Abstract
Radix puerariae, a traditional Chinese herbal medication, has been used to treat patients with diabetic nephropathy (DN). Several studies demonstrated that puerarin, the active compound of radix puerariae, reduces diabetic injury in streptozotocin (STZ)-induced diabetic rodent models. However, as STZ injection alone results in mild kidney injury, the therapeutic benefit afforded by puerarin in DN remained inconclusive. Thus we sought to clarify the role of puerarin by employing an accelerated DN model, STZ-induced diabetes in the endothelial nitric oxide synthase-null (eNOS−/−) mice. Puerarin treatment of diabetic eNOS−/− mice significantly attenuated albuminuria and diabetic kidney injury, which were associated with reduced oxidative stress and reduced NAPDH oxidase 4 (NOX4) in glomeruli of diabetic eNOS−/− mice. Puerarin treatment of murine podocytes culture in high glucose conditions led to reduced superoxide production and NOX4 expression. We further determined that that puerarin treatment increased both mRNA and protein levels of SIRT1 in podocytes and that puerarin led to SIRT1-mediated deacetylation of NF-κB and suppression of NOX4 expression. Our findings confirm the renoprotective effects of puerarin in an experimental model of advanced DN and provide a molecular mechanism by which puerarin exerts the anti-oxidative effects in podocytes in the diabetic milieu.
Similar content being viewed by others
Introduction
Diabetic nephropathy (DN) remains the most common cause of end-stage renal disease (ESRD) in US and worldwide1,2. The incidence of DN has also dramatically increased in China in the recent years due to the increasing prevalence of diabetes, and DN may soon become the leading cause of ESRD in China3,4. The treatment of DN has been limited to hyperglycemic control, blood pressure control, and renin-angiotensin system blockade. However, many patients on angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockades (ARB) continue to progress to ESRD5. Moreover, new clinical trials in patients with DN have either failed to show efficacy or were prematurely terminated due to significant side effects6,7, such that there has not been a new FDA-approved therapy against DKD progression for the past 20 years. Therefore, there is a large unmet need to develop potent and safer therapies against DN.
The early stage of DN is clinically defined by the appearance of persistent microalbuminuria8,9. Podocyte injury is a major contributor to the development of microalbuminuria in DN10, and the reduction in podocyte density is the strongest predictor of progressive DN11,12. Apoptosis and detachment from the glomerular basement membrane have been proposed as potential mechanisms of podocyte loss13, and oxidative stress is considered to be a major culprit in podocyte injury in DN14. Among the different NADPH oxidase (NOX) isoforms, NOX4 was shown to be the main enzyme contributing to the increased oxidative stress in podocytes, as either the genetic ablation of Nox4 or pharmacological inhibition of its activity was shown to attenuate DN in a rodent model of diabetes15. Further, podocyte-specific deletion of NOX4 was also shown to mitigate diabetes-induced podocyte injury and to confer renoprotection in DN16.
Traditional Chinese herbal medications have been used widely in China to treat patients with DN. However, their mechanisms of renoprotection in DN remain unclear. We previously reported that Chen’s Tangshen decoction, in which puerarin is a major component, is able to significantly reduce microalbuminuria in patients with early DN17. A recent meta-analysis suggests that treatment of DN patients with puerarin induces a further reduction of albuminuria in combination with ACEI18. We and others have also shown that puerarin improve albuminuria in rodent models of streptozotocin (STZ)-induced diabetes19,20,21, although the extent of kidney injury was relatively mild in these models to clearly delineate the renoprotective effects of puerarin. Several studies have also shown both in vitro and in vivo that puerarin exhibited an anti-oxidative activity19,20,22,23, and that it attenuated apoptosis of proximal tubular cells through the restoration of mitochondrial function24,25. However the detailed mechanisms by which puerarin exerts renoprotection in DN had not been explored. Here, we sought to confirm the renoprotective effects of puerarin in an accelerated DN mouse model and to explore the mechanism by which puerarin attenuates oxidative stress and podocyte injury under diabetic conditions.
Results
Puerarin treatment attenuates proteinuria and kidney injury in the diabetic eNOS−/− mice
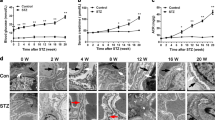
Diabetes was induced in 6-week old eNOS−/− mice using low-dose streptozotocin as described previously26, and age-matched citrate buffer-injected eNOS−/− mice served as controls. Both diabetic and control mice were treated with either puerarin (20 mg/kg of body weight) or vehicle starting at 10 weeks post- STZ injection when a significant albuminuria is present in diabetic mice. All mice were sacrificed at the age of 18 weeks for assessment of renal function and histopathology. As shown in the Table 1, there were no significant differences in blood glucose between mice treated with puerarin or vehicle in either non-diabetic controls or in diabetic mice. The kidney-to-body weight ratio increased in diabetic eNOS−/− mice in comparison to control eNOS−/− mice, but this increase was significantly attenuated in puerarin-treated diabetic eNOS−/− mice (Table 2).
As expected, urinary albumin to creatinine ratio (UACR) increased nearly 10-fold in diabetic eNOS−/− mice compared to control eNOS−/− mice (Fig. 1A). However, administration of puerarin significantly reduced the UACR and halted the further worsening of albuminuria over time in diabetic eNOS−/− mice (Fig. 1A). Histologically, we also observed a significant attenuation of glomerular hypertrophy and mesangial expansion in puerarin-treated diabetic mice in comparison to vehicle-treated diabetic mice (Fig. 1B,C), indicating that puerarin treatment reduced the glomerular injury in diabetic eNOS−/− mice.
Puerarin reduced albuminuria and kidney injury in STZ-induced diabetic eNOS knockout mice. (A) Both diabetic and non-diabetic mice were treated with either vehicle or puerarin when mice developed albuminuria for a total of 9 weeks. Urine samples were collected weekly after starting the treatments for determination of urinary albumin/creatinine ratio. Significant differences were observed starting at 2 weeks after puerarin treatment compared with vehicle-treated diabetic mice. *p < 0.05 and *p < 0.001 compared to vehicle-treated diabetic mice, n = 6. (B) Representative image of periodic acid-Schiff (PAS) stained kidneys. Scale bar: 20 μm. (C,D) Morphometric analysis was performed to determine the glomerular area (C) and % of mesangial area (D) in the glomerular cross sections. **p < 0.01 and ***p < 0.001, n = 6.
Puerarin reduces oxidative stress in diabetic mice
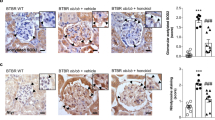
We next determined the effects of puerarin on the extent of oxidative stress in the diabetic glomeruli. Immunohistochemical staining showed a strong upregulation of nitrotyrosine expression in the glomeruli of vehicle-treated diabetic mice, which was not observed in nondiabetic control kidneys (Fig. 2A). Puerarin treatment led to a marked decrease of nitrotyrosine staining in the diabetic mouse kidneys (Fig. 2A). The attenuation of oxidative stress by puerarin was further confirmed by immunofluorescence staining of 8-oxoguanine (8-oxoG), a common DNA lesion resulting from oxidative stress, with co-staining of WT-1, a podocyte marker (Fig. 2B). A large extent of 8-oxoG-positive cells was also WT-1-positive, confirming an increase of oxidative stress in podocytes in diabetic milieu. We did note however that 8-oxoG-positive cells were not limited to podocytes, but were present in other glomerular cells in the diabetic eNOS−/− kidneys. Puerarin treatment significantly decreased the number of 8-oxoG-positive cells, suggesting that puerarin improves DN through the inhibition of oxidative stress. Figure 2C shows the quantification of the 8-oxoG and WT-1 staining in the nondiabetic and diabetic kidneys.
Puerarin improved oxidative stress in diabetic glomeruli. (A) Representative image of nitro-tyrosine immunostaining in paraffin-embedded kidney sections. Scale bar: 100 μm. (B) Representative images of 8-oxoG (green) and podocyte marker WT1 (red) immunofluorescence. DAPI was used as a counterstain. Scale bar: 20 μm. (C) Quantification of 8-oxoG immunofluorescence are shown. ***p < 0.001, n = 6 mice.
Because NOX4 is a main contributor of reactive oxygen species in the diabetic kidneys15,16, we next determined the mRNA and protein levels of NOX4 in the glomeruli of diabetic and nondiabetic mice treated with puerarin or vehicle. We found that NOX4 expression was indeed increased in vehicle-treated diabetic mice, but suppressed in puerarin-treated diabetic mice (Fig. 3A–C), indicating that puerarin attenuates oxidative stress through the inhibition of NOX4 expression.
Treatment of puerarin reduced expression of NOX4 in the glomeruli of diabetic mice. (A) Real-time PCR analysis of NOX4 mRNA levels from isolated glomeruli. **p < 0.01 and ***p < 0.001, n = 6 mice. (B) Representative western blot analyses showing NOX4 expression in glomerular lysates. (C) Quantification of NOX4 protein expression normalized to GAPDH. *p < 0.05, n = 6 mice.
Puerarin reduces oxidative stress in conditionally immortalized murine podocytes
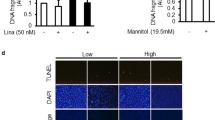
In order to confirm the above in vivo findings, we tested in vitro whether puerarin can directly reduce the superoxide production in podocytes cultured under the high glucose condition. Reactive oxygen species were detected using the cell permeant 2′, 7′ –dichlorofluorescin diacetate (DCFDA), a fluorogenic dye. Detection of oxidized 2′, 7′ –dichlorofluorescein (DCF) showed that puerarin treatment reduced the superoxide production in podocytes treated with high glucose (Fig. 4A,B). Furthermore, we found that puerarin suppressed NOX4 expression at both mRNA and protein levels (Fig. 4C–E). Together, these data suggest that puerarin inhibits NOX-dependent superoxide generation in podocytes cultured in high glucose condition.
Puerarin inhibited oxidative stress in murine podocytes cultured in high glucose. (A,B) Immortalized mouse podocytes were cultured in either normal glucose (NG: 5 mM glucose + 25 mM mannitol) or high glucose (HG: 30 mM glucose) treated with DMSO vehicle or 10 μM puerarin for 24 hours. Superoxide production was determined by incubation of cells with dichlorofluorescin diacetate (DCFDA) fluorogenic dye and by detection of oxidized 2′, 7′-dichlorofluorescein (DCF). Puerarin reduced superoxide generation in podocytes cultured in high glucose media. Representative image is shown in (A) and ratio of DCF+ cells/total (DAPI+) cells per field are shown in (B) (***p < 0.001, n = 3, 7 fields per group). (C) Real-time PCR analysis of NOX4 mRNA levels in podocytes cultured with NG or HG treated with vehicle or 10 μM puerarin for 4 hours. GAPDH mRNA was used as an internal control. ***p < 0.001, n = 3. (D) Representative western blot analyses of NOX4 expression in podocytes cultured in NG or HG conditions for 24 hours. The representative blots of three individual experiments are shown here. (E) Quantification of NOX4 protein expression normalized to GAPDH is shown. **p < 0.01 and ***p < 0.001, n = 3.
Puerarin regulates NOX4 expression through SIRT1-NF-κB pathway in podocytes
We previously found that the expression of NAD+-dependent histone deacetylase SIRT1 is reduced in human DN glomeruli27. Interestingly, we observed that the high-glucose-mediated suppression of SIRT1 protein expression in podocytes was alleviated by puerarin treatment at both low and high doses (1 and 10 μM, respectively) (Fig. 5A,B). Moreover, SIRT1 expression was inversely correlated with NOX4 expression (Fig. 5A,B), suggesting that SIRT1 negatively regulates NOX4 expression. Our earlier work showed that the activity of NF-κB is increased in podocytes in DN and that SIRT1 inhibits NF-κB activity through deacetylation28. We also observed that puerarin treatment led to the decreased NF-κB acetylation, likely through the stimulation of SIRT1 expression (Fig. 5A,B). In support of this, overexpression of SIRT1 in podocytes attenuated both NF-κB acetylation and NOX4 expression (Fig. 5C,D). Promoter analysis of NOX4 confirmed the presence of putative NF-κB binding sites, and others have shown in non-renal cells that NF-κB directly binds to the NOX4 promoter and regulates NOX4 expression29. Our previous studies indicated that NF-κB mutant with a lysine to arginine mutation of its acetylation site at Lys310 (p65K310R) serves as a dominant negative to block the activity of endogenous NF-κB28. We found that the overexpression of p65K310R mutant (K310R) in podocytes led to the reduced NOX4 expression as compared to overexpression of wildtype p65 (WT) (Fig. 6A,B), strongly suggesting that NF-κB acetylation is required for NOX4 expression and that SIRT1 suppresses NOX4 expression through deacetylation of NF-κB. We further confirmed that the overexpression of SIRT1 exhibited similar inhibition of superoxide production in podocytes as puerarin (Fig. 6C). Lastly, we found that puerarin also increased SIRT1 mRNA levels in podocytes, suggesting that puerarin stimulates SIRT1 expression at the transcriptional level (Fig. 6D).
Puerarin downregulated NOX4 expression through activation of SIRT1 in murine podocytes exposed to high glucose. (A,B) Murine podocytes cultured in NG or HG were treated with different amounts of puerarin as indicated. The cells were lysed for western blot analysis for SIRT1, NOX4, acetyl-p65, total-p65, and β-actin. Representative blots of three independent experiments are shown in A, and quantification is shown in (B). (C,D) Podocytes transfected with SIRT1 overexpression vector or control vector were cultured in NG and HG for 24 hours. The cells were lysed for western blot analysis for SIRT1, NOX4, acetyl-p65, total-p65, and β-actin. Representative blots of three independent experiments are shown in (C), and quantification is shown in (D).
Puerarin downregulated NOX4 expression through deacetylation of p65NF-κB in murine podocytes exposed to high glucose. (A,B) Podocytes transfected with either wildtype p65 (WT), p65 with mutation of K310 acetyl-residue (K310R) or control empty vector (EV) were treated with TNF-α for 24 hours. Cells were lysed for western blot analysis for p65, NOX4 and β-actin. Representative blots of three independent experiments are shown in A, and quantification is shown in (B). (C) Podocytes cultured in NG or HG were treated with 10 μM puerarin or 1 μM SIRT1 agonist SRT1720 for 24 hours. Superoxide production was then measured by the DCF fluorescence method. *p < 0.05 compared between groups n = 3. (D) Real-time PCR analysis of Sirt1 mRNA in podocytes treated with vehicle or puerarin in NG or HG conditions show increased Sirt1 expression by puerarin. *p < 0.05 compared between groups n = 3.
Discussion
In the current study, we confirmed that puerarin mitigates albuminuria and kidney injury in STZ-induced diabetic mice with eNOS-deficiency, a model which better mimics the advanced human DN. In addition, we showed that the anti-oxidative effect of puerarin is mediated through the suppression of NOX4 expression in diabetic kidney in vivo and in cultured murine podocytes in vitro. We also demonstrated that puerarin suppressed NOX4 expression through the upregulation of SIRT1, resulting in increased deacetylation of NF-κB, thus uncovering a new molecular mechanism by which puerarin exerts renoprotection in kidney cells under diabetic conditions. Lastly, we have shown that puerarin stimulates SIRT1 expression at the transcriptional level.
Puerarin is a major isoflavonoid component from the root of pueraria candollei of Leguminosae family. Puerarin has a structure of 7-hydroxy-3-(4-hydroxyphenyl)-1-benzopyran-4-one-8-β-D-glucopyranoside30. Although large randomized clinical trials are lacking, several small clinical studies suggest that puerarin treatment significantly reduced albuminuria in patients with the stage 3 DN18. The renoprotective effects of puerarin have been also reported in several animal studies19,20,21. However, these studies were reported in STZ-induced diabetic rodent models with very mild kidney injury. In this study, we took advantage of the accelerated DN in absence of eNOS in STZ-induced diabetic eNOS−/− mice31, which is a better-suited DN model to mimic the human diabetic kidney disease, to assess the effects of puerarin in DN progression. Using this advanced DN model, our results indeed confirm that the puerarin treatment commencing after the establishment of DN by detection of albuminuria significantly halted the progression of DN in diabetic eNOS−/− mice. These results are consistent with previous clinical findings18, and strongly suggest the potential therapeutic benefits of puerarin treatments in DN patients.
Anti-oxidative activity of puerarin has been demonstrated in cardiovascular32 and neurological diseases33. Several studies also suggest that puerarin has anti-oxidative effects in kidney cells19,20,22,23. However, the mechanism by which puerarin exerts these effects remained unclear. Here, we report that the anti-oxidative effect of puerarin to confer renoprotection is in part mediated through the suppression of NOX4 expression in podocytes. Previous studies have shown that NOX4 expression is increased in diabetic kidneys and that podocyte-specific knockout of NOX4 attenuates DN15,16, underscoring the importance of NOX4 regulation in DN. In addition to NOX4 suppression, our results demonstrated that puerarin upregulates SIRT1 expression. The role of SIRT1 in DN has been well documented28,34,35. We have previously demonstrated the anti-inflammatory role of SIRT1 via deacetylation of NF-κB in DN28. Our data now suggest that puerarin decreases NOX4 expression through the inhibition of NF-κB activity by SIRT1 upregulation. Our promoter analysis further confirmed that NOX4 has binding sites for NF-κB. Consistent with our data, previous studies suggest that NF-κB directly mediates NOX4 expression36,37.
In line with these findings, recent studies reported that SIRT1 expression is increased in the diabetic kidney from mice treated with puerarin20. However, it remained unclear how puerarin regulates SIRT1 in the kidney cells. Here, we found that puerarin can upregulate both mRNA and protein levels of SIRT1 in podocytes, suggesting a transcriptional regulation of SIRT1. Since SIRT1 regulates multiple biological pathways such as aging,38, metabolism39, cancer and inflammation40, we believe that puerarin and its analogues could have a broader application in its therapeutic usage. Moreover, the renoprotective effects of puerarin may not be limited to anti-oxidative effects. It has been shown that puerarin can attenuate the apoptosis of proximal tubular cells through the restoration of mitochondrial function25,41. We have previously shown that puerarin improves DN through regulation of metalloproteinase 9 (MMP9) in podocytes19.
In summary, we confirmed the renoprotective effects of puerarin treatment in a mouse model with established DN. We demonstrated that puerarin exerts anti-oxidative effects through the activation of SIRT1-mediated NF-κB deacetylation and that puerarin is a stimulator of SIRT1 expression in podocytes. Our study suggests that puerarin may be a potential drug to treat patients with DN. However, future clinical studies are required to confirm the therapeutic effects of puerarin in patients with DN.
Methods
Animal studies
8-week old eNOS-null male mice on a C57BL/6 background were purchased from The Jackson Laboratory (Bar Habor, ME). Diabetes was induced by intraperitoneal injection of freshly prepared streptozotocin (STZ) (Sigma-Aldrich, Saint Louis, MO) dissolved in 0.1 M citrate buffer, pH 4.5 at 50 mg/kg after 4–6 hours of food withdrawal for 5 consecutive days. Control mice were injected with sodium citrate buffer. Blood glucose was measured every week. 2 weeks after diabetes was confirmed in mice (blood glucose > 250 mg/dl), mice were given puerarin (Sigma-Aldrich, Saint Louis MO) dissolved in 5% DMSO by oral gavage at a dose of 20 mg/kg body weight/day, or 5% DMSO vehicle as control, for 8 weeks. Urine samples were collected every week until they were sacrificed. All experimental methods were performed in accordance with the approved guidelines as described in the Guide for the Care and Use of Laboratory Animals42. All animal studies were approved by the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai, New York, NY.
Urine albumin and creatinine measurement
Urine albumin was measured using an ELISA kit (Bethyl Laboratory, Houston, TX), and urine creatinine was measured using a colorimetric assay kit (Cayman, Ann Arbor, MI).
Kidney histology
Kidney samples were fixed in 10% formalin at room temperature for 16 hours and embedded in paraffin. Tissues were cut into 4 μm thick sections for periodic acid–Schiff (PAS) staining. Glomerular area and percentage of glomerular area were calculated as described (2).
Immunohistochemistry and immunofluorescence staining
Paraffin-embedded kidney sections were deparaffinized and rehydrated for immunostaining. Specific antibodies used in this study are as following: anti-nitro-tyrosine from Santa Cruz (SC-32757) at 1:100 dilution, anti-8-oxo-G from Japan Institute for the Control of Aging (N45.1) at 1:50 dilution, and anti-WT-1 antibody was from Santa Cruz at 1:50 dilution. The secondary and tertiary antibodies were obtained from Jackson Immunoresearch, Inc. and used at 1:200 dilution.
Glomerular RNA isolation
Mice glomeruli were isolated as previously reported (1). Total RNA was extracted from glomeruli using Trizol method. Purified RNA underwent DNA digestion using RNase-free DNase set (79254, Qiagen, Germantown, MD).
Cell culture
Conditionally immortalized murine podocytes were obtained from Dr. Peter Mundel and cultured at 37 °C for 7 days for full differentiation before being used as described43.
Reactive oxygen species measurement
Differentiated podocytes cultured with normal glucose (glucose 5.5 mM, supplemented with 24.5 mM mannitol as high osmolarity control) and high glucose (30 mM) were treated with 5% DMSO or puerarin for 24 hours. Cells were then incubated with 20 μm of DCFDA (Abcam, Cambridge, MA) for 30 min at 37 °C. Cells were viewed and photographed with a fluorescence microscopy with excitation and emission spectra of 495 nm and 529 nm respectively. DAPI was used for visualization of nuclear or phase-contrast pictures to visualize cells.
NADPH oxidase activity measurement
The lucigenin-enhanced chemiluminescence method was used to monitor superoxide production as total Nox activity. Podocytes were washed twice with HBSS (CaCl2-2H2O 1.25 mM, KCl 5.4 mM, KH2PO4 0.5 mM, MgCL2-6H2O 0.5 mM, MgSO4-7H2O 0.7 mM, NaCl 0.136 mM, NaHCO3 4 mM, Na2HPO4 0.4 mM, D-Glucose 5.5 mM, pH 7.2) and the culture plate was snap-frozen. Cells were scraped with ice-cold HBSS containing protease inhibitor cocktail. Cell suspensions were homogenized with 100 strkes in a Dounce homozinizer on ice. Protein content was quantified. 30ug of proteins were transferred to each well followed by adding 25uM Lucigenin (Santa Cruz, Lake Forest, CA) and 200 uM NADPH (Cayman Chemical, Ann Arbor, MI). After 10 minutes at 37 C for dark adaptation, the light emission was recorded. The NOX activity was expressed as relative light units per milligram of cellular protein in cells.
Podocyte transfection
Podocytes were transfected with 5 μg of empty vector or corresponding vectors as indicated in the figures using Viafect reagent (Promega, E4981; Madison, WI) according to the manufacturer’s protocol.
Western blot
Cells were lysed in M-PER mammalian protein extraction reagent (ThermoFisher, Waltham, MA) containing protease and phophastase inhibitor cocktail. Protein was separated on SDS-PAGE and transferred to PVDF membranes. Proteins were detected using specific antibodies: phospho-p65 (1:1000): Abcam, ab28856; total p65 (1:1000): Cell Signaling, 4764; Nox4 (1:2000): ab133303; GAPDH (1:2000): Cell Signaling 2118; beta-Actin (1:3000): Sigma A5136. The secondary antibodies were obtained from Jackson Immunoresearch Inc. and used at 1:10,000. Images of whole blots are included in the Supplementary Figure.
Real time PCR
RNA transcript quantification was performed by 7500 Real-Time PCR System. Gene level was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and expressed as fold change. The primer sets used are as follows: SIRT1 (5′-CACTGTAACTGGGGGCAACT-3′ and 5′-CACTTCTTGTCAGCGTCGAA-3′), Nephrin (5′-CAGCCTCTTGACCATCGCTAA-3′ and 5′-TGGTGGCCGTGCATTTG-3′), Podocin (5′-TGGAAGCTGAGGCACAAAGA-3′ and 5′-CCCCTTCGGCAGCAATC-3′), and GAPDH (5′-GGCATTGCTCTCAATGACAA-3′ and 5′-TGTGAGGGAGATGCTCAGTG-3′).
Statistical analysis
Data are expressed as mean ± SEM. The unpaired t-test was used to comparison between groups or ANOVA followed by Bonferroni correction was used when comparing between groups for treatment conditions using the GraphPad Prism software. P-value < 0.05 was considered statistically significant.
Change history
06 March 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
22 December 2017
A correction to this article has been published and is linked from the HTML version of this paper. The error has been fixed in the paper.
References
USRDS. Annual Data Report: Atlas of End-Stage-Renal-Disease in the United States. (2011).
World Health Organization. Global status report on noncommunicable diseases. volumes (World Health Organization, Geneva, Switzerland).
Zhang, L. et al. Trends in Chronic Kidney Disease in China. The New England journal of medicine 375, 905–906 (2016).
Yang, W. et al. Prevalence of diabetes among men and women in China. The New England journal of medicine 362, 1090–1101 (2010).
de Zeeuw, D. Unmet need in renal protection–do we need a more comprehensive approach? Contrib Nephrol 171, 157–160 (2011).
de Zeeuw, D. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. The New England journal of medicine 369, 2492–2503 (2013).
Packham, D. K. et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol 23, 123–130 (2012).
Parving, H. H., Chaturvedi, N., Viberti, G. & Mogensen, C. E. Does microalbuminuria predict diabetic nephropathy? Diabetes Care 25, 406–407 (2002).
Rossing, P., Hommel, E., Smidt, U. M. & Parving, H. H. Impact of arterial blood pressure and albuminuria on the progression of diabetic nephropathy in IDDM patients. Diabetes 42, 715–719 (1993).
Ijpelaar, D. H. et al. Glomerular hypertrophy precedes albuminuria and segmental loss of podoplanin in podocytes in Munich-Wistar-Fromter rats. Am J Physiol Renal Physiol 294, F758–767 (2008).
Meyer, T. W., Bennett, P. H. & Nelson, R. G. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42, 1341–1344 (1999).
Steffes, M. W., Schmidt, D., McCrery, R. & Basgen, J. M. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 59, 2104–2113 (2001).
Wolf, G., Chen, S. & Ziyadeh, F. N. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54, 1626–1634 (2005).
Susztak, K. et al. Molecular profiling of diabetic mouse kidney reveals novel genes linked to glomerular disease. Diabetes 53, 784–794 (2004).
Jha, J.C. et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 25, 1237–1254 (2014).
Jha, J. C. et al. Podocyte-specific Nox4 deletion affords renoprotection in a mouse model of diabetic nephropathy. Diabetologia 59, 379–389 (2016).
lian, S. et al. Clinical investigation of stage-IV diabetic nephropathy with treatment of Chen’s Tangshen decoction and conventional Western medicine. Shanghai Jouranl of Traditional Chinese Medicine 44, 3 (2010).
Wang, B. et al. The therapeutic effect and possible harm of puerarin for treatment of stage III diabetic nephropathy: a meta-analysis. Alternative therapies in health and medicine 21, 36–44 (2015).
Zhong, Y. et al. Puerarin attenuated early diabetic kidney injury through down-regulation of matrix metalloproteinase 9 in streptozotocin-induced diabetic rats. PLoS One 9, e85690 (2014).
Xu, X. et al. Puerarin, isolated from Pueraria lobata (Willd.), protects against diabetic nephropathy by attenuating oxidative stress. Gene 591, 411–416 (2016).
Zhang, Y., Wang, H., Yu, L. & Chen, J. The Puerarin improves renal function in STZ-induced diabetic rats by attenuating eNOS expression. Renal failure 37, 699–703 (2015).
Ma, J. Q., Ding, J., Xiao, Z. H. & Liu, C. M. Puerarin ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney through ERK/Nrf2/ARE pathway. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 71, 264–271 (2014).
Kim, K. M. et al. Puerarin suppresses AGEs-induced inflammation in mouse mesangial cells: a possible pathway through the induction of heme oxygenase-1 expression. Toxicology and applied pharmacology 244, 106–113 (2010).
Song, X. B., Liu, G., Wang, Z. Y. & Wang, L. Puerarin protects against cadmium-induced proximal tubular cell apoptosis by restoring mitochondrial function. Chemico-biological interactions 260, 219–231 (2016).
Wang, Z. K. et al. Alleviation of Lead-Induced Apoptosis by Puerarin via Inhibiting Mitochondrial Permeability Transition Pore Opening in Primary Cultures of Rat Proximal Tubular Cells. Biological trace element research 174, 166–176 (2016).
Fu, J. et al. Comparison of Glomerular and Podocyte mRNA Profiles in Streptozotocin-Induced Diabetes. J Am Soc Nephrol 27, 1006–1014 (2016).
Chuang, P. Y. et al. Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS One 6, e23566 (2011).
Liu, R. et al. Role of transcription factor acetylation in diabetic kidney disease. Diabetes 63, 2440–2453 (2014).
Manea, A., Tanase, L. I., Raicu, M. & Simionescu, M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem Biophys Res Commun 396, 901–907 (2010).
Duan, H. J. et al. Effects of puerarin on renal function, expressions of MMP-2 and TIMP-2 in diabetic rats]. Yao Xue Xue Bao 39, 481–485 (2004).
Brosius, F. C. III et al. Mouse models of diabetic nephropathy. Journal of the American Society of Nephrology: JASN 20, 2503–2512 (2009).
Wu, L., Qiao, H., Li, Y. & Li, L. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine 14, 652–658 (2007).
Zhang, H., Liu, Y., Lao, M., Ma, Z. & Yi, X. Puerarin protects Alzheimer’s disease neuronal cybrids from oxidant-stress induced apoptosis by inhibiting pro-death signaling pathways. Exp Gerontol 46, 30–37 (2011).
Chuang, P. Y. et al. In vivo RNA interference models of inducible and reversible Sirt1 knockdown in kidney cells. Am J Pathol 184, 1940–1956 (2014).
Wakino, S., Hasegawa, K. & Itoh, H. Sirtuin and metabolic kidney disease. Kidney Int 88, 691–698 (2015).
Xi, G., Shen, X., Wai, C., Vilas, C. K. & Clemmons, D. R. Hyperglycemia stimulates p62/PKCzeta interaction, which mediates NF-kappaB activation, increased Nox4 expression, and inflammatory cytokine activation in vascular smooth muscle. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 29, 4772–4782 (2015).
Zhao, Q. D. et al. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkappaB signaling pathways. Circulation 131, 643–655 (2015).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001).
Moynihan, K. A. et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell metabolism 2, 105–117 (2005).
Yang, H. et al. Multifaceted Modulation of SIRT1 in Cancer and Inflammation. Critical reviews in oncogenesis 20, 49–64 (2015).
Song, X., Li, Z., Liu, F., Wang, Z. & Wang, L. Restoration of autophagy by puerarin in lead-exposed primary rat proximal tubular cells via regulating AMPK-mTOR signaling. Journal of biochemical and molecular toxicology 31 (2017).
National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.) & National Academies Press (U.S.). Guide for the care and use of laboratory animals. xxv, 220 p (National Academies Press, Washington, D.C. (2011).
Mundel, P., Reiser, J. & Kriz, W. Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 8, 697–705 (1997).
Acknowledgements
YF Zhong is supported by National Natural Science Foundation of China (2015-81573768), Shanghai Shuguang Scholar (2016- 6SG37), Shanghai Three Years’ Pilot Project (ZY3-CCCX-2-1002, ZY3-CCCX-3-2001), Training Plan for Talent Investigator from Shanghai Department of Health (2017-2017BR023), Award from Chen Yiping’s National Research Studio for Famous Chinese Medicine Practitioners; J.C.H. is supported by NIH 1R01DK078897, NIH 1R01DK088541 and VA merit award.
Author information
Authors and Affiliations
Contributions
Y.Z., J.C.H., Y.D., Y.C., and K.L. designed the research project; X.L., W.C., B.L. and X.Z. performed experiments; X.L., W.C., J.C.H., and Y.Z. analyzed data; X.L., W.C., J.C.H., and Y.Z. prepared figures; J.C.H., and Y.Z. drafted manuscript; K.L., Y.D., and Y.C. edited and revised manuscript; Y.Z. and J.C.H. approved final version of manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Change History: A correction to this article has been published and is linked from the HTML version of this paper. The error has been fixed in the paper.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Cai, W., Lee, K. et al. Puerarin attenuates diabetic kidney injury through the suppression of NOX4 expression in podocytes. Sci Rep 7, 14603 (2017). https://doi.org/10.1038/s41598-017-14906-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-14906-8
This article is cited by
-
Puerarin alleviates chronic renal failure-induced pyroptosis in renal tubular epithelial cells by targeting miR-342-3p/TGF-β/SMAD axis
Genes & Genomics (2023)
-
The protective effect and mechanism of catalpol on high glucose-induced podocyte injury
BMC Complementary and Alternative Medicine (2019)
-
Role of sirtuin-1 in diabetic nephropathy
Journal of Molecular Medicine (2019)
-
Caspase/AIF/apoptosis pathway: a new target of puerarin for diabetes mellitus therapy
Molecular Biology Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.