Abstract
Chronic exposure of pancreatic β-cells to high glucose levels results in β-cell dysfunction and death. These effects can be protected by estrogen. The local pancreatic renin-angiotensin system (RAS) has been shown as a novel pathological pathway of high-glucose-induced cell death. The effect of estrogen on pancreatic RAS is still unknown. This study examines whether estrogen protects against pancreatic β-cell death caused by glucotoxicity via a decrease in the pancreatic β-cell RAS pathway. When INS-1 cells were cultured in a high glucose medium, cell death was significantly higher than when the cells were cultured in a basal glucose medium; similarly, there were also higher levels of AGTR1 and p47 ph ° x mRNA, and protein expression. Moreover, the addition of 10−8 M 17β-estradiol to INS-1 cells cultured in a high glucose medium markedly reduced cell death, AGTR1 and p47 ph ° x mRNA levels, and protein expression. Similar results were demonstrated in the pancreatic islets. The presence of 10−8 M 17β-estradiol, losartan, or a combination of both, in a high glucose medium had similar levels of reduction of p47 ph ° x mRNA and protein expression, compared with those cultured in high glucose. Taken together, estrogen protected pancreatic β-cells from high-glucose-induced cell death by reducing the AGTR1 pathway.
Similar content being viewed by others
Introduction
Chronic exposure of pancreatic β-cells to high glucose levels causes cellular dysfunction; the resulting β-cell impairment reduces insulin production, thereby causing hyperglycemia1. Chronic hyperglycemia and impaired pancreatic β-cell function eventually lead to β-cell death2. This condition is known as glucotoxicity. The mechanisms that cause pancreatic β-cell glucose toxicity have not been fully elucidated; however, it has been hypothesized that oxidative stress is a central mechanism for glucose toxicity and pancreatic β-cell damage3. Oxidative stress is a condition that results from reactive oxygen species (ROS) generation4. ROS is produced by several pathways, including the mitochondrial electron transport system5, advanced glycation end-product formation, and glucose autoxidation6. The pancreatic β-cell renin-angiotensin system (RAS) is another pathway that induces ROS production through Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) complexes7. Culturing pancreatic β-cells under high glucose increased angiotensin II receptor (AGTR) mRNA levels and protein expression, which induced the formation of NADPH oxidase complexes7. Altogether, the evidence suggests that the pancreatic β-cell RAS plays a role in pancreatic β-cell apoptosis.
The local RAS has been shown to be involved in the pathophysiology of several organs, including the liver and pancreas, while the systemic RAS has been shown to control blood pressure and fluid homeostasis8. The pancreatic β-cell RAS component enzymes – including renin, angiotensinogen, and angiotensin converting enzyme (ACE) – are found in the pancreatic acinar and islet cells of both human and murine pancreatic islets9. AGTR1 and AGTR2 are expressed in different pancreatic islet cell types: AGTR1 is expressed by the pancreatic β-cells, while AGTR2 is expressed by the pancreatic α- and δ-cells. While the local acinar cell RAS regulates the exocrine function, the local islet cell RAS regulates glucose-induced insulin secretion7. RAS inhibitors, ACE inhibitors or angiotensin receptor blockers (ARB) prevent type 2 diabetes both in humans and animals10,11. ARB-mediated type 2 diabetes protection is supported by cell line experiments in which the AGTR1 blocker increased insulin secretion and proinsulin synthesis12.
Estrogen is a steroid hormone that plays an important role in the female reproductive system. Estrogen also regulates glucose homeostasis by improving insulin sensitivity, increasing glucose-stimulated insulin secretion, and increasing glucose transporter expression13. Additionally, estrogen replacement in post-menopausal women decreased type 2 diabetes risk14. In our previous study, we showed that estrogen treatment improved glucose-stimulated insulin secretion from mouse pancreatic islets that were cultured in a high glucose medium15. The role of estrogen on AGTR1 expression has been examined in several tissues. In ovariectomized rats, AGTR1 expression was increased in aortic tissue and cultured vascular smooth muscle cells16. Estrogen decreased the AGTR1 expression by inhibiting AGTR1 translation in the rat adrenal cortex while decreasing AGTR1 transcription in the pituitary gland17. Conversely, estrogen increased cardiac AGTR1 expression in ovariectomized rats18. Thus, the effects of estrogen on AGTR1 expression are cell type- or tissue-specific. However, the effects of estrogen on the pancreatic β-cell AGTR1 pathway are not known.
We hypothesized that high glucose enhances pancreatic AGTR1 expression, which in turn induces pancreatic β-cell apoptosis. Estrogen or an AGTR1 inhibitor might protect pancreatic β-cells from glucotoxicity by decreasing the pancreatic AGTR1 pathway. Therefore, the current study investigated the role of estrogen or an AGTR1 inhibitor on pancreatic β-cell apoptosis, AGTR1 and NADPH oxidase expression in pancreatic β-cells cultured under high glucose conditions.
Materials and Methods
INS-1 cell culture
INS-1 cells were cultured in RPMI 1640 containing 11.1 mM glucose, supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin, at 37 °C in humidified air containing 5% CO2. The medium was changed every 2 days.
Animals
The work using animals was approved by Siriraj Animal Care and Use Committee (SI-AUCC). Male ICR outbred 8–12 weeks mice were purchased from the National Laboratory Animal Center, Mahidol University, Bangkok, Thailand. Mice were kept at in a 12-h light/dark cycle environment at 25 ± 2 °C.
Mouse pancreatic islet isolation
Pancreatic islets were isolated by collagenase digestion by using a modified method of Lacy and Kostianovsky19, and Gotoh20. Briefly, pancreases were infused with collagenase-P and digested at 37 °C. Islets were separated by using histopaque gradient, and manually picked under a stereomicroscope. All methods were carried out in accordance with ACUC guidelines. The animal experimentation protocol was approved by the Institutional Animal Care and Use Committee, Faculty of Medicine Siriraj Hospital, Mahidol University (Approval No: SI-ACUP 002/2553).
Isolated islets were cultured for 24 hours and the medium was changed to basal or high glucose with or without 10−8 M 17β-estradiol for 10 days. Then, mRNA and protein were extracted to perform real-time RT-PCR and Western blot analysis.
Propidium iodide (PI) staining
INS-1 cells were plated into 6-well plates for 24 h. The medium was replaced with one containing either the basal glucose level (11.1 mM) or a high glucose level (40 mM), with or without 10−8 M 17β-estradiol, and cells were further incubated for 72 h. After incubation, the cells were trypsinized and washed. Ice-cold, 70% ethanol was then added to the cells and gently mixed by vortexing. The cells were kept at −20 °C for 1 h. After incubation, the cells were pelleted by centrifugation, and resuspended in 100 μl PBS with RNase. The cells were then transferred to flow cytometry tubes, and PI was added immediately before injecting the cells into a FACScan (Beckton Dickinson, USA) for flow cytometric analysis. The sub-G1 DNA content histogram was estimated.
Caspase 3 activity assay
INS-1 cells were cultured either in normal or high glucose RPMI 1640 media, with or without 10−8 M 17 β-estradiol, for 72 h; caspase 3 activity was determined using a caspase 3 colorimetric protease assay (Invitrogen, USA). The assay was performed following the manufacturer’s protocol. Briefly, cells were lysed to obtain protein. The protein concentrations were determined. Fifty μg of protein samples were added with 2X reaction buffer and DEVD-pNA substrate, and then incubated at 37 °C for 2 hours in the dark. After incubation, the caspase 3 activities were determined by spectrophotometer at a wavelength of 400 nm.
MTT assay
INS-1 cells were cultured either in normal or high glucose RPMI 1640 media, with or without 10−8 M 17 β-estradiol and/or 1 µM losartan, in a 96-well plate for 72 h. Cell viability was determined using a colorimetric MTT assay. In brief, 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide thiazolyl blue (MTT) was added to the cells, and incubated at 37 °C, 5% CO2, for 4 h. The medium was removed, and 0.1 N HCl acidic isopropanol was added to each well and mixed. The cells were then incubated at 37 °C, 5% CO2, for 1 h. Absorbance was measured at 570 and 650 nm, using a PowerWaveTM microplate scanning spectrophotometer (BIO-TEK, USA.) Cell viability was calculated from the average corrected 570 nm absorbance value using the following equation:
Measurement of intracellular superoxide generation
The superoxide production was detected by Nitroblue tetrazolium (NBT) assay. Briefly, INS-1 cells were cultured in 11.1 mM or 40 mM glucose, with or without 10−8 M 17 β-estradiol, for 72 h. After incubation, the cells were incubated with NBT for 90 minutes. The cells were then lysed in potassium hydroxide (KOH) and dissolved in DMSO. The amount of superoxide production was measured by optical density (OD) at a wavelength of 630 nm, using a PowerWave microplate scanning spectrophotometer (BIO-TEK, USA).
RNA isolation and real time-polymerase chain reaction (RT-PCR)
The total RNA was extracted from INS-1 cells using a High Pure RNA Isolation Kit (Roche Diagnostic Corporation, USA) according to the manufacturer’s instructions. The total RNA concentration was measured with a ND-1000 Spectrophotometer (Nanodrop, USA). The first strand complementary DNA (cDNA) was generated from 1 μg total RNA, using SuperScript III reverse transcriptase (RT) and random hexamer primers (Invitrogen, USA), and in accordance with the manufacturer’s instructions. The primers were synthesized by Sigma-Aldrich (Sigma-Aldrich, USA). Real-time PCR primers for AGTR1 and p47 phox were used, as described in a previous study12. The β-Actin primers were 5′-ATG AAG TGT GACGTTGACATCGTC-3′ and 5′-CCTAGAAGCATTTGCGGTGCACGATG-3′. A real-time PCR was performed to amplify specific cDNA sequences with the Brilliant® II SYBR® Green QPCR Master Mix (Agilent Technologies, USA). The PCR programs were 95 °C incubation for 10 min, 95 °C denaturation for 30 sec, 57 °C annealing for 15 sec, and 72 °C extension for 30 sec. The gene expression was calculated by the 2−∆∆Ct method, and was presented as fold change compared with the control.
Whole cell protein extraction
The total protein was extracted from INS-1 cells or mouse pancreatic islets that had been treated under experimental conditions for 48 h with a radioimmunoprecipitation assay (RIPA) buffer containing Halt Protease Inhibitor Cocktail (Pierce, USA). Briefly, cold RIPA buffer was added to the culture plate and shaken on ice for 5 minutes. Cell lysates were then transferred to Eppendorf tubes, and centrifuged to obtain whole cell proteins. The protein concentration was measured with a MicroBCATM protein reagent kit.
Western blot analysis
The total protein (50 µg) was loaded onto a polyacrylamide gel and separated by electrophoresis. The separated proteins were then transferred onto a nylon membrane with an electroblotting apparatus. After that, the membrane was blocked with 5% skimmed milk. The membrane was incubated with one of the following primary antibodies: rabbit polyclonal anti-AGTR1 (Santa Cruz Biotechnology, USA), goat polyclonal anti-p47 phox (Santa Cruz Biotechnology, USA), or mouse monoclonal anti-β-actin (Santa Cruz Biotechnology, USA), overnight at 4 °C. Then, the membrane was washed and incubated with HRP-conjugated secondary antibody (Santa Cruz Biotechnology, USA) at room temperature in the dark for 1 h. The protein bands were detected with SuperSignal Pico Chemiluminescence Luminol Substrate (Pierce, USA) and X-ray film exposure, followed by scanning and quantification with ImageJ software.
Statistical analysis
The data were analyzed with SPSS software, version 17 (SPSS Inc., USA), and expressed as the mean ± standard deviation (S.D). Differences between the groups were determined by one-way ANOVA followed by Tukey’s post-hoc test. A p-value of less than 0.05 was considered to be statistically significant.
Results
Estrogen reduced cell death of INS-1 cells cultured in high glucose medium
To investigate the effect of estrogen on INS-1 cell death, cells were cultured in basal or high glucose media, with or without 10−8 M 17β-estradiol. Dead cells were detected by propidium iodine (PI) staining, and were analyzed by flow cytometer. The total percentage of dead cells was significantly increased in INS-1 cells cultured in the high glucose medium compared to those cultured in the basal glucose medium. However, the presence of 10−8 M 17β-estradiol in the INS-1 cells cultured in the high glucose medium significantly reduced the percentage of cell deaths (Fig. 1A). In contrast, the addition of 10−8 M 17β-estradiol to the INS-1 cells cultured in the basal glucose medium showed no difference in the proportion of cell deaths compared to the proportion for the INS-1 cells cultured in the basal glucose medium alone.
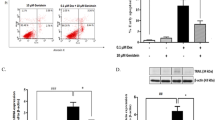
Cell death was measured using FACS to analyze PI staining and caspase 3 activity. (A) The percentage cell death in INS-1 cells cultured in experimental conditions. (B) Caspase 3 activity in INS-1 cells cultured in experimental conditions. The mean ± S.D. of 4 independent experiments are shown. *P < 0.05 and ***P < 0.001.
To confirm cell death, the caspase 3 activities were measured in INS-1 cells cultured in basal or high glucose media, with or without 10−8 M 17β-estradiol. As expected, the caspase 3 activity was significantly increased when the INS-1 cells were cultured in a high glucose medium compared to INS-1 cells cultured in a basal glucose medium. The addition of 10−8 M 17β-estradiol to the INS-1 cells cultured in a high glucose medium significantly reduced the caspase3 activity compared to when the INS-1 cells were cultured only in a high glucose medium (Fig. 1B). These results indicated that a high glucose medium increased β-cell death, which was ameliorated by the estrogen treatment.
Estrogen decreased AGTR1 mRNA and protein expression in INS-1 cells cultured in high glucose medium
We further examined the effect of estrogen on AGTR1 mRNA expression by real-time PCR. Our results showed that AGTR1 mRNA expression was significantly increased in INS-1 cells cultured in a high glucose medium compared with those cultured in a basal glucose medium (Fig. 2A). The addition of 10−8 M 17β-estradiol in INS-1 cells cultured in a high glucose medium significantly reduced AGTR1 mRNA expression, compared to those cultured in a high glucose medium alone. In contrast, 10−8 M 17β-estradiol supplementing INS-1 cells cultured in a basal glucose medium did not have any effect on AGTR1 mRNA levels, compared to those cultured in just a basal glucose medium. This finding suggests that estrogen down-regulates the AGTR1 mRNA expression in high glucose conditions.
AGTR1 mRNA and protein expression from INS-1 cells and mouse isolated pancreatic islets that were cultured in normal and high glucose. (A) Fold change of AGTR1 mRNA from INS-1 cells as normalized to β-actin mRNA at 48 hrs after treatment with high glucose media. (B) Above picture is a representative Western blot of AGTR1 and β-actin proteins from INS-1 cells. The bar graph below demonstrates fold change in AGTR1 protein normalized to β-actin protein. (C) Fold change of AGTR1 mRNA from isolated islets as normalized to β-actin mRNA at 10 days after treatment with high glucose media. (D) Above picture is a representative Western blot of AGTR1 and β-actin proteins from isolated islets. The bar graph below demonstrates fold change in AGTR1 protein normalized to β-actin protein. The data are presented as the mean ± S.D. of 3–4 independent experiments. *P < 0.05 and **P < 0.01. All full-length blots are presented in Supplementary Figure 1.
To determine whether estrogen also decreased AGTR1 protein levels, the AGTR1 protein levels from experimental conditions were determined by Western blot analysis. INS-1 cells cultured in a high glucose medium significantly increased AGTR1 protein expression (by approximately 3.53 fold), compared with those cultured in a basal glucose medium. However, a supplement of 10−8 M 17β-estradiol added to the INS-1 cells cultured in a high glucose medium significantly reduced the AGTR1 protein expression (Fig. 2B). On the other hand, there was no difference in the AGTR1 protein expression in INS-1 cells cultured in a basal glucose medium, with or without the addition of 10−8 M 17β-estradiol.
Estrogen reduced AGTR1 mRNA and protein expression in pancreatic β-cells cultured in high glucose medium
To verify whether estrogen attenuates AGTR1 expression in ex vivo, AGTR1 mRNA and protein expressions in pancreatic islets cultured in basal and high glucose media, and with or without 10−8 M 17β-estradiol, were assessed by real-time PCR and Western blot analysis. Similar to previous results in INS-1 cells, AGTR1 mRNA and protein expressions significantly increased in pancreatic islets cultured with a high glucose medium compared to the control. The presence of 10−8 M 17β-estradiol in pancreatic islets cultured with a high glucose medium markedly reduced the AGTR1 mRNA levels and protein expressions, compared to those cultured only in a high glucose medium. By comparison, there was no significant change in AGTR1 mRNA levels and protein expressions in pancreatic islets cultured in basal glucose media, with or without 10−8 M 17β-estradiol (Fig. 2C and D).
Estrogen, but not losartan, decreased AGTR1 protein expression in INS-1 cells cultured in high glucose medium with AngII
To examine whether AngII has an effect on AGTR1 expression in basal and high glucose media, AngII was added to INS-1 cells cultured in basal and high glucose media. The results indicated that AngII had no effect on the AT1R expression in the basal glucose medium, but AngII increased the AT1R expression in the high glucose medium (Fig. 3A). We further investigated whether estrogen reduced the AGTR 1 expression in the presence of AngII in a high glucose medium. Our results showed that AngII cultured with a high glucose medium significantly increased the AT1R expression. Estrogen cultured with a high glucose medium alone, or cultured with AngII in a high glucose medium, reduced the AGTR 1 expression (Fig. 3B). Lastly, we further examined whether losartan could reduce the AGTR 1 expression in the presence of AngII in a basal or a high glucose medium. The results showed that losartan did not decrease the AGTR 1 expression in the presence of AngII in either a basal or a high glucose medium (Fig. 3C). The addition of AngII in basal glucose had a trend to increase p47 phox expression when compared with basal glucose condition. AngII in high glucose has no additive effect on p47 phox expression when compared with high glucose alone. AngII showed a similar pattern of expression in both AGTR1 and p47 phox (Fig. 3D).
Effects of basal & high glucose media, Ang II and estrogen on AGTR1 protein expressions in INS-1 cells. (A) INS-1 cells were cultured with basal glucose medium, with or without 1 μM Ang II, for 72 h. Above picture is a representative Western blot of AGTR1 and β-actin proteins. The bar graph below demonstrates fold change in AGTR1 protein normalized to β-actin protein. (B) INS-1 cells were cultured in high glucose medium in the present or absent 1 μM Ang II, with or without 10−8 M 17β-estradiol, for 72 h. Above picture is a representative Western blot of AGTR1 and β-actin proteins. The bar graph below shows fold change in AGTR1 protein to control group, and normalized to β-actin protein. (C) INS-1 cells were cultured with basal glucose or high glucose medium, with or without 10−8 M 17β-estradiol, in the presence or absence of 10 μM losartan. Above picture is a representative Western blot of AGTR1 and β-actin proteins. The bar graph below shows fold change in AGTR1 protein to control group, and normalized to β-actin protein. (D) INS-1 cells were cultured with basal glucose or high glucose medium, with or without 1 μM Ang II. Above picture is a representative Western blot of p47phox and β-actin proteins. The bar graph below shows fold change in p47phox protein to control group, and normalized to β-actin protein. All data are expressed as mean ± S.D. of 3 independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001. NS is not significant. All full-length blots are presented in Supplementary Figure 3.
Estrogen decreased AGTR1 downstream signaling in INS-1 cells cultured in high glucose medium
We further examined the role of estrogen in AGTR1 downstream signaling by measuring a NADPH oxidase enzyme subunit, p47 phox, mRNA and protein expression. A high glucose medium significantly increased the p47 phox mRNA expression compared to those cultured in a basal glucose medium. As expected, 10−8 M 17β-estradiol co-cultured with a high glucose medium brought the p47 phox mRNA expression back to a similar level as in the control condition. There were no changes in the p47 phox mRNA expressions in the INS-1 cells cultured in a basal glucose medium either in the presence or the absence of 10−8 M 17β-estradiol (Fig. 4A). These results indicate that 10−8 M 17β-estradiol down-regulates p47 phox mRNA expression in high glucose conditions.
p47 phox mRNA and protein expression analysis and superoxide production. INS-1 cells were cultured in normal and high glucose media, with or without 10−8 M 17β-estradiol. (A) Fold change p47 phox mRNA normalized to β-actin mRNA. (B) Above picture is a representative Western blot of p47 phox and β-actin proteins. The bar graph below shows fold change in p47 phox protein to control group, and normalized to β-actin protein. (C) Superoxide production was measured using NBT assay. Amount of superoxide was determined by optical density (OD). The bar graph shows production of superoxide in INS-1 cells. The data are presented as the mean ± S.D. of 4 independent experiments. *P < 0.05 and **P < 0.01. All full-length blots are presented in Supplementary Figure 4.
To verify whether estrogen decreases p47 phox protein levels, the levels of p47 phox protein from INS-1 cells cultured in experimental conditions were determined by Western blot analysis. Similar to the mRNA expression, the p47 phox protein expression increased significantly in INS-1 cells that were cultured in high glucose conditions and was reduced markedly by estrogen treatment. There were no differences in the p47 phox protein expressions in INS-1 cells cultured in basal glucose conditions, with or without 10−8 M 17β-estradiol (Fig. 4B). These results confirm that estrogen reduced AGTR1 downstream mRNA levels and protein expressions in high glucose conditions.
Estrogen decreased oxidative stress in INS-1 cells cultured in high glucose medium
NADPH oxidase complexes produce superoxide as a downstream product of AGTR1 pathway activation. We examined whether estrogen reduced NADPH oxidase activity in INS-1 cells cultured in a high glucose medium. The superoxide anion in INS-1 cells cultured in each experimental condition was measured by an NBT assay. The superoxide production was increased significantly in the INS-1 cells cultured in a high glucose medium, compared with those cultured in a basal glucose medium. Estrogen added to INS-1 cells cultured in a high glucose medium lowered the superoxide production relative to the control level. In comparison, the addition of estrogen to INS-1 cells cultured in a basal glucose medium had no effect on superoxide production, relative to the control (Fig. 4C).
Estrogen and losartan increased cell viability in INS-1 cells cultured in high glucose medium
To investigate whether estrogen and the angiotensin II receptor blocker, losartan, have a similar effect on cell viability, the viability was assessed in INS-1 cells cultured in basal and high glucose media, with or without 10−8 M 17β-estradiol, 1 µM losartan, or a combination of both. Approximately 85% of the cells in the high glucose medium were viable compared with those that were cultured in a normal glucose medium. When, cells that were cultured in a high glucose medium were treated with 10−8 M 17β-estradiol, the percentage of viable cells increased significantly to 94%, which was similar to those that were treated with 1 µM losartan, or with a combination of both 10−8 M 17β-estradiol and 1 µM losartan. In contrast, the percentage of viable cells did not differ for INS-1 cells cultured in a basal glucose medium with 10−8 M 17β-estradiol, 1 µM losartan, or a combination of both (Fig. 5A). These results suggested that both estrogen and losartan increased cell survival to similar levels under high glucose conditions.
Cell viability and p47 phox mRNA and protein expression from INS-1 cells cultured in basal and high glucose media in the presence or absence of estrogen with or without losartan. (A) Cell viability was measured by MTT assay. Each condition was performed in triplicate for each experiment. (B) p47 phox mRNA and protein expression from INS-1 cells cultured in basal and high glucose media, with or without 10−8 M 17β-estradiol, or 1μM losartan alone, or in combination. (C) A. Fold change of p47 phox mRNA as normalized to β-actin mRNA. *P < 0.05 and **P < 0.01. (B) Representative Western blot analysis of p47 phox protein as normalized to β-actin protein. The data are presented as the mean ± S.D. of 3–4 independent experiments. *P < 0.05, **P < 0.01and ***P < 0.001. All full-length blots are presented in Supplementary Figure 5.
Estrogen and losartan decreased p47 phox mRNA and protein expression in INS-1 cells cultured in high glucose medium
To investigate whether estrogen and losartan act through the AGTR1 signaling pathway, p47 phox mRNA and protein expression were measured in INS-1 cells that were cultured in normal and high glucose media, with or without estrogen or losartan alone or in combination. The p47 phox mRNA expression was increased significantly under high glucose conditions, whereas treatment with estrogen, losartan, or both in combination reduced p47 phox mRNA expression significantly. To determine whether p47 phox protein levels were also reduced, Western blots were performed. It was determined that p47 phox protein levels decreased under high glucose conditions, similar to the mRNA levels. Treatment with estrogen, losartan, or a combination of both significantly reduced p47 phox protein expression, and to a similar extent. These results suggest that estrogen and losartan have no additive or synergistic effects to reduce p47 phox mRNA and protein expression (Fig. 5B and C). As well, the results indicate that estrogen and losartan ameliorate pancreatic β-cell death by reducing the AGTR1 pathway.
Discussion
Chronic hyperglycemia has been observed as a pathological cause of pancreatic β-cell death in type 2 diabetic patients, which is known as glucotoxicity3,21. The mechanism of glucotoxicity has been investigated over several decades. A number of studies have proposed that the central mechanism of glucotoxicity is the overproduction of oxidative stress in pancreatic β-cells3,4,6,21. This oxidative stress occurs due to the increased production of reactive oxygen species (ROS) or reactive nitrogen species (RNS)22. Local angiotensin II (Ang II) produces ROS by AGTR activation23. The components of RAS are found in both human and murine pancreatic islets24,25. The protective effect of estrogen against diabetes has been reported in several studies26,27,28,29. However, the effect of estrogen on local pancreatic RAS has not been studied.
This study showed that a high glucose medium increased pancreatic cell death, AGTR1 mRNA levels and protein expression. Our results were comparable to the findings from another study in which a high glucose medium induced AGTR1 mRNA and protein expression12. Local RAS has been proposed as a pathological process to induce pancreatic β-cell death via oxidative stress30. Our previous study showed that knock down AGTR1 reduced caspase 3 expression in a high glucose medium31, which suggested that high glucose might induce pancreatic β-cell apoptosis via the local pancreatic RAS. The local RAS has been demonstrated as a pathological process in diabetic complication32,33,34. For example, high glucose activated the RAS by increasing Ang II and AT1R expression, which causes damage to, and the loss of, podocytes in diabetic nephropathy35. RAS also plays an important role in the pathogenesis of atherosclerosis36.
It has been proposed that high glucose increases AngII production in rodent pancreatic β-cells37 and human islets38. Thus, it is possible that AngII might act as a local hormone and induce pancreatic β-cell death. Our previous study showed that AngII cannot increase the percentage of pancreatic β-cell apoptosis in basal glucose conditions, and AngII slightly increased the proportion of pancreatic β-cell apoptosis in high glucose conditions31. This suggested that AngII required upregulated AGTR1 expression to induce pancreatic β-cell death. This study examined this notion by adding AngII in experimental conditions. Our results showed that AngII did not increase AGTR1 expression in a basal glucose medium, but AngII significantly stimulated AGTR1 expression in high glucose conditions. On the other hand, estrogen significantly decreased AGTR1 expression in a high glucose medium, with or without AngII. This is evidence that AGTR1 expression plays an important role in inducing pancreatic β-cell apoptosis. This notion corresponded with the findings of several studies that a blocker of the pancreatic local RAS can prevent pancreatic β-cell death both in vitro and in vivo 10,11,39,40.
Estrogen in a high glucose medium significantly decreased pancreatic β-cell death and AGTR1 mRNA and protein expression in INS-1 cells. The effect of estrogen in a high glucose medium was also examined using mouse pancreatic islets. A similar result was shown: that estrogen reduced AGTR1 mRNA levels and protein expression in the mouse pancreatic islets. It is known that activated AGTR1 induces NADPH oxidase enzyme complexes, which consist of two membrane-bound subunits (gp91 phox and p22 phox), three cytosolic subunits (p40 phox, p47 phox and p67 phox), and a low molecular weight G-protein (Rac2 or Rac1)41. This study further examined the effect of high glucose and estrogen on p47 phox, an AGTR1 downstream protein. Our results showed that a high glucose medium increased p47 phox mRNA and protein expression, whereas estrogen co-cultured with high glucose brought p47 phox mRNA and protein expression back to levels similar to those in the control condition. Our results were comparable with those from other studies that reported an upregulation of NADPH oxidase subunits p47 phox, gp91 phox and p22 phox in diabetic animal rodents42. Altogether, estrogen seems to reduce both AGTR1 and its downstream signaling protein. Activated NADPH oxidase produces ROS in cells. To verify NADPH oxidase enzyme activity, the superoxide production in each experimental condition was measured. Again, a high glucose medium increased superoxide production, but the presence of estrogen with a high glucose medium reversed this effect. Estrogen-inhibited NADPH oxidase expression and activity has also been reported in endothelial and monocyte cells43,44. Although, this study did not measured AngII production in the medium, previous study showed that local RAS enzymes, renin, angiotensinogen and ACE, were up-regulated by high glucose9. Whether estrogen decreased local AngII production is still unknown. There is an in vivo study demonstrated that estrogen replacement in ovariectomized rat reduced plasma ACE and AngII levels45. Whether estrogen decreased local pancreatic AngII production is required further investigation.
The study compared the protective effects of estrogen and the AGTR1 inhibitor, losartan, on high-glucose-induced pancreatic β-cell death. Our results demonstrated that estrogen and losartan reduced pancreatic β-cell death and p47 phox expression. Estrogen and losartan did not have an additive effect to protect against high-glucose-induced pancreatic β-cell death. It is possible that estrogen and losartan might exploit similar pathways. The effect of estrogen on the inhibition of AGTR1 expression has been shown in several tissues, including vascular smooth muscle16, the heart18 and the adrenal glands17. However, it is worth noting that estrogen has been shown to reduce pancreatic β-cell apoptosis through several pathways, including an antioxidant effect18,29 and the reduction of ER stress26. Estrogen inhibited-AGTR1 expression might be another pathway by which estrogen prevents pancreatic β-cell apoptosis due to high glucose levels. For a future direction, these results should be verified in animal model of type 2 diabetes. The molecular mechanisms of estrogen on AT1R down-regulation should be explored, which should be tested for both genomic and non-genomic pathways. The better understanding of these mechanisms may lead to the identification of novel substances for treatment of diabetes.
In conclusion, we demonstrated that estrogen reduces high-glucose-induced pancreatic β-cell death through the reduction of the AGTR1 pathway both in cell line and rodent islets. The molecular mechanism of estrogen-inhibiting AGTR1 activation was not revealed in this study. Identification of the molecular mechanism by which estrogen decreases the AGTR1 pathway merits further investigation.
References
Donath, M. Y., Gross, D. J., Cerasi, E. & Kaiser, N. Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes 48, 738–744 (1999).
Butler, A. et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 52, 102–110 (2003).
Robertson, R. P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem 279, 42351–42354 (2004).
Evans, J. L., Goldfine, I. D., Maddux, B. A. & Grodsky, G. M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23, 599–622 (2002).
Andreyev, A., Kushnareva, Y. & Starkov, A. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc). 70, 200–214 (2005).
Robertson, R., Zhou, H., Zhang, T. & Harmon, J. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem Biophys. 48, 139–146 (2007).
Leung, P. S. The physiology of a local renin-angiotensin system in the pancreas. J Physiol. 580, 31–37 (2007).
Leung, P. & Carlsson, P. Tissue renin-angiotensin system: its expression, localization, regulation and potential role in the pancreas. J Mol Endocrinol. 26, 155–164 (2001).
Lau, T., Carlsson, P. O. & Leung, P. S. Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia. 47, 240–248 (2004).
Chu, K. Y., Lau, T., Carlsson, P. O. & Leung, P. S. Angiotensin II type 1 receptor blockade improves beta-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes 55, 367–374 (2006).
Andraws, R. & Brown, D. Effect of inhibition of the renin-angiotensin system on development of type 2 diabetes mellitus (meta-analysis of randomized trials). Am J Cardiol. 99, 1006–1012 (2007).
Leung, K. K. & Leung, P. S. Effects of hyperglycemia on angiotensin II receptor type 1 expression and insulin secretion in an INS-1E pancreatic beta-cell line. JOP 9, 290–299 (2008).
Riant, E. et al. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 150, 2109–2117 (2009).
Borissova, A. et al. Effect of hormone replacement therapy on insulin secretion and insulin sensitivity in postmenopausal diabetic women. Gynecol Endocrinol. 16, 67–74 (2002).
Kooptiwut, S., Semprasert, N. & Chearskul, S. Estrogen increases Glucose-Induced Insulin Secretion from Mouse Pancreatic Islets cultured in a Prolonged High Glucose Condition. J Med Assoc Thai. 90, 956–961 (2007).
Nickenig, G. et al. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation. 9, 197–201 (1998).
Wu, Z. et al. Estrogen regulates adrenal angiotensin AT1 receptors by modulating AT1 receptor translation. Endocrinology 144, 3251–3261 (2003).
Ricchiuti, V. et al. Estradiol increases angiotensin II type 1 receptor in hearts of ovariectomized rats. J Endocrinol. 200, 75–84 (2009).
Lacy, P. E. & Kostianovsky, M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 16, 35–39 (1967).
Gotoh, M., Maki, T., Kiyoizumi, T., Satomi, S. & Monaco, A. An improved method for isolation of mouse pancreatic islets. Transplantation. 40, 437–438 (1985).
Wu, L. et al. Oxidative stress is a mediator of glucose toxicity in insulin-secreting pancreatic islet cell lines. J Biol Chem 279, 12126–12134 (2004).
Evans, J., Goldfine, I., Maddux, B. & Grodsky, G. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 52, 1–8 (2003).
Tikellis, C., Cooper, M. E. & Thomas, M. C. Role of the renin-angiotensin system in the endocrine pancreas: implications for the development of diabetes. Int J Biochem Cell Biol 38, 737–751 (2006).
Lam, K. Y. & Leung, P. S. Regulation and expression of a renin-angiotensin system in human pancreas and pancreatic endocrine tumours. Eur J Endocrinol 146, 567–572 (2002).
Lau, T., Carlsson, P. O. & Leung, P. S. Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia 47, 240–248 (2004).
Kooptiwut, S. et al. Estrogen reduces endoplasmic reticulum stress to protect against glucotoxicity induced-pancreatic β-cell death. J Steroid Biochem Mol Biol. 139, 25–32 (2014).
Andersson, B. et al. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 82, 638–643 (1997).
Zhu, L. et al. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes. 62, 424–434 (2013).
Le May, C. et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 103, 9232–9237 (2006).
Leung, P. S. & Carlsson, P. O. Pancreatic islet renin angiotensin system: its novel roles in islet function and in diabetes mellitus. Pancreas. 30, 293–298 (2005).
Kooptiwut, S. et al. Testosterone reduces AT1R expression to prevent β-cell and islet apoptosis from glucotoxicity. J Endocrinol. 224, 215–224 (2015).
Wang, Z., Rao, P., Shillcutt, S. & Newman, W. Angiotensin II induces proliferation of human cerebral artery smooth muscle cells through a basic fibroblast growth factor (bFGF) dependent mechanism. Neurosci Lett. 373, 38–41 (2005).
Rajagopalan, S. et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 97, 1916–1923 (1996).
Papp, M., Li, X., Zhuang, J., Wang, R. & Uhal, B. Angiotensin receptor subtype AT(1) mediates alveolar epithelial cell apoptosis in response to ANG II. Am J Physiol Lung Cell Mol Physiol 282, L713–718 (2002).
Peng, H. et al. High glucose induces activation of the local renin-angiotensin system in glomerular endothelial cells. Mol Med Rep. 9, 450–456 (2014).
Nickenig, G. & Harrison, D. G. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: Part II: AT(1) receptor regulation. Circulation 105, 530–536 (2002).
Madec, A. et al. Losartan, an angiotensin II type 1 receptor blocker, protects human islets from glucotoxicity through the phospholipase C pathway. FASEB J. 27, 5122–5130 (2013).
Lupi, R. et al. The direct effects of the angiotensin-converting enzyme inhibitors, zofenoprilat and enalaprilat, on isolated human pancreatic islets. Eur J Endocrinol. 154, 355–361 (2006).
Hasegawa, G. et al. Telmisartan, an angiotensin II type 1 receptor blocker, prevents the development of diabetes in male Spontaneously Diabetic Torii rats. Eur J Pharmacol 605, 164–169 (2009).
Iwase, M. et al. Angiotensin II type 1 receptor antagonists prevent glucose-induced increases in islet blood flow in rats. Scand J Clin Lab Invest 69, 145–150 (2009).
Babior, B. NADPH oxidase. Curr Opin Immunol. 16, 42–47 (2004).
Lee, J., Kim, W., Yeo, J. & Jung, M. ER stress is implicated in mitochondrial dysfunction-induced apoptosis of pancreatic beta cells. Mol Cells. 30, 545–549 (2010).
Sumi, D. et al. 17beta-estradiol inhibits NADPH oxidase activity through the regulation of p47phox mRNA and protein expression in THP-1 cells. Biochim Biophys Acta. 1640, 113–118 (2003).
Wagner, A., Schroeter, M. & Hecker, M. 17beta-estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J. 15, 2121–2130 (2001).
Brosnihan, K., Senanayake, P., Li, P. & Ferrario, C. Bi-directional actions of estrogen on the renin-angiotensin system. Braz J Med Biol Res. 32, 373–381 (1999).
Acknowledgements
This work was supported by Siriraj Research Development Grant and Siriraj Chalearmprakiat Fund (to SK, NS, and PY), Siriraj Graduate Thesis Scholarship (to KW), and Thailand Research Fund (TRF IRG5980006) Grant to the Department of Research and Development. We also thank Pitchnischa Mahawong, Wanthanee Hanchang, Suchada Kaewin, Malika Churintaraphan and Samarn Onreabroi for their technical assistance.
Author information
Authors and Affiliations
Contributions
S.K. C.S. and P.Y. were responsible for the study concept and design and K.W., N.S. and S.K. for acquisition of data. K.W., N.S. and S.K. were responsible for analysis and interpretation of experimental data. S.K. drafted the manuscript. P.Y. C.S. and S.K. critically revised the manuscript for important intellectual content. S.K. was responsible for the study supervision. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kooptiwut, S., Wanchai, K., Semprasert, N. et al. Estrogen attenuates AGTR1 expression to reduce pancreatic β-cell death from high glucose. Sci Rep 7, 16639 (2017). https://doi.org/10.1038/s41598-017-15237-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15237-4
This article is cited by
-
Cotton rat (Sigmodon hispidus) develops metabolic disorders associated with visceral adipose inflammation and fatty pancreas without obesity
Cell and Tissue Research (2019)
-
Attenuating effect of silibinin on palmitic acid-induced apoptosis and mitochondrial dysfunction in pancreatic β-cells is mediated by estrogen receptor alpha
Molecular and Cellular Biochemistry (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.