Zhang, L., Jha, A. K. & Nies, L. Linking performance with microbial community characteristics in an anaerobic baffled reactor. Appl Biochem Biotechnol 169, 1822–1836, https://doi.org/10.1007/s12010-013-0105-6 (2013).
Hahn, M. J. & Figueroa, L. A. Pilot scale application of anaerobic baffled reactor for biologically enhanced primary treatment of raw municipal wastewater. Water Res 87, 494–502, https://doi.org/10.1016/j.watres.2015.09.027 (2015).
Galib, M., Elbeshbishy, E., Reid, R., Hussain, A. & Lee, H. S. Energy-positive food wastewater treatment using an anaerobic membrane bioreactor (AnMBR). J Environ Manage 182, 477–485, https://doi.org/10.1016/j.jenvman.2016.07.098 (2016).
Moset, V., Poulsen, M., Wahid, R., Hojberg, O. & Moller, H. B. Mesophilic versus thermophilic anaerobic digestion of cattle manure: methane productivity and microbial ecology. Microb Biotechnol 8, 787–800, https://doi.org/10.1111/1751-7915.12271 (2015).
Ahring, B. K. Methanogenesis in thermophilic biogas reactors. Antonie van Leeuwenhoek 67, 91–102, https://doi.org/10.1007/bf00872197 (1995).
Zhang, J. et al. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour Technol 200, 253–261, https://doi.org/10.1016/j.biortech.2015.10.037 (2016).
Shimada, T. et al. Syntrophic acetate oxidation in two-phase (acid–methane) anaerobic digesters. Water Science and Technology 64, 1812–1820, https://doi.org/10.2166/wst.2011.748 (2011).
Luton, P. E., Wayne, J. M., Sharp, R. J. & Riley, P. W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology-Sgm 148, 3521–3530 (2002).
Takahashi, S., Tomita, J., Nishioka, K., Hisada, T. & Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS One 9, e105592, https://doi.org/10.1371/journal.pone.0105592 (2014).
Caporaso, J. et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods 7, 335–336 (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics, btu170 (2014).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Liu, B. et al. COPE: an accurate k-mer-based pair-end reads connection tool to facilitate genome assembly. Bioinformatics 28, 2870–2874 (2012).
Perera, K. R. J., Ketheesan, B., Gadhamshetty, V. & Nirmalakhandan, N. Fermentative biohydrogen production: Evaluation of net energy gain. International Journal of Hydrogen Energy 35, 12224–12233, https://doi.org/10.1016/j.ijhydene.2010.08.037 (2010).
Acknowledgements
This work was supported by the National Science Fund Projects (Nos 41371266 and 31670507), the Innovation in Cross-functional Team Program of the Chinese Academy of Sciences (No. 2015) and the National Science and Technology Major Project of China (Nos 2015ZX07206-006 and 2014ZX07204-005).
Author information
Authors and Affiliations
Contributions
S.X. and C.J. conceived of and performed the study, and wrote the paper. S.M. performed parts of the research. X.Z., G.Z., Z.B., and S.W. contributed to the discussion and analyzed the experiments. All the authors reviewed and/or modified the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, S., Jiang, C., Ma, S. et al. The performance and archaeal community shifts in a modified anaerobic baffled reactor treating sweet potato starch wastewater at ambient temperatures. Sci Rep 7, 14734 (2017). https://doi.org/10.1038/s41598-017-15421-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15421-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.