Abstract
American Tegumentary Leishmaniasis is a chronic infection caused by Leishmania protozoan. It is not known whether genetic variances in NOD-like receptor (NLR) family members influence the immune response towards Leishmania parasites and modulate intracellular killing. Using functional genomics, we investigated whether genetic variants in NOD1 or NOD2 influence the production of cytokines by human PBMCs exposed to Leishmania. In addition, we examined whether recognition of Leishmania by NOD2 contributes to intracellular killing. Polymorphisms in the NOD2 gene decreased monocyte- and lymphocyte-derived cytokine production after stimulation with L. amazonensis or L. braziliensis compared to individuals with a functional NOD2 receptor. The phagolysosome formation is important for Leishmania-induced cytokine production and upregulation of NOD2 mRNA expression. NOD2 is crucial to control intracellular infection caused by Leishmania spp. NOD2 receptor is important for Leishmania recognition, the control of intracellular killing, and the induction of innate and adaptive immune responses.
Similar content being viewed by others
Introduction
American Tegumentary Leishmaniasis (ATL) is a vector-borne parasitic disease caused by Leishmania protozoan that is characterized by lesions of the skin and oral or nasopharyngeal mucosa. Among the species of Leishmania and Viannia subgenus, L. (L.) amazonensis and L. (V.) braziliensis cause ATL leading to different clinical forms, which are dependent on the parasite species and the immune system of the host1. Both L. amazonensis and L. braziliensis can cause localized cutaneous leishmaniasis (LCL). In the most severe cases of Leishmaniasis, L. amazonensis can cause diffuse cutaneous lesions (DCL) and L. braziliensis can cause mucocutaneous lesions (ML)2.
Innate immune cells such as macrophages, neutrophils, and natural killer cells recognize microorganisms through interaction between microbial ligands (MAMPs) with their pattern recognition receptors (PRRs). These PRRs include Toll-like receptors (TLRs) and NOD-like receptors (NLRs)3,4. The Leishmania membrane has lipophosphoglycans (LPG), glycoinositolphospholipids (GIPLs), and glycoprotein 63 (gp63) as the main molecules that are recognized by innate immune cells5,6. Recent studies have shown the involvement of TLRs in recognizing some of these molecules. LPG and GIPLs are recognized by TLR4 and TLR2, respectively. TLR9 also plays a role in recognizing the DNA of Leishmania spp. These mechanisms lead to induction of cytokines and microbicidal molecules after exposure to Leismania spp.7,8,9,10,11. In addition to, NLRs are important receptors for recognition of several microorganisms12. However, few studies have described their role in Leishmania recognition. Lima Junior et al 13 have shown that the NLRP3 inflammasome plays an important protective role during L. amazonensis infection in a mouse model. Conversely, it was showed that NLRP3 activation followed by IL-1β production mediates the detrimental CD8+ T cell-mediated cytotoxicity in tegumentary leishmaniasis, causing lesions/chronic inflammation in mouse model of infections caused by L. braziliensis or L. major 14. NLRP3 can also contribute to mouse susceptibility to L. major by increasing neutrophils recruitment, which plays a crucial role in the development of nonhealing lesions15. Recently, in patients with visceral leishmaniasis and in a murine infection model with L. infantum, the NOD2-RIPK2 pathway has been found to be involved in the development of the Th1-type response16, the most important response against Leishmania spp. Because the innate immune response is important in driving the acquired immune response, the efficient recognition of the parasite by PRRs improves the host resistance16.
Leishmania recognition by monocytes or macrophages through TLRs leads to the production of proinflammatory cytokines such as tumor necrosis factor (TNFα), interleukin (IL)-6, and interferon gamma (IFNγ), which all contribute for the exacerbated inflammation in leishmaniasis lesions11,17,18,19. Following NLRP3 inflammasome activation, IL-1β can be produced and contributes to the control of murine Leishmania infection13. Besides inflammation, these proinflammatory cytokines are important for controlling the Leishmania infection by inducing microbicidal molecules, such as reactive oxygen and nitrogen intermediates (ROI, RNI), which are crucial for the parasite killing10,19,20,21,22.
Since it is known that the immunogenetic background of the patients is one of the most important factors to determine the clinical outcome of leishmaniasis23, we investigated the role of genetic variants in genes of the NLR family for the Leishmania-induced immune response.
Results
Individuals heterozygous for NOD2 mutation produce less cytokines after Leishmania spp. exposure compared to individuals with wild-type NOD2
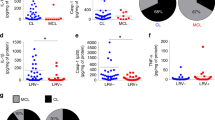
We explored whether SNPs in NLR family members NOD1 and NOD2 influence the production of cytokines after stimulation with Leishmania parasites. Through these analyses, we found that the NOD2 receptor plays an important role in the immune response against Leishmania spp. (Fig. 1). The results demonstrated that individuals heterozygous for NOD2 Leu1007insC polymorphism displayed significantly lower production of TNFα, IL-1β, IL-6, IL-8 and IFNγ for either L. amazonensis or L. braziliensis stimulation (Fig. 1A,B). Interestingly, NOD2 was only important for IL-17 induced by L. amazonensis, while L. braziliensis practically did not induce IL-17 production (Fig. 1B). Confirming the relevance of this NOD2 mutation for cytokine induction, the NOD2 agonist MDP induced lower cytokine production when added to PBMCs from individuals with the Leu1007insC variance, compared to the control subjects (Fig. 1A). Evaluation of other common polymorphisms in the NOD2 gene revealed no differences in the Leishmania-induced cytokine production (Figs S1 and 2). In addition to NOD2, we also investigated whether the NOD1 receptor could play a role in the cytokine induction after Leishmania exposure. In contrast to NOD2, no differences in monocyte- or lymphocyte-derived cytokine production were noted when PBMCs homozygous for NOD1 Glu796Lys were stimulated with L. amazonensis or L. braziliensis (Fig. 1C). Using the NOD1 agonist FK156, we demonstrated that Glu796Lys variance results in loss of function of NOD1 (Fig. 1C).
NOD2 but not NOD1 plays an important role in monocyte-and lymphocyte-derived cytokines induced by after Leishmania pecies stimulation. Peripheral blood mononuclear cells (PBMCs, 5 × 105 cells/100 μL) from healthy individuals genotyped for NOD1 (Glu796Lys) and NOD2 (1007finsC) were stimulated with different stimuli including lysates of Leishmania spp. (50 μg/mL, L. (L.) amazonensis: L. amaz; L. (V.) braziliensis: L. braz), FK156 (10 μg/mL), MDP (10 μg/mL); Medium: non-stimulated cells. TNFα, IL-6, IL-1β and IL-8 concentrations were measured in supernatants by ELISA after 24 h of incubation. IFNγ and IL-17 were determined after 7 days of incubation: the NOD2 (A and B) and NOD1 (C) genotype. Bars represent individuals carrying no SNP (wild type, Wt, white bars), heterozygous SNP carries (He, black bars), or homozygous variation (Ho, grey bars). Data represent the mean ± SEM. *p < 0.05; Mann-Whitney U-test (A and B; Wt vs He); (C; Wt vs Ho).
Phagolysosome formation is important for Leishmania-induced cytokine production and upregulation of NOD2 mRNA expression
In order to investigate whether Leishmania could upregulate the mRNA expression of NOD2, human PBMCs were incubated for 24 h with either parasite lysates or live promastigote forms of both L. amazonensis or L. braziliensis. The use of both forms of Leishmania stimuli was determined by the need to examine whether NOD2 is activated by Leishmania fragments which means that the parasites needs to be degraded intracellularly. Figure 2A showed that lysates as well as intact parasites of both Leishmania spp. were able to increase the NOD2 mRNA expression but not NOD1 mRNA. As positive controls FK-156 and MDP both could upregulation NOD1 or NOD2 mRNA expression respectively (Fig. 2A).
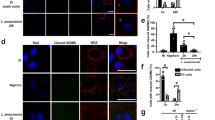
Leishmania species induce NOD2 mRNA expression and phagolysosome formation is important for Leishmania-induced cytokine production. (A and B) Peripheral blood mononuclear cells (PBMCs, 5 × 105 cells/100 μL) from healthy individuals were stimulated with either lysates (50 μg/mL) or promastigotes (1 × 105 parasites) of Leishmania species (L. (L.) amazonensis: L. amaz; L. (V.) braziliensis: L. braz); Medium (non-stimulated cells), FK156 (10 μg/mL), MDP (10 μg/mL) and LPS (10 ng/mL) were used as controls. (B) In some experiments cells were incubated in the absence (white) or presence (gray) of bafilomycin A1 (250 nM). Medium plus Vehicle (DMSO). (A) NOD1 and NOD2 mRNA expression were determined by quantitative real-time PCR after 24 h of incubation. (B) TNFα, IL-6 and IL-1β concentrations were determined in supernatants by ELISA after 24 h of incubation. Data represent the mean ± SEM, *p < 0.05; Wilcoxon test (A) (Medium vs stimuli) (B) (Vehicle vs Bafilomycin) (n = 6, in 2 independent experiments done in duplicates). (C) Embryonic kidney (HEK)-293 cells (1 × 106 cells/100 µL) transfected or not with NOD2 were stimulated for 24 h with lysates of Leishmania species (50 μg/mL) or promastigote forms (2 × 106 parasites) in the stationary growth phase of L. amaz; L. braz. Medium, LPS (10 ng/mL), and MDP (10 μg/mL) were included as controls. Protein levels of IL-8 were determined by ELISA in supernatants. Data represent the mean ± SEM of three independent experiments, *p < 0.05; Paired t-test (HEK-293 vs HEK + NOD2).
To examine whether degradation of the parasite by the phagolysosome is essential for Leishmania-induced cytokine production we inhibited the formation of phagolysosomes. PBMCs were incubated in the absence or presence of bafilomycin A1, a chemical compound that prevents maturation of parasitophorus vacuoles by inhibiting fusion between phagosomes and lysosomes24. Figure 2B shows a remarkable decrease in TNFα and IL-1β production after exposure to promastigote forms of either L.amazonensis and L. braziliensis in the presence of bafilomycin A1 (Fig. 2B). For IL-6, a decrease was observed after exposure to L. amazonensis and despite a tendency to reduction after exposure to L. braziliensis the difference did not achieve statistical significance (p = 0.0938). No differences in TNFα, IL-1β or IL-6 concentrations were observed when lysates of both Leishmania spp. were used to activate PBMCs in absence or presence of bafilomycin A1 (Fig. 2B).
To confirm the pivotal role of NOD2 in the recognition of Leishmania, HEK-293 cells overexpressing NOD2 were exposed to either lysates or live promastigote forms of either L. amazonensis or L. braziliensis. We observed a strong increase in IL-8 production after stimulation with either lysates or promastigote forms of Leishmania pecies when NOD2 was present in the HEK-293 cells (Fig. 2C). A similar effect in IL-8 production was observed when NOD2 agonist MDP was added as a positive control. A potent TLR4 agonist (LPS) was used as negative control, showing no elevated IL-8 production. To validate the HEK-293-NOD2 reporter cells, we determined NOD2 mRNA expression before and after MDP exposure by quantitative real-time PCR (Fig. S3).
PBMCs isolated from patients bearing NOD2 (3020insC) mutation confirmed the crucial role of NOD2 for Leishmania-induced cytokine production
Additional experiments were performed to confirm the role of NOD2 in Leishmania recognition. To this end, PBMCs carrying the NOD2 frameshift (3020insC) mutation were stimulated with live parasites or lysates of parasites for 24 h. The production of cytokines was compared with PBMCs isolated from healthy individuals (wt). As expected, cytokine concentrations were complete absent in individuals homozygous for the 3020insC frameshift mutation when PBMCs where stimulated with MDP (Fig. 3A). LPS-induced cytokine production was similar between PBMCs isolated from subjects carrying the 3020insC frameshift mutation and healthy controls (Fig. 3A). Production of TNFα, IL-1β, IL-6, IL-8, IFNγ was significantly decreased in PBMCs bearing the NOD2 3020insC frameshift mutation compared to PBMCs of individuals without this mutation after exposure to lysates or promastigote forms of both L. amazonensis and L. braziliensis (Fig. 3A and B). Of high interest, a reduction of IL-17 production was only observed after exposure to L. amazonensis (Fig. 3B).
NOD2 plays an important role in the cytokines after Leishmania stimulation. (A,B) Peripheral blood mononuclear cells (PBMCs, 5 × 105 cells/100 μL) from healthy individuals (n = 8) carrying no mutation in NOD2 (3020insC) (Wt; white bars) and from individuals carrying a mutation in NOD2 receptor (n = 4; grey bars) were stimulated with either lysates (50 μg/mL) or promastigotes (1 × 105 parasites) of Leishmania species (L. (L.) amazonensis: L. amaz; L. (V.) braziliensis: L. braz); Medium (non-stimulated cells), MDP (10 μg/mL) and LPS (10 ng/mL) were used as controls. (A) TNFα, IL-6 and IL-1β concentrations were determined in supernatants by ELISA after 24 h incubation. (B) IFNγ and IL-17 were determined after 7 days of incubation in supernatants by ELISA; Data represent the mean ± SEM, *p < 0.05; Mann-Whitney U-test (Wt vs 3020insC). (C) Peripheral blood mononuclear cells (PBMCs, 5 × 105 cells/100 μL) from healthy individuals were stimulated with either lysates (50 μg/mL) or promastigotes (1 × 105 parasites) of Leishmania species (L. (L.) amazonensis: L. amaz; L. (V.) braziliensis: L. braz) in the presence (grey bars) or absence (white bars) of the Ponatinib (100 nM); Medium (non-stimulated cells) plus Vehicle (DMSO) and MDP (10 μg/mL) were used as controls. TNFα, IL-6 and IL-1β concentrations were determined by ELISA, after 24 h incubation. Data represent the mean ± SEM, *p < 0.05; (DMSO vs Ponatinib; n = 6, in 2 independent experiments done in duplicates, by Wilcoxon test).
To investigate the downstream pathway of NOD2 signalling, we stimulated human PBMCs in the absence or presence of Ponatinib, a drug that potently inhibits the phosphorylation of RIPK225. RIPK2 is the important kinase for the NOD2 signalling cascade26. A significant reduction in TNFα, IL-1β and IL-6 production was observed when PBMCs were incubated with lysates or promastigote forms of both L. amazonensis and L. braziliensis in the presence of Ponatinib (Fig. 3C). A dose-response experiment with Ponatinib was performed and no cytotoxicity of Ponatinib to human cells or parasites was found (Fig. S4).
NOD2 is important to control intracellular infection caused by Leishmania spp
We investigated whether NOD2 plays a role in controlling Leishmania spp. infection in human primary macrophages. To this end, monocyte-derived macrophages from individuals homozygous for the NOD2 3020insC polymorphism were compared with macrophages from healthy individuals. Both types of macrophages were infected with either promastigote forms of L. amazonensis or L. braziliensis. Figure 4A shows an increase of the macrophage infection index in cells isolated from individuals carrying the NOD2 loss-of-function mutation compared with healthy controls (wt). Similar results were found in primary human macrophages pre-treated with Ponatinib, which showed an increase in the infection index compared to vehicle-treated macrophages (Fig. 4B). The results demonstrated that the NOD2 receptor plays an important role in the control of Leishmania infection in human macrophages.
NOD2 plays a role in control of Leishmania spp. infection. Monocyte-derived macrophages were obtained from PBMCs from wild type (wt) individuals (n = 4) carrying no mutations in NOD2 receptor (3020insC) (black circles) and individuals carrying mutations in NOD2 (n = 2, black squares) (A), after 5 days of differentiation. On day 5, macrophages (5 × 105 cells) were infected with promastigote forms of either L. (V.) braziliensis and L. (L.) amazonensis (2.5 × 105 parasites) during 4 h. (B) Macrophages from wt individuals (n = 4) were incubated in the absence (black balls - Vehicle DMSO) or presence (black squares) of Ponatinib (100 nM) 1 h before. Cells were washed out to remove the non-internalized parasites after 4 h, Ponatinib was added again and cells were incubated for 48 h. Coverslips were collected to determine macrophage infection index. Data represent individual values and horizontal lines represent medians. *p < 0.05; Wilcoxon test (DMSO vs Ponatinib).
Discussion
In the present study, we investigated the role of the NLR family members NOD1 and NOD2 in the induction of cytokines in human PBMCs after exposure to New World Leishmania spp. Results showed that whereas NOD2 plays an important role in parasite-induced cytokines, NOD1 is not relevant. Moreover, NOD2 is important for microbicidal activity of human macrophages contributing to the control of Leishmania spp. infection. This is the first report describing the NOD2 involvement in activation of human cells by New World Leishmania spp. Recently, a study in mice with L. infantum demonstrated that the activation of NOD2-RIP2 pathway drives the development of a Th1 instead of Th17 immune response16. These results demonstrated an important role of NOD2 in controlling Leishmania infection.
SNPs in the NOD2 receptor are related to autoinflammatory diseases such as Crohn’s disease and Blau syndrome27,28,29. In general, as a consequence, individuals carrying these mutations have a reduced NF-κB activation after recognition of specific pathogen-associated NOD2 ligands, which interfere in cytokine production30. Mutations in NOD1 lead to a reduced capacity to detect their ligands31. To evaluate the role of genetic variance in NOD1 and NOD2 in the immune response to Leishmania antigens, we applied a functional genomics approach as reported recently32,33,34. Using whole exome sequencing data of 100 subjects, we selected SNPs that are frequently present in NOD1 or NOD2 with a predicted loss or gain of function35. Here, we showed that one particular genetic variance in NOD2 (Leu1007finsC) downregulates the production of monocyte-derived cytokines such as TNFα, IL-1β, IL-6, IL-8 as well as IFNγ after exposure to lysates of either L. amazonensis or L. braziliensis. Remarkably, IL-17 was induced after exposure to lysates of L. amazonensis, but not L. braziliensis in a NOD2-dependent manner. The results indicate that in addition to its role in activation of the innate immunity, NOD2 shapes the adaptive immune response against Leishmania spp. Our results are partially in agreement with the results demonstrated by Nascimento et al.16, in which, the authors showed that in a murine model of L. infantum infection, the development of Th1 responses and the production of IFNγ was dependent on NOD2. However, the authors showed that the NOD2 pathway was not relevant for Th17 development. In contrast, we demonstrated that in human PBMCs, IL-17 production is NOD2-dependent only after exposure to L. amazonensis. These results could be ascribed to the differences among Leishmania spp. as well as to differences in human and mouse immune responses36. Here, besides Leishmania antigens (parasite lysates) or live promastigotes increased cytokines in a NOD2-dependent manner they upregulated NOD2, but not NOD1 mRNA expression in PBMCs. These results strengthened the involvement of NOD2 but not NOD1 in Leishmania spp.-induced immune responses.
To confirm that NOD2 is relevant for Leishmania-induced cytokine production, we overexpressed NOD2 in HEK-293 cells and showed that these NOD2-transfected cells produced significantly higher IL-8 concentrations after exposure to parasite lysates or promastigote forms of either L. amazonensis or L. braziliensis, compared to control HEK-293 cells. Although, NOD2 is known as a receptor that recognizes structures present in the bacteria cell wall37,38, the results presented here suggest that the NOD2 receptor also recognizes protozoan structures.
It is well described that PBMCs from patients with Crohn’s disease carrying the NOD2 (NOD2 3020insC) mutation display a reduction in cytokine production after exposure to several microbial ligands or pathogens39,40. In our study, we also used PBMCs from subjects carrying NOD2 3020insC mutation and demonstrated a significant decrease in the production of proinflammatory cytokines after Leishmania spp. exposure. These data are in line with the results of the NOD2 genetic variance Leu1007finsC and the HEK-NOD2 cells, underlining the pivotal role of NOD2 in the recognition of Leishmania spp.
An additional line of evidence that the NOD2 pathway is important for Leishmania-induced immune responses, are our results with the RIPK2 inhibitor (Ponatinib). The inhibition of RIPK2 led to almost complete abolishment of Leishmania cytokine production. These data demonstrate previously unknown role for human NOD2 in the recognition of L. amazonensis or L. braziliensis and make it tempting to speculate that individuals carrying NOD2 polymorphisms might be more susceptible to ATL.
The ligand of Leishmania parasites that binds to NOD2 remains unknown but we showed that degradation of Leishmania is a prerequisite for cytokine production. Bafilomycin A1, a specific inhibitor of the phagosome acidification and blocker of phagosome maturation impaired the proinflammatory cytokine production only after exposure to live promastigote forms of L. amazonensis or L. braziliensis, whereas no effect was observed after exposure to lysates of both these species. Enzymes present in the phagolysosomes, such as lysozyme, may degrade Leishmania to release NOD2 ligands in a similar process that happens with Listeria monocytogenes 41. Future studies are warranted to investigate which microbial components of Leishmania are recognized by NOD2.
It is known that the production of proinflammatory cytokines can play a double role in infection caused by Leishmania spp., contributing to the control of the infection but also favoring inflammation and tissue destruction42,43. Here we demonstrated that loss of NOD2 signalling impairs intracellular Leishmania killing. The exact mechanisms involved in NOD2-mediated Leishmania killing need to be further explored, although it is known that proinflammatory cytokines promote the induction of microbicidal molecules after infection with Leishmania spp.10,19,20,21,22,44. Moreover, it is known that NOD2 plays an important role in induction of microbicidal mechanisms including autophagy, antimicrobial peptides and ROS production, which are essential to control infections caused by intracellular pathogens45,46,47.
To conclude, our study showed for the first time that the human NOD2 pathway is important for Leishmania recognition, the induction of innate and adaptive immune responses, and the control of intracellular killing of the parasite (Fig. 5). Our findings provide the first evidence that genetic variances in NOD2 are associated with differences in the human immune response towards Leishmania spp. The relevance of the NOD2 pathway in the susceptibility to or severity of human ATL needs to be investigated in the near future.
Schematic overview of the role of NOD2 receptor in Leishmania spp. recognition and control. (A) General overview of NOD2 pathway: The NOD2 receptor is engaged by its respective ligand, driving the activation of RIPK2, which is followed by NF-κB activation and translocation to the nucleus leading to inflammatory gene transcription. In addition, microbicidal molecules can be produced. NOD2 genetic variances can lead to a non-functional NOD2 receptor, as a consequence, the cytokine and microbicidal molecule production mediated by NOD2 can be affected. (B) Phagolysosome-mediated degradation of Leishmania spp. promastigote forms leads to parasite antigen release in the cytoplasm of infected cells. This may drive the NOD2 activation and subsequently induction of NF-κB gene transcription and microbicidal molecule production. L. (L.) amazonensis and L.(V.) braziliensis-NOD2-mediated proinflammatory cytokines produced by innate and adaptive cells might contribute for controlling of the infection.
Methods
Ethics Statement
The study was approved by Ethics Committee of Radboud University Nijmegen, the Netherlands (no. 42561.091.12). Experiments were conducted according to the principles expressed in the Declaration of Helsinki. All individuals gave written informed consent to donate blood.
200FG cohort
Healthy individuals with a Dutch European genetic background that were recruited as part of the 200FG study34 participated in the study. The volunteers were between 23–73 years old, and consisted of 77% men and 23% women.
Isolation of genomic DNA and single-nucleotide polymorphism (SNP) analysis
DNA was isolated from whole blood by using the isolation Gentra Pure Gene Blood kit (Qiagen), according to the manufacturer’s protocol. SNPs in the analyzed receptor genes were selected from the National Center for Biotechnology Information SNP database (http://www.ncbi.nlm.nih.gov/snp/) upon previously described associations with human diseases and a minor allele frequency of at least 5% among different populations. In total, 7 SNPs in NOD1 and NOD2 receptors were selected and genotyped (Supplementary Table 1). Gene fragments were amplified by commercially available TaqMan® SNP Genotyping Assays according to the manufacturer’s protocol on the AB StepOnePlus polymerase chain reaction system (Applied Biosystems). Quality control was performed by the incorporation of positive and negative controls and duplication of random samples across different plates.
Leishmania cultures and lysates
L. amazonensis (IFLA/BR/67/PH8) reference strain and MHOM/BR/2003/IMG L. braziliensis, a clinical isolate obtained from a LCL patient (Leishbank IPTSP/UFG)48, were used. Promastigote forms were cultured in Grace’s Insect Medium, (Gibco - Life Technologies) and prepared for experiments as described by dos Santos et al.19. To obtain lysates of Leishmania, promastigotes (1 × 109 cells/mL) were lysed by 5 freeze-thaw cycles in liquid nitrogen and water bath at 37 °C followed by protein quantification using the Pierce BCA protein assay (Thermo Scientific). For cell stimulations, parasites were suspended in RPMI 1640 medium (Sigma) and added to the cultures as described below.
Human embryonic kidney cell line (HEK) stimulation
Transfection of HEK-293 cells with human NOD2 was performed as previously described by Laayouni et al.49 and culture conditions are described as supplementary material. Non-transfected or NOD2-transfected HEK-293 cells (1 × 106 cells) were added to 96-well flat-bottom plates (Greiner) in the presence of either E. coli lipopolysaccharide (LPS, O111:B4, 10 ng/mL; Sigma), further purified based on50, muramyl dipeptide (MDP, 10 μg/mL; Sigma), lysates or promastigotes (2 × 106 parasites) of L. braziliensis or L. amazonensis in a final volume of 200 μL. After 24 h of incubation, at 37 °C and 5% CO2, supernatants were collected and stored at −20 °C until analysis of IL-8 production.
Isolation of human peripheral blood mononuclear cells (PBMC) and treatments
Venous blood was obtained from eight healthy individuals bearing the wild-type allele of NOD2 (wild type, wt) and from four homozygous individuals for the NOD2 3020insC mutation presenting Crohn’s disease. Isolation of PBMCs was performed as described previously39, by density gradient centrifugation of blood diluted 1:1 in pyrogen-free saline overlayed on Ficoll-Paque (Pharmacia Biotech). PBMCs were suspended in RPMI 1640 medium with 50 mg/mL gentamicin, 2 mmol/L L-glutamine, and 1 mmol/L pyruvate (Invitrogen). The number was adjusted to 5 × 106 cells/mL. Cells (5 × 105 cells/mL) were added to round-bottom 96-well plates (Greiner) and incubated with either culture medium (negative control), MDP (10 μg/ml), FK-156 (10 μg/mL, Sigma), ultra-pure E. coli LPS as described by Battisti & Minnick50 (O111:B4 serotype, 10 ng/mL; Sigma) or 50 μg/mL of both Leishmania pp. lysates or promastigotes (1 × 105 parasites) of L. braziliensis and L. amazonensis in the presence or absence of 10% of human pool serum. In some experiments, PBMCs were pre-incubated for 1 h with a RICK inhibitor (Ponatinib, 100 nM/mL; Selleckchem) and a phagolysosome inhibitor (Bafilomycin A1, 250 nM; Invivogen) before cells were stimulated with lysates or promastigotes. After 24 h or 7 days, the supernatants were collected and stored at −20 °C until further analysis. The cell monolayers were collected by adding 200 µL of TRIzol and stored at −80 °C until being used for mRNA extraction.
Measurement of cytokines
Human TNFα, IL-1β, IL-6, IL-8, IL-17A and IFNγ were determined in culture supernatants using commercial Enzyme-Linked Immunosorbent Assay (ELISA) kits (Sanquin, Amsterdam, and R&D Systems, Minneapolis), according to the manufacturer’s protocols.
Evaluation of mRNA expression by quantitative real-time PCR (qPCR)
RNA isolation was carried out based on the method reported by Chomzynski & Sacchi51 and the qPCR method is shortly described in supplementary material. Primer sequences (Supplementary Table 2) for NOD1 and NOD2 receptors were obtained from the Harvard Primerbank database. Primers were purchased from Biolegio. Relative expression of mRNA levels was calculated and normalized for the housekeeping gene GAPDH.
Evaluation of Macrophage infection
Monocyte-derived macrophages from healthy volunteers and from homozygous individuals for the NOD2 3020insC mutation were obtained from PBMCs (5 × 105 cells/well) during 5 days at 37 °C, 5% CO2. PBMCs were counted and plated into 24-well plates (Costar) over coverslips in the presence of RPMI-1640 medium supplemented as described above and added with 10% of human pool serum. Medium was refreshed every 48 h. On day 5, macrophages were infected with promastigote forms of either L. braziliensis or L. amazonensis (2.5 × 105 parasites) during 4 h. Cells were washed to remove non-internalized parasites and incubated for 48 h. Additionally, is some experiments monocyte-derived macrophages were pre-treated or not with Ponatinib (100 nM/mL; Selleckchem) 1 h before infection and the drug was replaced after washings. Coverslips were collected to determine the macrophage infection index after cells were fixed and stained with Giemsa (Merck Millipore), according to52 and briefly described in supplementary material.
Statistical Analysis
Data are expressed as median, interquartile, minimal and maximal values, unless otherwise indicated. Differences between experimental groups were tested by Mann-Whitney U test or unpaired t test according to the data, or by Wilcoxon paired test, using Prism software (version 6.0; GraphPad; San Diego, CA, USA). Differences with p < 0. 05 were considered significant.
References
Gontijo, B. & de Carvalho, MdeL. R. [American cutaneous leishmaniasis]. Rev. Soc. Bras. Med. Trop. 36, 71–80 (2003).
Reithinger, R. et al. Cutaneous leishmaniasis. Lancet. Infect. Dis. 7, 581–96 (2007).
Kumar, H., Kawai, T. & Akira, S. Pathogen recognition in the innate immune response. Biochem. J. 420, 1–16 (2009).
Moreira, L. O. & Zamboni, D. S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 3, 328 (2012).
Turco, S. J. & Sacks, D. L. Control of Leishmania-sand fly interactions by polymorphisms in lipophosphoglycan structure. Methods Enzymol. 363, 377–381 (2003).
McConville, M. J. & Ferguson, M. A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem. J. 305–24 (1993).
Becker, I. et al. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol. Biochem. Parasitol. 130, 65–74 (2003).
Liese, J., Schleicher, U. & Bogdan, C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur. J. Immunol. 37, 3424–3434 (2007).
Assis, R. R., Ibraim, I. C., Noronha, F. S., Turco, S. J. & Soares, R. P. Glycoinositolphospholipids from Leishmania braziliensis and L. infantum: modulation of innate immune system and variations in carbohydrate structure. PLoS Negl. Trop. Dis. 6, e1543 (2012).
Ibraim, I. C. et al. Two biochemically distinct lipophosphoglycans from Leishmania braziliensis and Leishmania infantum trigger different innate immune responses in murine macrophages. Parasit. Vectors 6, 54 (2013).
Nogueira, P. M. et al. Lipophosphoglycans from Leishmania amazonensis Strains Display Immunomodulatory Properties via TLR4 and Do Not Affect Sand FlyInfection. PLoS Negl. Trop. Dis. 10, e0004848 (2016).
Davis, B. K., Wen, H. & Ting, J. P.-Y. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu. Rev. Immunol. 29, 707–735 (2011).
Lima-Junior, D. S. et al. Inflammasome-derived IL-1β production induces nitric oxide–mediated resistance to Leishmania. Nat. Med. 19, 909–915 (2013).
Novais, F. O. et al. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1β production. PLoS Pathog. 13, e1006196 (2017).
Charmoy, M. et al. The Nlrp3 inflammasome, IL-1β, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice. Eur. J. Immunol. 46, 897–911 (2016).
Nascimento, M. S. L. et al. NOD2-RIP2–Mediated Signaling Helps Shape Adaptive Immunity in Visceral Leishmaniasis. J. Infect. Dis. 214, 1647–1657 (2016).
Faria, M. S. et al. Role of protein kinase R in the killing of Leishmania major by macrophages in response to neutrophil elastase and TLR4 via TNF and IFN. FASEB J. 28, 3050–3063 (2014).
Galdino, H. et al. Leishmania (Viannia) braziliensis amastigotes induces the expression of TNFα and IL-10 by human peripheral blood mononuclear cells in vitro in a TLR4-dependent manner. Cytokine 88, 184–192 (2016).
dos Santos, J. C. et al. Cytokines and microbicidal molecules regulated by IL-32 in THP-1-derived human macrophages infected with New World Leishmania species. PLoS Negl. Trop. Dis. 11, e0005413 (2017).
Díaz, N. L., Arveláez, F. A., Zerpa, O. & Tapia, F. J. Inducible nitric oxide synthase and cytokine pattern in lesions of patients with American cutaneous leishmaniasis. Clin. Exp. Dermatol. 31, 114–7 (2006).
Carvalho, L. P., Passos, S., Schriefer, A. & Carvalho, E. M. Protective and pathologic immune responses in human tegumentary leishmaniasis. Front. Immunol. 3, 301 (2012).
Carneiro, P. P. et al. The Role of Nitric Oxide and Reactive Oxygen Species in the Killing of Leishmania braziliensis by Monocytes from Patients with Cutaneous Leishmaniasis. PLoS One 11, e0148084 (2016).
Sophie, M. et al. SLC11A1 polymorphisms and host susceptibility to cutaneous leishmaniasis in Pakistan. Parasit. Vectors 10, 12 (2017).
Yamamoto, A. et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 23, 33–42 (1998).
Canning, P. et al. Inflammatory Signaling by NOD-RIPK2 Is Inhibited by Clinically Relevant Type II Kinase Inhibitors. Chem. Biol. 22, 1174–84 (2015).
Park, J.-H. et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 178, 2380–6 (2007).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411, 599–603 (2001).
Bonen, D. K. et al. Crohn’s disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology 124, 140–146 (2003).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411, 603–606 (2001).
Inohara, N. et al. Host Recognition of Bacterial Muramyl Dipeptide Mediated through NOD2: Implications for Crohn’s Disease. J. Biol. Chem. 278, 5509–5512 (2003).
Girardin, S. E. et al. Identification of the Critical Residues Involved in Peptidoglycan Detection by Nod1. J. Biol. Chem. 280, 38648–38656 (2005).
ter Horst, R. et al. Host and Environmental Factors Influencing Individual Human Cytokine Responses. Cell 167, 1111–1124.e13 (2016).
Oosting, M. et al. Functional and Genomic Architecture of Borrelia burgdorferi-Induced Cytokine Responses in Humans. Cell Host Microbe 20, 822–833 (2016).
Li, Y. et al. A Functional Genomics Approach to Understand Variation in Cytokine Production in Humans. Cell 167, 1099–1110.e14 (2016).
Shah, T. S. et al. optiCall: a robust genotype-calling algorithm for rare, low-frequency and common variants. Bioinformatics 28, 1598–603 (2012).
Gollob, K. J., Viana, A. G. & Dutra, W. O. Immunoregulation in human American leishmaniasis: balancing pathology and protection. Parasite Immunol. 36, 367–376 (2014).
Girardin, S. E. et al. Peptidoglycan Molecular Requirements Allowing Detection by Nod1 and Nod2. J. Biol. Chem. 278, 41702–41708 (2003).
Girardin, S. E. et al. Nod2 Is a General Sensor of Peptidoglycan through Muramyl Dipeptide (MDP) Detection. J. Biol. Chem. 278, 8869–8872 (2003).
Oosting, M. et al. Recognition of Borrelia burgdorferi by NOD2 Is Central for the Induction of an Inflammatory Reaction. J. Infect. Dis. 201, 1849–1858 (2010).
van Heel, D. A. et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn’s disease. Lancet 365, 1794–1796 (2005).
Herskovits, A. A., Auerbuch, V. & Portnoy, D. A. Bacterial Ligands Generated in a Phagosome Are Targets of the Cytosolic Innate Immune System. PLoS Pathog. 3, e51 (2007).
Antonelli, L. R. V. et al. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol. Lett. 101, 226–30 (2005).
Oliveira, F. et al. Lesion size correlates with Leishmania antigen-stimulated TNF-levels in human cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 85, 70–3 (2011).
Khouri, R. et al. IFN-beta impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J. Immunol. 182, 2525–31 (2009).
Kobayashi, K. S. et al. Nod2-Dependent Regulation of Innate and Adaptive Immunity in the Intestinal Tract. Science. 307, 731–734 (2005).
Homer, C. R., Richmond, A. L., Rebert, N. A., Achkar, J. & McDonald, C. ATG16L1 and NOD2 Interact in an Autophagy-Dependent Antibacterial Pathway Implicated in Crohn’s Disease Pathogenesis. Gastroenterology 139, 1630–1641.e2 (2010).
Cooney, R. et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 16, 90–7 (2010).
Dorta, M. L. et al. Improvements in obtaining New World Leishmania sp from mucosal lesions: Notes on isolating and stocking parasites. Exp. Parasitol. 132, 300–303 (2012).
Laayouni, H. et al. Convergent evolution in European and Rroma populations reveals pressure exerted by plague on Toll-like receptors. Proc. Natl. Acad. Sci. 111, 2668–2673 (2014).
Battisti, J. M. & Minnick, M. F. Laboratory maintenance of Bartonella quintana. Curr. Protoc. Microbiol. Chapter 3, Unit 3C.1.1-3C.1.13 (2008).
Chomzynski, P. & Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 162, 156–159 (1987).
Morato, C. I. et al. Essential role of leukotriene B4 on Leishmania (Viannia) braziliensis killing by human macrophages. Microbes Infect. 16, 945–953 (2014).
Acknowledgements
The authors thank CNPq by financial support (F.R.-D., L.A.B.J.) grant n. 465771/2014-9 - INCT - National Institute of Science and Technology of the strategies in host-pathogen interaction, Brazil). F.R-D. is research fellow of CNPq. The work of J.C.S. was financially supported by CAPES and CNPq, Brazil. C.S.A. was financially supported by CNPq. M.G.N. was supported by an ERC Consolidator Grant (#310372) and a POC-FUSE grant of the Romanian National Agency for Scientific Research. We thank Dr. Dimitri A. Diavatopoulos for having kindly donated the HEK-NOD2 cells.
Author information
Authors and Affiliations
Contributions
L.A.B.J., F.R.-D. and M.G.N., designed the study; J.C.S., M.S.M.A.D., M.O., D.J.J., B.H., C.S.A., performed the experiments. J.C.S., M.S.M.A.D., M.O., R.S.G., L.A.B.J., F.R.-D. wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
dos Santos, J.C., Damen, M.S.M.A., Oosting, M. et al. The NOD2 receptor is crucial for immune responses towards New World Leishmania species. Sci Rep 7, 15219 (2017). https://doi.org/10.1038/s41598-017-15412-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15412-7
This article is cited by
-
IL-1 and CD40/CD40L platelet complex: elements of induction of Crohn’s disease and new therapeutic targets
Archives of Pharmacal Research (2021)
-
Differential immune response modulation in early Leishmania amazonensis infection of BALB/c and C57BL/6 macrophages based on transcriptome profiles
Scientific Reports (2019)
-
Leishmaniasis and glycosaminoglycans: a future therapeutic strategy?
Parasites & Vectors (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.