Abstract
Routine laboratory lipid assays include simple measurements of total cholesterol, triacylglycerols and HDL. However, lipids are a large group of compounds involved in many metabolic pathways, and their alterations may have serious health consequences. In this study, we used 1H NMR to analyze lipids extracted from sera of 16 obese patients prior to and after bariatric surgeries. We observed a post-surgery decrease in serum concentrations of lipids from various groups. The hereby presented findings imply that 1H NMR is suitable for rapid, simple and non-invasive detection of lipids from 30 structural groups, among them triacylglycerols, phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, total phospholipids, total, free and esterified cholesterol, total and unsaturated fatty acids. NMR-based analysis of serum lipids may contribute to a substantial increase in the number of routinely determined markers from this group; therefore, it may find application in clinical assessment of obese subjects prior to and after bariatric surgeries, as well as in the examination of patients with other metabolic diseases.
Similar content being viewed by others
Introduction
Since the discovery of nuclear magnetic resonance (NMR) phenomenon and publications of E.M. Purcell et al.1 and F. Bloch et al.2 from 1946, due to progress in multi-dimensional, multi-quantum Fourier spectroscopy and magnetic resonance tomography, NMR has found application in many scientific disciplines3. Nowadays, NMR spectroscopy is widely used in clinical trials and everyday practice as a diagnostic tool to detect various biomarkers, especially those related to metabolic disorders4,5,6,7. Moreover, this technique is suitable for structural examination and rapid qualitative and quantitative analyses of non-derivatized lipids extracted from membranes, tissues and biofluids5,8,9,10. An advantage of NMR is its high reproducibility, which makes this method suitable for identification and validation of biomarkers11. Another important feature of NMR spectroscopy is a possibility to analyze the sample without its destruction; as a result, the sample can be used for repeated analyses and/or for sequential analyses of various analyte species12. However, according to some authors, NMR-based analyses should be accompanied by examination with MS techniques to improve the accuracy of results obtained in complex biological systems11,12,13.
Lipidomics is a research discipline analyzing whole lipidome with high throughput and on a large scale14. In lipidomic studies, analytical technique is adjusted for matrix type, and according to some authors, NMR spectroscopy is the most promising method used for biofluid analysis12,15. The results obtained by lipidomic analyses may be applied to prognosis, prevention, diagnosis and treatment of various metabolic diseases (Fig. 1). Only few lipid biochemical markers, triacylglycerols (TGs), total cholesterol and HDL cholesterol, are assayed in routine practice, whereas other, more diverse groups of lipids, such as various classes of polar lipids, are not determined at all. In this study, we determined the levels of phospholipids (PL), including phosphatidylcholine (PC), phosphatidylethanolamine (PE) and sphingomyelin (SM), based on their characteristic head groups. Polar lipids, among them phospholipids, are crucial for structural integrity and interactions between molecules16. However, little is known about PL composition in obese patients, and the role of this lipid group in pathogenesis of metabolic diseases is still not fully understood17. Changes in polar lipid profile are observed during the course of many metabolic diseases, including obesity, and may be reflected by altered properties of plasma membranes17. Previous studies documented some changes in serum lipidome of obese patients, such as alterations of fatty acid composition18 and fatty acid content in phospholipids17. Furthermore, changes in SM and ceramide levels contribute to obesity-induced endothelial dysfunction and cardiovascular disease19,20. SM and ceramides are also involved in control of cell proliferation and/or cancer prevention21. Consequently, knowledge of specific lipid groups present in the serum is not only vital for the identification of related lipid disorders, but may also help to understand their impact on various metabolic processes.
Obesity is a major public health problem. In 2014, more than 600 million subjects worldwide were classified as obese, and more than 1.9 billion adults were overweight22. Epidemiological data suggest that in 2030, obesity may be present in 50% of general population23. This justifies research on novel treatment options, and puts emphasis on prevention of side effects of currently available therapies. Complex morbid obesity can be managed by dietary treatment, pharmacotherapy and bariatric surgery. Other than the two former strategies, bariatric surgery is highly effective, resulting in a significant reduction of food intake and/or decrease in digestion and absorption24. Furthermore, bariatric treatment was shown to significantly reduce cardiovascular risk and even more importantly, may contribute to remission of type 2 diabetes mellitus25. However, as any other therapy, also bariatric surgery may produce some adverse effects, such as peripheral neuropathy, metabolic bone disease, micronutrient deficiency and protein-calorie malnutrition26,27. Therefore, the aim of this study was to analyze the impact of bariatric surgery on the levels of lipids from selected groups being vital for health of obese patients. To achieve this goal, we used a new approach, NMR spectroscopy, as this method offers many advantages which are not available in the case of conventional laboratory techniques.
To this date, polar lipid contents in obese patients were determined with several techniques. Complex lipids, such as PC, PS, PE, PI and SM, were separated with EDTA vacutainers28, HPLC29, TLC30,31 and SPE32, whereas blood composition of FA in PHL was determined by means of GC28,30,31,32, LC-MS/MS33 and UPLC-MS34. All these techniques are suitable for detection, identification and quantification of lipids from several groups, but the analysis is time-consuming and includes multiple stages35. Moreover, we still lack an accurate method for rapid determination of whole spectrum of blood lipids. 1H NMR may be helpful in early detection of disorders in various groups of serum lipids being associated with a plethora of diseases, among them obesity. The main objective of this study was to develop a simple and rapid method capable to determine as many groups of serum lipids as possible. The study included patients with morbid obesity, examined prior to and after bariatric surgery; however, our hereby presented diagnostic method is likely also applicable to other metabolic diseases. We showed that extraction with chloroform and methanol mixture, followed by NMR analysis lasting no longer than a few minutes, is a time-sparing method that can detect up to 10 various groups of lipids, including TG, PC, PE, SM, total PL, total fatty acids and unsaturated fatty acids (UFA), as well as total, free and esterified cholesterol. Moreover, we were able to detect some specific lipid compounds, such as 7-lathosterol, oleic acid and linoleic acid.
Results
1H NMR analysis of sera from obese patients prior to and after bariatric surgery
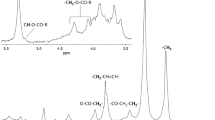
The analysis included 16 sera samples from obese patients who underwent bariatric surgery. A representative 1H NMR spectrum of patient’s serum is shown on Fig. 2 (upper panel). A total of 30 structural groups of serum lipid extracts were identified and quantified (Table 1). A set of 2D NMR experiments (COSY, TOCSY, HSQC, HSQC-TOCSY, and HMBC) was recorded to confirm the chemical shifts assignment in 1H NMR spectra. The spectrum HSQC (Fig. 2 – bottom panel, and Supplementary Figure 1) showed linkages (through one bond) between protons and corresponding carbon atoms. The identification of signals in a complex 1H NMR spectrum using a combination of 1H and13C chemical shifts obtained from HSQC experiments is much more reliable. Moreover the section of TOCSY spectrum (Supplementary Figure 2) allowed to distinguish protons of TG and PL (two different spin systems - red and blue lines).
Upper panel: A representative 1H NMR spectrum of serum from obese patient, with resonance signals assigned to respective groups of lipids; bottom panel: HSQC spectrum. All detected structural groups are listed in Table 1.
Verification of 1H NMR analysis accuracy against routine laboratory lipid assays
Two groups of lipids, total cholesterol and triacylglycerols, were determined both by means of NMR and using routine laboratory methods. To verify the accuracy of NMR analysis, we analyzed correlations between the results obtained with both methods. As shown on Fig. 3, two NMR signals for total cholesterol correlated strongly with each other, and showed a strong positive correlation with serum concentration of cholesterol determined using routine laboratory method. Similarly, three NMR signals specific for triacylglycerols correlated strongly with one another, as well as with serum concentration of triacylglycerols determined routinely at our laboratory (Fig. 4).
Associations between C18H3 and C26H3 1H NMR signals for total cholesterol (A), C18H3 1H NMR signal for total cholesterol and serum cholesterol concentration determined routinely at a clinical laboratory (B), C26H3 1H NMR signal for total cholesterol and serum cholesterol concentration determined routinely at a clinical laboratory (C) in the study subjects.
Associations between C3H2 and C1H2 1H NMR signals for triacylglycerol (A), C3H2 and C1H2 1H NMR signals for triacylglycerol (B), C1H2 and C2H1H NMR signals for triacylglycerol (C), C3H2 1H NMR signal for triacylglycerol and serum triacylglycerol concentration determined routinely at a clinical laboratory (D), C1H2 1H NMR signal for triacylglycerol and serum triacylglycerol concentration determined routinely at a clinical laboratory (E), C2H1H NMR signal for triacylglycerol and serum triacylglycerol concentration determined routinely at a clinical laboratory (F) in the study subjects.
Changes in serum lipids after bariatric surgery
As shown in Table 1, bariatric surgery contributed to a significant decrease in the levels of all analyzed lipids, by 57% for triacylglycerols, by 40–45% for SM, PC, PE and by 42% for total PL. The levels of total, free and esterified cholesterol decreased by 43–48%, and the levels of total fatty acids and specific fatty acids, including UFA, 18:1, 18:2, 20:4 and 22:6, by about 50%. The most evident decrease (by about 80%) was observed in the case of C18H3 group in total 7-lathosterol (Table 1).
Discussion
1H NMR as a simple method for rapid detection of changes in many groups of serum lipids in morbidly obese patients after bariatric surgery
Although NMR has been previously applied to the analysis of lipid contents in adipose tissue36,37 and blood serum38, this technique is relatively infrequently used in lipidology, mainly as a method to determine lipoprotein levels16,39,40. Only few previous studies used NMR for metabolomic analysis of sera from obese subjects prior to and after bariatric surgery41,42,43,44. In these studies, no lipid extraction was made and NMR analysis was conducted with intact blood sera, which resulted in identification of only few or none lipid species. In our present study, we first extracted serum lipids with Folch’ method45, and then analyzed the lipid extracts by means of NMR. This enabled us to detect 30 various structural groups of serum lipids. High accuracy of our method was confirmed by strong positive correlations between NMR signal intensity for 2 groups representing total cholesterol/3 groups representing triacylglycerols and serum concentrations of these metabolites determined using routine enzymatic methods. Postoperative changes in cholesterol and triacylglycerol contents were more evident when estimated on the basis of NMR spectra (see: Tables 1 and 2). This implies that our NMR analysis is more suitable to estimate the direction and magnitude of postoperative changes in various lipid species, than to determine their absolute concentrations. According to some authors, due to its low sensitivity and problems with obtaining satisfactory quality of a multi-parallel analysis of complex lipid extracts with hundreds of metabolites, NMR should not be used without a supporting MS, especially in the case of low-abundance analytes46. However, our present study showed that despite these potential limitations, NMR is suitable for rapid lipidomic analysis of whole groups of polar lipids: PC, PE, SM and total PL. Moreover, this technique may provide an information about a global direction of changes in lipidome of patients after bariatric surgeries. Obtaining such information by means of MS would be much more costly and time-consuming. All this makes NMR lipid analysis more promising from a clinical perspective. Another NMR approach was employed by researchers from Computational Medicine Research Team from Finland, who has developed a high-throughput serum NMR metabolomics platform, which by combining NMR analysis of three molecular windows: LMWM (low- molecular-weight metabolites), LIPO (lipoproteins) and LIPID (extracted lipids) is able to measure total of 233 variables, including serum lipids47,48. This platform has been employed for metabolomics analyses in many clinical studies conducted in patients with early atherosclerosis, coronary heart disease, metabolic syndrome, type 1 and 2 diabetes mellitus and diabetic nephropathy47. It would be certainly worth doing such metabolomics analyses in morbidly obese patients before and after bariatric surgery. In turn, our method is quite simple, but also allows for rapid identification of the most important groups of serum lipids. Moreover it allows to measure some additional serum lipids – PE as well as lathosterol, which can serve as a marker of cholesterol production rate in human organism49. In Fig. 5 we present the overview of the methodology used in this study.
A conceptual image presenting the overview of the methodology used in this study. The most important advantage of this approach is significantly increased number of analyzed serum lipid species comparing to standard serum lipid assay in clinical laboratory. The measurement of these lipid species (e.g. phospholipids) is not possible by standard enzymatic assays. Moreover their measurement by our NMR method can be performed in just a few minutes.
Bariatric surgery contributes to a decrease in serum lipid content
Our study demonstrated that in morbidly obese patients, bariatric surgery contributed to a significant decrease in all analyzed groups of serum lipids. However, the degree of the reduction was group-specific. The most evident decrease (by 82.5%) was observed in the case of 7-lathosterol, a precursor of cholesterol. 7-lathosterol is a marker of cholesterol production rate49, and therefore, our findings suggest that bariatric surgery results in a substantial decrease in cholesterol synthesis. This observation is consistent with the results published recently by Vuono et al.50, who using GC-MS, also demonstrated a significant decrease in serum lathosterol level in patients after sleeve gastrectomy. The same study revealed that lower level of serum lathosterol was associated with a decrease in cholesterol synthesis. After bariatric surgery, the levels of total, esterified and free cholesterol in our patients were approximately 45% lower than prior to the procedure. However, even more pronounced (57%) postoperative reduction was observed in the case of triacylglycerol content. Noticeably, also the analysis with routine enzymatic methods demonstrated that the postoperative decrease in TG was greater than the reduction of total cholesterol level (Table 2). The key deliverable of our study is the development of a method for rapid detection of quantitative changes in many groups of polar lipids, including PC, PE, SM and total PL, as well as UFA in sera from morbidly obese patients after bariatric surgeries. UFA are the major component of PL51. The intensities of UFA and PL signals after bariatric surgery were shown to correlate strongly (R = 0.95; p < 0.01), which implies that the postoperative decrease in PL might result from a lesser availability of UFA. Morbid obesity is associated with enhanced oxidative stress52, and UFA are particularly prone to peroxidation51. Products of lipid peroxidation play a role in the pathogenesis of insulin resistance, diabetes mellitus53, chronic inflammation, atherosclerosis, cardiovascular diseases51 and some disorders incorporated into metabolic syndrome54; all these conditions may be also associated with morbid obesity. Therefore, the postoperative decrease in UFA and PL may contribute to attenuation of oxidative stress, a phenomenon observed after bariatric surgeries55. Aside from the role in oxidative stress propagation, PL composition and content may also influence the risk of diabetes mellitus29. Therefore, a method for routine determination of their serum levels in obese subjects would have unquestioned clinical value. Although polar lipids seem to be vital for health of obese subjects17, only few previous studies addressed bariatric surgery-related changes in serum phospholipids. Kayser et al.56 used HPLC-MS/MS to document a decrease in various species of PC, PE and PG at 3 months after gastric bypass procedure. Also in that study, a decrease in the level of SFA-containing species of SM co-existed with an increase in the content of PUFA-containing SM56. A decrease in PC, PE and SM was also reported by Graessler et al.57 three months after RYGB procedure. Both studies mentioned above were based on MS techniques and therefore, provided an information about the contents of specific species from each group of polar lipids, which was not possible in the case of our method. However, in contrast to the MS-based technique, our method is suitable for rapid, non-destructive analysis of a sample; thus, it provides an information about the changes in overall PC, PE and SM contents in the sample, which can be then re-used for more accurate MS analyses, if necessary. Contrary to our hereby presented findings, Lopes et al.42,43 reported an increase in PC at 12 months after RYGB. Perhaps, PC content increases with time elapsed since the bariatric procedure, due to a raise in HDL concentration. Our study demonstrated a ca. 50% postoperative decrease in the levels of total and unsaturated fatty acids, as well as a decrease in the contents of two specific fatty acids, oleic and linoleic. This is not surprising, owing that fatty acids are the main component of other, more complex lipids. Interestingly, Forbes et al.32 reported an increase in serum concentration of FFA at one month after bariatric surgery, which might correspond to enhanced postoperative catabolism of lipids; however, FFA have eventually returned to their baseline level at 6 months post-procedure. FFA constitute only a small proportion of all FA building other serum lipids, including PL and TG. Oleic acid is synthetized primarily in the liver, by stearoyl-CoA desaturase (SCD1)58. Therefore, a postoperative decrease in the concentration of this FA might reflect lower rate of liver lipogenesis and FA desaturation after bariatric surgery. On the other hand, linoleic acid is an essential fatty acid and thus, its decreased content after bariatric surgery was likely associated with a reduced food intake.
In conclusion, this study showed that extraction of serum lipids, followed by NMR analysis, lasting no longer than a few minutes, are suitable for rapid detection of various serum lipids, including total TG, PC, PE, SM, total PL, total, free and esterified cholesterol, total and unsaturated fatty acids, as well as 7-lathosterol, oleic acid, and linoleic acid, in obese patients prior to and after bariatric surgeries. A postoperative decrease in serum levels of various lipids likely reflected their reduced dietary intake, as well as lower rate of cholesterol production, and less intense liver synthesis and desaturation of fatty acids in obese patients subjected to bariatric surgeries. However, a concomitant decrease of some other lipid species being important for patient health, such as PUFA and PL, points to a necessity of their dietary supplementation in patients after bariatric surgery, in order to prevent deficiencies. NMR-based analysis of serum lipids may contribute to a substantial increase in the number of routinely determined markers from this group; therefore, it may find application in clinical assessment of obese subjects prior to and after bariatric surgeries.
Material and Methods
Patients
Sixteen obese patients (7 men and 9 women with mean BMI of 41 ± 1.1 kg/m2) aged 27–61 years (mean age, 44 ± 3.1 years) underwent bariatric surgery (BS) at the Department of General, Endocrine and Transplant Surgery, Medical University of Gdansk (Poland). Six patients were subjected to sleeve gastrectomy (SG), another 6 to Roux-en-Y gastric bypass (RYGB), and 4 to omega-loop gastric bypass (OLGB). Patients included in the study didn’t present clinical evidence of endocrine, cardiac, hepatic or renal diseases, additionally all of them were non-smokers. Anthropometric and laboratory parameters of the study subjects were determined twice, prior to the surgery and 6 months thereafter. Blood samples for NMR analysis were obtained after an overnight fast. Standard laboratory parameters were determined at the Central Clinical Laboratory, Medical University of Gdansk. The study conformed to the principles of the Declaration of Helsinki of the World Medical Association. Protocol of the study was approved by the Local Bioethics Committee at the Medical University of Gdansk (decision no. NKEBN/281/2014), and prior to the enrolment, written informed consent was sought from all the study subjects. All tests used in this study followed relevant protocols and guidelines. Selected biochemical and anthropometric characteristics of the study subjects are presented in Table 2.
Sample preparation
Total lipids were extracted from whole serum samples with a chloroform-methanol mixture (2:1, v/v), as described previously59. The extraction process lasted 30 minutes. Prior to lipidomic analysis, serum analytes were dissolved in 600 µl of deuterated chloroform and deuterated methanol mixture (2:1; v/v) with 3 mM TMS. After centrifugation, the samples were transferred to 5-mm NMR tubes, and stored at 4 °C until the analysis.
NMR measurement and data processing
All NMR spectra were recorded with Bruker Avance III 500 MHz spectrometer at 298 K and referenced to TMS signal (0.00 ppm). The 1H NMR spectra of lipid extracts from patient sera were recorded using zg30 pulse sequence. Each spectrum was comprised of 64 consecutive scans, with acquisition time of 4 s, FID size of 65 k and spectral width of 15 ppm.
2D NMR homonuclear 1H, 1H COSY (correlation spectroscopy) and TOCSY (total correlation spectroscopy), as well as 2D NMR heteronuclear 1H,13C HSQC (heteronuclear single quantum coherence), HSQC-TOCSY (heteronuclear single quantum coherence - total correlation spectroscopy) and HMBC (heteronuclear multiple bond correlation) experiments were recorded to confirm the chemical shifts assignment.
The obtained 1H NMR spectra were processed with 0.3 Hz line broadening, manually phased and corrected for baseline distortion with MestReNova software (Mestrelab Research v. 11.0). The essential signals in 1H NMR spectra were integrated manually. All obtained integrals were scaled according to the area of the TMS reference signal.
Statistical analysis
Statistical significance of differences in pre- and postoperative values of the study variables was verified with paired t-test. The differences were considered significant at p < 0.05. Relationships between pairs of variables were determined on linear regression analysis. All statistical calculations were carried out with Sigma Stat software.
References
Purcell, E. M., Torrey, H. C. & Pound, R. V. Resonance Absorption by Nuclear Magnetic Moments in a Solid. Phys. Rev. 69, 37–38 (1946).
Bloch, F., Hansen, W. W. & Packard, M. The Nuclear Induction Experiment. Phys. Rev. 70, 474–485 (1946).
Fayed, N., Olmos, S., Morales, H. & Modrego, P. J. Physical Basis of Magnetic Resonance Spectroscopy and its Application to Central Nervous System Diseases. Am. J. Appl. Sci. 3, 1836–1845 (2006).
Waguri, T., Goda, T., Kasezawa, N. & Yamakawa-Kobayashi, K. The combined effects of genetic variations in the GPR120 gene and dietary fat intake on obesity risk. Biomed. Res. 34, 69–74 (2013).
X, L. et al Identification of metabolic biomarkers in patients with type 2 diabetic coronary heart diseases based on metabolomic approach. Nat. Publ. Gr. 30785, https://doi.org/10.1038/srep30785 (2016).
Dawiskiba, T. et al. Serum and urine metabolomic fngerprinting in diagnostics of inflammatory bowel diseases. World J. Gastroenterol. 20, 163–174 (2014).
Kimura, T. et al. Identification of biomarkers for development of end-stage kidney disease in chronic kidney disease by metabolomic profiling. Sci. Rep. 6, 26138 (2016).
Adosraku, R. K., Choi, G. T., Constantinou-Kokotos, V., Anderson, M. M. & Gibbons, W. A. NMR lipid profiles of cells, tissues, and body fluids: proton NMR analysis of human erythrocyte lipids. J. Lipid Res. 35, 1925–1931 (1994).
Hammerschmidt, S., Wirtz, H. & Arnold, K. Lipid analysis of bronchoalveolar lavage fluid (BAL) by MALDI – TOF mass spectrometry and 31 P NMR spectroscopy. Chem. Phys. Lipids 112, 67–79 (2001).
Wang, C. et al. Plasma phospholipid metabolic profiling and biomarkers of type 2 diabetes mellitus based on high-performance liquid chromatography/electrospray mass spectrometry and multivariate statistical analysis. Anal. Chem. 77, 4108–4116 (2005).
Gowda, G. N. et al. Metabolomics-based methods for early disease diagnostics. Expert Rev. Mol. Diagn. 8, 617–633 (2008).
Teo, C. C. et al. Advances in sample preparation and analytical techniques for lipidomics study of clinical samples. TrAC - Trends Anal. Chem. 66, 1–18 (2015).
Fuchs, B. Mass spectrometry and inflammation — MS methods to study oxidation and enzyme-induced changes of phospholipids. Anal. Bioanal. Chem. 406, 1291–1306 (2014).
Wang, J. et al. Applications of Mass Spectrometry for Cellular Lipid Analysis. Mol. Biosyst. 34, 4358–4367 (2016).
Jelonek, K., Ros, M. & Pietrowska, M. Cancer biomarkers and mass spectrometry-based analyses of phospholipids in body fluids. Clin. Lipidol. 8, 137–150 (2013).
Soininen, P. et al. 1H NMR at 800 MHz facilitates detailed phospholipid follow-up during atherogenic modifications in low density lipoproteins. Biochem. Biophys. Res. Commun. 360, 290–294 (2007).
Mika, A. & Sledzinski, T. Alterations of specific lipid groups in serum of obese humans: a review. Obes. Rev. 18, 247–272 (2017).
Mika, A. et al. Obesity A Comprehensive Study of Serum Odd- and Branched-Chain Fatty Acids in Patients with Excess Weight. Obes. (Silver Spring) 24, 1669–1676 (2016).
Chaurasia, B. & Summers, S. A. Ceramides - Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol. Metab. 26, 538–550 (2015).
Choi, S. & Aj, S. Sphingolipids in High Fat Diet and Obesity Related Diseases. PubMed Commons. Mediators Inflamm. 2015, 26648664 (2015).
Ogretmen, B. Sphingolipids in cancer: Regulation of pathogenesis and therapy. FEBS Lett. 580, 5467–5476 (2006).
WHO 2015. WHO | Obesity and overweight. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/. (Accessed: 1st February 2016).
Levi, J., Vinter, S., St Laurent, R. & Segal, L. F as in Fat. How Obesity Threatens America’s Future 2012. Trust Am. Heal. (2012).
Wyleżo, M. et al. Polish recommendations for bariatric surgery. Wideochirurgia i inne Tech. małoinwazyjne/Videosurgery Other Miniinvasive Tech. Suppl. 4, 8 (2009).
Proczko-Markuszewska, M., Stefaniak, T., Kaska, L., Kobiela, J. & Sledziński, Z. Impact of Roux-en-Y gastric bypass on regulation of diabetes type 2 in morbidly obese patients. Surg. Endosc. 26, 2202–7 (2012).
Thodiyil, Paul, Rogula, Tomasz, Mattar, Samer, S. P. In Laparoscopic Bariatric Surgery. Inabnet W. B., Eric, J., Ikramuddin, S. Lippincott Williams & Wilkins 225–237 (2004).
Rogala Tomasz. In Prevention and Management of laparoendoscopic surgical complications (ed. Capella, R.F., Iannace, V.A., C. J.) 291–308 (Society of Laparoendoscopic Surgeons. Miami, Florida, 2005).
del Genio, G. et al. Morbid Obesity is Associated to Altered Fatty Acid Profile of Erythrocyte Membranes. J. Diabetes Metab. 6, 582 (2015).
Zeghari, N. et al. Adipocyte and erythrocyte plasma membrane phospholipid composition and hyperinsulinemia: a study in nondiabetic and diabetic obese women. Int. J. Obes. Relat. Metab. Disord. 24, 1600–7 (2000).
Elizondo, A. et al. Effects of weight loss on liver and erythrocyte polyunsaturated fatty acid pattern and oxidative stress status in obese patients with non-alcoholic fatty liver disease. Biol. Res. 41, 59–68 (2008).
Elizondo, A. et al. Polyunsaturated fatty acid pattern in liver and erythrocyte phospholipids from obese patients. Obesity (Silver Spring). 15, 24–31 (2007).
Forbes, R. et al. Essential Fatty Acid Plasma Profiles Following Gastric Bypass and Adjusted Gastric Banding Bariatric Surgeries. Obes. Surg. 26, 1237–1246 (2016).
Anjani, K. et al. Circulating phospholipid profiling identifies portal contribution to NASH signature in obesity. J. Hepatol. 62, 905–912 (2015).
Arora, T. et al. Roux-en-Y gastric bypass surgery induces early plasma metabolomic and lipidomic alterations in humans associated with diabetes remission. PLoS One 10, 1–10 (2015).
Choi, G. T. Y., Casut, M. & Gibbons, W. A. N. m. r. lipid profiles of cells, tissues and body fluids. Biochem. J. 296, 717–721 (1993).
Branca, R. T. & Warren, W. S. In vivo NMR detection of diet-induced changes in adipose tissue composition. J. Lipid Res. 52, 833–839 (2011).
Verma, S. K. et al. Differentiating brown and white adipose tissues by high-resolution diffusion NMR spectroscopy. J. Lipid Res. 58, 289–298 (2017).
Zhang, Y. et al. Associations between intensive diabetes therapy and NMR-determined lipoprotein subclass profiles in type 1 diabetes. J. Lipid Res. 57, 310–7 (2016).
Corso, G. et al. Effect of low density lipoprotein fatty acid composition on copper-induced peroxidation: 1H-nuclear magnetic resonance analysis. Clin. Chim. Acta. 258, 193–200 (1997).
Lodge, J. K., Sadler, P. J., Kus, M. L. & Winyard, P. G. Copper-induced LDL peroxidation investigated by 1H-NMR spectroscopy. Biochim. Biophys. Acta 1256, 130–40 (1995).
Kwon, H. N. et al. Prediction of glycated hemoglobin levels at 3 months after metabolic surgery based on the 7-day plasma metabolic profile. PLoS One 9, e109609 (2014).
Lopes, T. I. B. et al. ‘Omics’ Prospective Monitoring of Bariatric Surgery: Roux-En-Y Gastric Bypass Outcomes Using Mixed-Meal Tolerance Test and Time-Resolved 1H NMR-Based Metabolomics. Omi. A J. Integr. Biol. 20, 415–423 (2016).
Lopes, T. I. B. et al. Blood Metabolome Changes Before and After BariatricSurgery: A 1 H NMR-Based Clinical Investigation. Omi. A J. Integr. Biol. 19, 318–327 (2015).
Gralka, E. et al. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am. J. Clin. Nutr. 102, 1313–22 (2015).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Wu, Z., Shon, J. C. & Liu, K.-H. Mass Spectrometry-based Lipidomics and Its Application to Biomedical Research. J. lifestyle Med. 4, 17–33 (2014).
Soininen, P., Kangas, A. J., Würtz, P., Suna, T. & Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 8, 192–206 (2015).
Soininen, P. et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 134, 1781 (2009).
Wolthers, B. G. et al. Use of determinations of 7-lathosterol (5 alpha-cholest-7-en-3 beta-ol) and other cholesterol precursors in serum in the study and treatment of disturbances of sterol metabolism, particularly cerebrotendinous xanthomatosis. J. Lipid Res. 32, 603–12 (1991).
De Vuono, S. et al. Laparoscopic sleeve gastrectomy modifies cholesterol synthesis but not cholesterol absorption. Obes. Res. Clin. Pract. 11, 118–122 (2017).
Repetto, M., Semprine, J. & Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. J. Free Radic. Biol. Med. 1, 3–30 (2012).
Donovan, E. L., Pettine, S. M., Hickey, M. S., Hamilton, K. L. & Miller, B. F. Lipidomic analysis of human plasma reveals ether-linked lipids that are elevated in morbidly obese humans compared to lean. Diabetol. Metab. Syndr. 5, 24 (2013).
Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes. Obes. 18, 139–43 (2011).
Mattson, M. P. M. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp. Gerontol. 44, 625–633 (2009).
Sledzinski, T., Goyke, E., Smolenski, R. T., Sledzinski, Z. & Swierczynski, J. Decrease in serum protein carbonyl groups concentration and maintained hyperhomocysteinemia in patients undergoing bariatric surgery. Obes. Surg. 19, 321–326 (2009).
Kayser, B. D. et al. Serum lipidomics reveals early differential effects of gastric bypass compared with banding on phospholipids and sphingolipids independent of differences in weight loss. Int. J. Obes. https://doi.org/10.1038/ijo.2017.63 (2017).
Graessler, J. et al. Lipidomic profiling before and after Roux-en-Y gastric bypass in obese patients with diabetes. Pharmacogenomics J. 14, 201–7 (2014).
Mika, A. et al. Visceral and subcutaneous adipose tissue stearoyl-CoA desaturase-1 mRNA levels and fatty acid desaturation index positively correlate with BMI in morbidly obese women. Eur. J. Lipid Sci. Technol. 117, 926–932 (2015).
Sledzinski, T. et al. Identification of cyclopropaneoctanoic acid 2-hexyl in human adipose tissue and serum. Lipids 48, 839–48 (2013).
Acknowledgements
This study was supported by the Medical University of Gdansk (grants no. ST40, ST89), Ministry of Science and Higher Education of the Republic of Poland (DS 530-8727-D503-17, DS 530-8615-D690-17) and by the National Science Centre of Poland (grant no. NCN 2016/21/D/NZ5/00219).
Author information
Authors and Affiliations
Contributions
M.K., M.P.-S. and L.K. collected research material and analyzed clinical data; A.M., and T.S. designed research; A.M. and Z.K. performed research; A.M., T.S. P.S. analyzed data; and A.M. and T.S. wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mika, A., Kaczynski, Z., Stepnowski, P. et al. Potential Application of 1H NMR for Routine Serum Lipidome Analysis –Evaluation of Effects of Bariatric Surgery. Sci Rep 7, 15530 (2017). https://doi.org/10.1038/s41598-017-15346-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15346-0
This article is cited by
-
Plasma lipid metabolites as potential biomarkers for identifying individuals at risk of obesity-induced metabolic complications
Scientific Reports (2023)
-
Increased Amount of Polyunsaturated Fatty Acids in the Intestinal Contents of Patients with Morbid Obesity
Obesity Surgery (2023)
-
Metabolomic signatures after bariatric surgery – a systematic review
Reviews in Endocrine and Metabolic Disorders (2022)
-
La2Zr2O7 Doping Effects on MOD-SmBCO-Coated Conductors
Journal of Superconductivity and Novel Magnetism (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.