Abstract
We previously demonstrated thatprenatal caffeine exposure (PCE) suppressed fetal adrenal steroidogenesis and resulted in developmental programming changes in offspring rats. However, whether these changes play a role in adrenal corticosterone synthesis under high-fat diet (HFD) and unpredictable chronic stress (UCS) remains unknown. In present study, rat model was established by PCE (120 mg/kg.d), and male offspring were provided normal diet or HFD after weaning. At postnatal week 21, several rats fed HFD were exposed to UCS for 3 weeks and sacrificed. The results showed that compared with the corresponding control group, the serum corticosterone levels and adrenal steroid synthetase expression of the PCE offspring without UCS were reduced. Moreover, the glucocorticoid (GC)-activation system was inhibited, and insulin-like growth factor 1 (IGF1) signaling pathway expression was increased. With UCS exposure in the PCE offspring, serum corticosterone levels and adrenal steroid synthetase expression were increased, the activity of GC-activation system was enhanced, and adrenal IGF1 signaling pathway expression was decreased. Based on these findings, PCE induced adrenal hypersensitivity in adult male offspring rats, as shown by the reduced corticosterone levels under HFD conditions but significantly enhanced corticosterone levels with UCS, in which GC-IGF1 axis programming alteration may play an important role.
Similar content being viewed by others
Introduction
Caffeine is a methylxanthine alkaloid widely found in coffee, tea, soft drinks, food and certain analgesic drugs1,2,3,4. Worldwide, 50% of all adults consume coffee, and in the US, the average daily caffeine intake during pregnancy is 110 mg/d5,6. Epidemiological and animal studies have shown that prenatal caffeine exposure (PCE) has adverse effects on reproductive and embryo toxicity, such as intrauterine growth retardation (IUGR)7,8. Another report found that the incidence of adult metabolic syndrome (MS) is 2.53-fold higher in IUGR-affected infants than in those not affected by IUGR9. Our previous studies demonstrated that PCE in rodents could lead to overexposure to maternal glucocorticoids (GCs) in IUGR-affected rodent fetuses and hypothalamic-pituitary-adrenal (HPA) axis-associated neuroendocrine metabolic programming alteration. The fetuses showed low basal activity of the HPA axis, enhanced sensitivity of the HPA axis to chronic stress, GC-dependent alterations of blood glucose and lipid, and an increased risk of MS after birth10,11,12,13.
The adrenal gland is the earliest and fastest developing organ of the HPA axis, which is responsible for the synthesis of GC14,15. GC have key roles in altering fetal tissue morphology and function, and steroidogenic acute regulatory protein (StAR) and cholesterol side-chain lyase (CYP11A1) are the key rate-limiting enzymes in steroid hormone synthesis16,17. A previous report showed that 11β-hydroxysteroid dehydrogenase (11βHSDs) is distributed widely in the adrenal gland and other tissues in the body. Moreover, 11βHSD can effectively control the active GC concentration in local tissue (represented by cortisol in humans and corticosterone in rodents) by catalyzing reciprocal transformation between inactive and active GCs18. Corticoid receptors (CRs), including the mineralocorticoid receptor (MR) and glucocorticoid receptor (GR), are ligand-dependent nuclear transcription factors19. The insulin-like growth factor 1 (IGF1) signaling pathway is the core of the endocrine regulatory system, modulates adrenal cell proliferation, differentiation and metabolism, which play important roles in morphology and functional development20,21. Our recent studies have found that PCE could cause adrenal function abnormity in offspring rats, which was related to low- expression of adrenal steroid synthetase (the first type of programming)12,22 and GC-IGF1 axis programming (the second type of programming) induced by fetal overexposure to maternal GCs23.
Many environmental factors (such as high nutritional status and social or mental pressure) can induce and aggravate MS and related metabolic diseases. Given the role of high nutritional status, a high-fat diet (HFD) is a common cause of MS and related metabolic diseases in adults. Research has shown that an HFD is associated with nearly all MS phenotypes, such as hypertension, insulin resistance, diabetes and obesity24,25. An HFD can also significantly increase the damage caused by GCs, such as induction of type 2 diabetes26. IUGR-affected offspring are more likely to develop metabolic diseases when receiving an HFD27. Furthermore, chronic stress due to high social pressure was shown to be another common cause of MS and related metabolic diseases in adults28,29. Chronic stress can induce and aggravate the risk of metabolic diseases and mental disorders30,31. A recent study demonstrated that reduced birthweight or stress in pregnancy alter mRNA levels of steroid synthetase in male but not female offspring. These effects were associated with metabolic dysfunction32. Our previous study demonstrated that serum glucose and lipid levels showed GC-dependent changes in adult offspring rats with PCE under HFD conditions, which led to increased susceptibility to MS in a gender-specific manner33. However, whether the adrenal steroid synthesis of male offspring with PCE is changed with HFD or HFD/chronic stress conditions after birth, and the underlying mechanisms mediating the development of abnormal blood corticosterone levels are unclear.
The present study sought to explore the adrenal changes in IUGR-affected male offspring rats caused by PCE by detecting alterations in the steroidal synthetase system, IGF1 signaling pathway and GC-activation system (11βHSDs/CR levels) of the adrenal axis with normal diet, HFD and HFD/chronic stress conditions after birth. Furthermore, based on the two types of intrauterine programming of adrenal developmental toxicity, we elucidated the mechanisms of adrenal functional change, and below, we discuss its significance.
Results
Serum corticosterone levels
Using ELISA technique, we determined serum corticosterone concentrations in adult male offspring in the normal diet, HFD and HFD/chronic stress groups. As shown in Fig. 1, compared with their respective control groups, the serum corticosterone concentrations in the PCE groups showed decreases with a normal diet or HFD and increases (P < 0.01) with UCS exposure under HFD conditions.
Adrenal steroid synthesis function
To determine whether the changes in serum corticosterone levels were due to the adrenal steroid synthetase system, we tested the relevant indexes. As shown in Fig. 2A, compared with the respective control, the mRNA expression levels of StAR were decreased and of CYP21 were increased in the PCE group fed a normal diet (P < 0.05, P < 0.01). Adrenal steroidal synthetase levels in the PCE group were significantly reduced or presented a decreasing trend under HFD condition, including the levels of StAR, CYP11A1, 3β-HSD and CYP11B1 (P < 0.01). With HFD/UCS exposure, the adrenal steroid synthetase levels in the PCE group were significantly enhanced, including the levels of StAR, CYP11A1 and CYP11B1 (P < 0.05, P < 0.01). Adrenal immunohistochemical analyses are shown in Fig. 2B–D. Compared with their respective controls, the protein expression levels of StAR and CYP11A1 were decreased in the PCE groups under normal diet and HFD conditions (P < 0.05) and were increased with UCS (P < 0.05, P < 0.01). These results suggested that the low adrenal steroid synthetase expression continued into adulthood under HFD conditions and was enhanced with UCS exposure in offspring with IUGR induced by PCE.
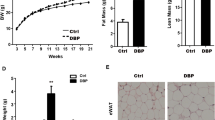
Effects of prenatal caffeine (PCE, 120 mg/kg.d) exposure on the mRNA and protein expression of adrenal steroidogenic enzymes with normal and high-fat diets without and with unpredictable chronic stress in adult male rat offspring. Mean ± S.E.M., n = 5 (the adrenal samples from two litters were counted as one sample for adrenal mRNA). * P < 0.05, ** P < 0.01 vs respective control. (A) The mRNA expression of adrenal steroidogenic enzymes; (B) The mean optical density (MOD) of steroidogenic acute regulatory (StAR) or cytochrome P450 cholesterol side chain cleavage (CYP11A1) in the adrenal cortex; (C) The protein expression of StAR (Immumohistochemical staining, ×200); (D) The protein expression of CYP11A1 (Immumohistochemical staining, ×200). 3β-HSD: 3β-hydroxysteroid dehydrogenase; CYP21: steroid 21-hydroxylase; CYP11B1: steroid 11β-hydroxylase.
Adrenal IGF1 signaling pathway
As shown in Fig. 3, compared with the respective control, only the mRNA expression level of IGF1 was increased in the PCE group fed a normal diet (P < 0.05). The mRNA levels of all the IGF1 signal pathway components were increased or showed an increasing trend under HFD conditions, such as the levels for IGF1, IGF1R, AKT1 and steroidogenic factor 1 (SF1) (P < 0.05, P < 0.01). With UCS exposure, the mRNA levels of IGF1 and IGF1R were decreased compared with the levels in the controls (P < 0.05, P < 0.01). These results suggested that the expression of the adrenal IGF1 signal pathway was enhanced without UCS exposure but decreased with UCS in male offspring with IUGR induced by PCE.
Effects of prenatal caffeine exposure (PCE, 120 mg/kg.d) exposure on the mRNA expression of adrenal insulin-like growth factors 1 (IGF1) pathway with normal and high-fat diets without and with unpredictable chronic stress in adult male rat offspring. Mean ± S.E.M., n = 5, the adrenal samples from two litters were counted as one sample. * P < 0.05, ** P < 0.01vs respective control. IGF-1R: insulin-like growth factor-1 receptor; AKT1: protein kinase B; SF1: steroidogenic factor 1.
Adrenal 11βHSD/CR system expression
As shown in Fig. 4, compared with their respective controls, the PCE group fed a normal diet exhibited increased expression of 11βHSD2 (P < 0.01), which resulted in a decreased 11βHSD1/11βHSD2 ratio (P < 0.01), and the PCE group fed an HFD exhibited increased expression of 11βHSD2 (P < 0.01) as well as decreased expression of 11βHSD1, a decreased 11βHSD1/11βHSD2 expression ratio and decreased MR expression (P < 0.05, P < 0.01). With UCS exposure, the PCE group exhibited decreased expression of 11βHSD2 (P < 0.01) as well as increased expression of 11βHSD1, an increased 11βHSD1/11βHSD2 expression ratio and increased MR expression (P < 0.05, P < 0.01). These results demonstrated that the adrenal 11βHSD/CR system was suppressed under HFD conditions and activated with UCS conditions in male offspring with IUGR induced by PCE.
Effects of prenatal caffeine (PCE, 120 mg/kg.d) exposure on the mRNA expression of adrenal 11β-hydroxysteroid dehydrogenases (11βHSDs), glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) with normal and high-fat diets without and with unpredictable chronic stress in adult male rat offspring. Mean ± S.E.M., n = 5, the adrenal gland samples from two litters were counted as one sample. * P < 0.05, ** P < 0.01 vs respective control.
Discussion
Developmental toxicity refers to permanent changes in morphology and function caused by damage in the early developmental stages34. The alteration of intrauterine programming is a permanent change in morphology and function caused by damage in utero, which is usually maintained from the developmental period into adulthood and even throughout the individual’s lifetime, leading to multiple effects on the adults35. Research has shown that high concentration of GC (dexamethasone) exposure during pregnancy can affect the sensitivity of the adrenal gland, mainly by increasing the mRNA expression of the ACTH receptor36. Our previous studies confirmed that PCE led to a low birthweight in fetal offspring rats and a high IUGR rate, and that offspring rats with PCE could undergo a period of catch-up growth after birth11,12,13. Furthermore, in the PCE-induced IUGR models, we found that the expression of StAR and CYP11A1 were reduced in the fetal adrenals due to overexposure to the maternal GCs22. Furthermore, when the PCE male offspring rats were fed a normal diet after birth, serum corticosterone levels presented a decreasing trend, the adrenal GC-activation system was inactivated, the expression of adrenal IGF1 signal pathway was enhanced, and the expression of the steroid synthetase system was increased. Hence, we had proposed a “two-programming” mechanism for PCE-induced adrenal developmental abnormality in the offspring rats, which included the low-function programming of adrenal de novo steroidogenesis and GC-IGF1 axis programming-mediated compensatory changes of steroidogenesis before and after birth23.
Studies have shown that an HFD can also affect the basic activity and stress sensitivity of the HPA axis37,38, and HFD exposure during pregnancy or lactation increased stress reactivity and serum corticosterone levels in spontaneously hypertensive adult rats39. Our previous study demonstrated that PCE induced a low expression of adrenal GC synthetase in the fetal rats and low basal activity of the HPA axis after birth12,23. Moreover, we had demonstrated an interaction between HFD after birth and PCE, which could affect the serum corticosterone levels33. In the present study, we found that the mRNA expression of the adrenal steroid synthetase system (including StAR, CYP11A1, 3β-HSD and CYP11B1) was reduced under HFD conditions. The immunohistochemical results showed that the protein levels of StAR and CYP11A1 were consistent with the gene expression, and the serum corticosterone concentration also showed a decreasing trend. These results suggested that the inhibited adrenal function in male offspring with PCE under HFD conditions may be the effect of programming, which was caused by the low basal activity of the HPA axis and the adrenal function inhibition. At the same time, the adrenal GC-activation system showed significant inactivation (reflected by effects such as decreased expression of 11β-HSD1 and increased expression of 11β-HSD2 as well as a decreased 11β-HSD1/11β-HSD2 ratio and decreased MR expression). Furthermore, the expression of the adrenal IGF1 signaling pathway components (including IGF1, IGF1R and AKT1) was notably increased, and the expression of the transcription factor for steroid synthetase, SF1, also showed an increasing trend. These results indicated that under HFD conditions, the inhibition of the GC-activation system may be related to the low serum GC level and may participate in the enhancement of the activity of the adrenal IGF1 signaling pathway (including SF1 expression) and steroid synthesis function. Hence, we hypothesized that the inactivation of GC-activation system increased adrenal steroidogenesis by enhancing the expression of the adrenal IGF1 signaling pathway, which then promoted local adrenal corticosterone synthesis and increased the serum corticosterone levels.
Studies have shown that chronic stress can affect the body’s homeostasis, such as by interfering with the synthesis and secretion of adrenal GCs40, and chronic stress has a long-term impact on the activity of the HPA axis41. Additionally, chronic stress can seriously affect the body’s endocrine and metabolic balance, increasing susceptibility to a variety of diseases, including cardiovascular disease and type 2 diabetes42,43,44. Our previous study found that ethanol exposure during pregnancy could change HPA axis activity in offspring under HFD and UCS conditions, which increased the susceptibility of the offspring to MS45. Caffeine exposure during pregnancy also could result in high stress sensitivity of the HPA axis in offspring rats after birth under normal diet conditions11. In the present study, we found that compared with the HFD control, the serum GC level and adrenal synthetase system expression were increased in the PCE male offspring with UCS, which suggested that UCS could improve adrenal steroid synthesis, leading to an increased serum GC level. Furthermore, the adrenal GC-activation system was triggered and the IGF1 signaling pathway was inhibited with UCS. These results showed that the UCS-induced high serum GC level could inhibit the adrenal IGF1 signaling pathway via negative feedback by activating the GC-activation system, which then decreased local corticosterone synthesis to maintain homeostasis.
Numerous experiments have shown that high blood GC levels increased the blood glucose level and increased the risk of insulin resistance, diabetes and other metabolic diseases46,47,48. Our previous additional study found that blood glucose and lipid levels and liver and pancreatic function were all damaged by PCE under normal diet conditions34. Meanwhile, an HFD could change HPA axis function in offspring rats with PCE, leading to a GC-associated blood glucose and lipid changes, which increased the susceptibility of the offspring to MS in a gender-specific manner33. In the present study, regardless of HFD or UCS conditions, the greater adrenal sensitivity of male offspring rats caused by PCE led to abnormal changes in adrenal synthesis, in which the GC-IGF1 axis played an important role by maintaining the stability of the serum GC level, serving a possible endocrine negative feedback axis. Furthermore, the serum GC level in the PCE group with HFD and UCS exposure was higher than in any other group (such as the normal diet group and the HFD group), which suggested that the compensatory effect maintained adrenal synthesis, and it could have easily caused the depletion and decompensation of adrenal function as well. Once decompensated, the glucose and lipid metabolic functions were affected, resulting in an enhanced susceptibility to MS and related metabolic diseases.
It is worth mentioning that gender differences in intrauterine programming of fetal development related research is commonly reported32,33,36. Meanwhile, another study indicated that prenatal corticosterone exposure induced offspring adrenal function abnormity in male but not female offspring49. Although only the male offspring rats were included in the present study, we tested the female rats at the same time. We were surprised to see some obvious gender differences in the GC-activation system and IGF1 signaling pathway of the PCE offspring rats under normal diet and HFD conditions, especially in the females, there was even disorder after UCS (data not shown). In view of the importance of gender differences in fetal origin adult diseases, we will strengthen the gender difference and its potential mechanism-associated research in the future.
One limitation of the present study is that the adrenal steroid synthetase system changes only were measured in gene expression not protein expression based on the limited amount of samples, but the immunohistochemistry of StAR and P450scc were included in the results. The other limitations are intragastric administration and litter size correction after birth. As a major confounding factor, caffeine administration by gavage is a stimulus and it can affect the corticosterone secretion of the pregnant rats, although the animals of the control group were given equal dose of saline also by gavage, which could relatively eliminate the gavage influence. Additionally, the litter size probably changed the offspring growth profile and metabolic health, therefore, we had corrected the litter size in both the experimental and control groups after birth, which would minimize the individual difference.
Conclusions
PCE induced greater adrenal sensitivity in adult male offspring rats, as shown by the reduced corticosterone synthesis under HFD conditions and the significantly enhanced corticosterone levels with UCS exposure. Adrenal GC-IGF1 axis programming changes may play an important role in these alterations by maintaining the stability of the blood GC level via regulation of local corticosterone synthesis through negative feedback. The adaptive changes resulting from the GC-IGF1 axis programming may maintain blood GC level within a certain range. This study provides an experimental basis for clarifying PCE-caused adrenal dysfunction and abnormal circulating GC levels, which would be beneficial in addressing GC-associated diseases in the IUGR-affected offspring.
Materials and Methods
Chemicals and reagents
Caffeine (CAS #58-08-2, N99% purity) was purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Isoflurane was purchased from Baxter Healthcare Co. (Deerfield, IL, USA). A rat corticosterone ELISA kit was obtained from Assay-pro LLC. (Saint Charles Missouri, USA). Reverse transcription and real-time quantitative PCR (RT-qPCR) kits were purchased from TaKaRa Biotechnology Co., Ltd. (Dalian, China). The oligonucleotide primers for rat genes were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). All other chemicals and agents were of analytical grade.
Animals and treatment
Specific pathogen-free (SPF) Wistar rats (with weights of 200–240 g for females and 260–300 g for males) were obtained from the Experimental Center of Hubei Medical Scientific Academy (No. 2008–0005, Hubei, China). The animal experiments were performed at the Center for Animal Experiment of Wuhan University (Wuhan, China), which has been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). All animal experimental procedures were approved by and performed in accordance with the Guidelines for the Care and Use of the Animal Welfare Committee (AWC) of Wuhan University and the International Council on Research Animal Care; the AWC specifically oversees the university’s animal programs, equipment and procedures.
Animals were held under temperature-controlled conditions on a 12 h light: dark cycle and had ad libitum access to standard chow and tap water at all times. After one week of acclimation, two females were mated with one male for one night. Upon confirmation of mating by the appearance of sperm in a vaginal smear, the day was defined as gestational day (GD) 0. Pregnant females were then transferred to individual cages.
Pregnant rats were randomly divided into two groups: a control group and a PCE group. Ten dams were in each group. Starting from GD11 until term delivery (GD20), the PCE group was administered 120 mg/kg.d caffeine via oral gavage, as previously reported12. The control group was administered the same volume of vehicle. A schematic of the treatments for maternal and offspring rats is shown in Fig. 5. The pregnant rats were allowed to deliver spontaneously at term.
On postnatal day (PD) 1, the number of pups in each litter was set to 8 (♂:♀ = 1:1) randomly; to ensure adequate and standardized nutrition until weaning50. At postnatal week (PW) 4, 60 male pups from 20 different mothers (3 male pups were chosen from each mother) were selected randomly for each group (30 pups with IUGR from the caffeine group and 30 normal pups from the control group), and all pups were fed a normal diet or HFD ad libitum before being sacrificed. The standard rodent chow purchased from the Experimental Centre of Hubei Medical Scientific Academy contained 21% kcal from protein, 68.5% kcal from carbohydrate and 10.5% kcal from fat. The HFD was previously described by our laboratory51 and contained 88.0% corn flour, 11.5% lard, and 0.5% cholesterol, which provided 18.9% kcal from protein, 61.7% kcal from carbohydrate and 19.4% kcal from fat.
At PW-21, 40 male rats fed a normal diet or HFD were anesthetized with isoflurane and decapitated in a room separate from where the other animals were kept. To minimize the effect of corticosterone circadian rhythm, all the rats were sacrificed within 9:00 am to 11:00 am. Trunk blood was collected, and serum was prepared by centrifugation at 17,205 g for 15 min at 4 °C and stored at −80 °C until use for detection of the corticosterone concentration. The adrenal glands were dissected for RT-PCR and fixed in 4% formaldehyde for histological examination. The remaining 20 male rats fed an HFD were exposed to UCS for 3 weeks52, with the stress administered once daily between 8:30 am and 10:30 am with lights -on except for the stressor with 24 h duration. The stressors consisted of ① food deprivation for 24 h; ② water deprivation for 24 h; ③ tail pinch (2 cm from the end of the tail) for 5 min; ④ heating in an oven at 45 °C for 5 min; ⑤ cold swimming at 4 °C–8 °C for 4 min, after which the rats were towel dried; ⑥ reversed day-and-night cycles; and ⑦ social isolation (one rat per cage) for 24 h. Every stressor was administered randomly at an interval of 7 days, for a total of three times within 21 days. On the last day of stress (PW-24), after 12 h fasting, all rats were obliged to swim at 4 °C–8 °C for 4 min and were then towel -dried. One hour after swimming, the rats were anesthetized with isoflurane and decapitated in a room separate from where the other animals were kept. Trunk blood was collected, and serum was prepared by centrifugation at 17,205 g for 15 min at 4 °C and stored at −80 °C until use for detection of the corticosterone concentration. The adrenal glands were dissected for RT-qPCR and fixed in 4% formaldehyde for histological examination.
Analysis of blood samples
The serum corticosterone concentration was detected using an ELISA kit following the manufacturer’s protocol. The limit of detection for the corticosterone concentration was 0.39 ng/mL. The intra-assay and inter-assay coefficients of variation for corticosterone were 5.0% and 7.2%, respectively. The cross-reactivity of the corticosterone ELISA, as defined by the manufacturer, was 2% for progesterone and 2% for aldosterone.
RT-qPCR detection
Total RNA was isolated from the rat adrenal glands using TRIzol reagent according to the manufacturer’s protocol. The same tissues of littermates were pooled for homogenization. The concentration and purity of the isolated RNA were determined by spectrophotometry and adjusted to 1 μg/μl. Total RNA was stored in diethyl pyrocarbonate-H2O (DEPC-H2O) at −80 °C.
For RT-qPCR analysis, single-stranded cDNA was prepared from 2 μg of total RNA according to the protocol of the ExScript RT reagent kit. Primers were designed using Primer Premier 5.0, and their sequences are shown in Table 1. Relative standard curves were constructed for the target genes (Table 1), using the corresponding RT-qPCR products, isolated using a DNA extraction kit, at different concentrations, ranging from 10–10,000 pg per reaction. PCR was performed in 96-well optical reaction plates using the ABI Step One real-time PCR thermal cycler (ABI StepOne, NY, USA) in a 20 µl reaction mixture. To quantify the gene transcripts more precisely, the mRNA level of the housekeeping gene glyceraldehyde phosphate dehydrogenase (GAPDH) was measured and used as a quantitative control, with each sample normalized against GAPDH mRNA content. The PCR cycling conditions used were as follows: pre-denaturation, 95 °C for 30 s; denaturation, 95 °C for 5 s; annealing, the conditions for each gene are listed in Table 1; and elongation, 72 °C for 30 s (the elongation step was performed for the 3β-HSD reactions).
Immunohistochemical examination
The adrenal glands were fixed in 4% paraformaldehyde solution overnight and then processed using the paraffin-sectioning technique. The immunohistochemical procedures were performed using a streptavidin-peroxidase (SP)-conjugation method according to the manufacturer’s instructions. Paraffin-embedded tissues were cut into 5 μm-thick serial sections and stained for StAR and CYP11A1 with a goat monoclonal antibody (1:200 dilution; sc-7846, Santa Cruz Biotechnology). At least five random fields from each section were examined. The signal was visualized using light microscopy and imaged, and the positively stained areas were analyzed using the Photo Imaging System (Nikon H550S, Japan). The number of Ki67-stained nuclei in each image was also counted with the Photo Imaging System.
Statistical analysis
SPSS 17 (SPSS Science Inc., Chicago, IL, USA) and Prism (GraphPad Software, La Jolla, CA, USA) were used for data analysis. All data presented are expressed as the mean ± S.E.M. The significant differences between different treatment groups were assessed with one-way analysis of variance (ANOVA). The gain rates of serum parameters were transformed if necessary before one-way ANOVA evaluations. Statistical significance was set at P < 0.05.
References
Sugiura, C. et al. Catechins and Caffeine Inhibit Fat Accumulation in Mice through the Improvement of Hepatic Lipid Metabolism. J Obes. 2012, 510–20 (2012).
Panchal, S. K., Wong, W. Y., Kauter, K., Ward, L. C. & Brown, L. Caffeine attenuates metabolic syndrome in diet-induced obese rats. Nutrition. 28, 1055–62 (2012).
Carrieri, M. P. et al. Elevated coffee consumption and reduced risk of insulin resistance in HIV-HCV coinfected patients (HEPAVIH ANRS CO-13). Hepatology. 56, 2010–19 (2012).
van Dam, R. M. [Coffee consumption and the decreased risk of diabetes mellitus type 2]. Ned Tijdschr Geneeskd. 150, 1821–5 (2006).
Hewlett, P. & Smith, A. Correlates of daily caffeine consumption. Appetite. 46, 97–9 (2006).
Knight, C. A., Knight, I., Mitchell, D. C. & Zepp, J. E. Beverage caffeine intake in US consumers and subpopulations of interest: estimates from the Share of Intake Panel survey. Food Chem Toxicol. 42, 1923–30 (2004).
Kuczkowski, K. M. Caffeine in pregnancy: a cause for concern? Ann Fr Anesth Reanim. 28, 605–7 (2009).
Fortier, I., Marcoux, S. & Beaulac-Baillargeon, L. Relation of caffeine intake during pregnancy to intrauterine growth retardation and preterm birth. Am J Epidemiol. 137, 931–40 (1993).
Silveira, V. M. & Horta, B. L. [Birth weight and metabolic syndrome in adults: meta-analysis]. Rev Saude Publica. 42, 10–8 (2008).
Huang, J. et al. Role of p53-dependent placental apoptosis in the reproductive and developmental toxicities of caffeine in rodents. Clin Exp Pharmacol Physiol. 39, 357–63 (2012).
Xu, D. et al. A hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in offspring rats of IUGR induced by prenatal caffeine ingestion. Toxicol Appl Pharmacol. 264, 395–403 (2012).
Xu, D. et al. Caffeine-induced activated glucocorticoid metabolism in the hippocampus causes hypothalamic-pituitary-adrenal axis inhibition in fetal rats. PLoS One. 7, e44497 (2012).
Liu, Y. et al. Fetal rat metabonome alteration by prenatal caffeine ingestion probably due to the increased circulatory glucocorticoid level and altered peripheral glucose and lipid metabolic pathways. Toxicol Appl Pharmacol. 262, 205–16 (2012).
Ishimoto, H. & Jaffe, R. B. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr Rev. 32, 317–55 (2011).
Mesiano, S. & Jaffe, R. B. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 18, 378–403 (1997).
Son, G. H., Chung, S. & Kim, K. The adrenal peripheral clock: glucocorticoid and the circadian timing system. Front Neuroendocrinol. 32, 451–65 (2011).
Anagnostis, P., Athyros, V. G., Tziomalos, K., Karagiannis, A. & Mikhailidis, D. P. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 94, 2692–701 (2009).
Hadoke, P. W., Kipari, T., Seckl, J. R. & Chapman, K. E. Modulation of 11beta-hydroxysteroid dehydrogenase as a strategy to reduce vascular inflammation. Curr Atheroscler Rep. 15, 320 (2013).
Djikic, D. et al. Ethanol and nitric oxide modulate expression of glucocorticoid receptor in the rat adrenal cortex. Pharmacol Rep. 64, 896–901 (2012).
Raha, D. et al. IGF-I enhances cortisol secretion from guinea-pig adrenal gland: in vivo and in vitro study. Int J Mol Med. 20, 91–5 (2007).
Weber, M. M., Auernhammer, C. J., Kiess, W. & Engelhardt, D. Insulin-like growth factor receptors in normal and tumorous adult human adrenocortical glands. Eur J Endocrinol. 136, 296–303 (1997).
Ping, J. et al. Prenatal caffeine ingestion induces aberrant DNA methylation and histone acetylation of steroidogenic factor 1 and inhibits fetal adrenal steroidogenesis. Toxicology. 321, 53–61 (2014).
He, Z. et al. Glucocorticoid and insulin-like growth factor 1 (GC-IGF1) axis programming might mediate prenatal caffeine exposure-induced adrenal developmental abnormality in male offspring rats. Toxicol Res. 5, 388–98 (2016).
Fraulob, J. C., Ogg-Diamantino, R., Fernandes-Santos, C., Aguila, M. B. & Mandarim-de-Lacerda, C. A. A Mouse Model of Metabolic Syndrome: Insulin Resistance, Fatty Liver and Non-Alcoholic Fatty Pancreas Disease (NAFPD) in C57BL/6 Mice Fed a High Fat Diet. J Clin Biochem Nutr. 46, 212–23 (2010).
Huang, K., Song, Y. T., He, Y. H. & Xing, Lin FengLangdon. Health system strengthening and hypertension management in China. Global Health Research and Policy 1, 13–22 (2016).
Auvinen, H. E. et al. Glucocorticoid excess induces long-lasting changes in body composition in male C57Bl/6J mice only with high-fat diet. Physiol Rep. 1, e00103 (2013).
Rueda-Clausen, C. F. et al. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes. 60, 507–16 (2011).
Peters, A. & McEwen, B. S. Stress habituation, body shape and cardiovascular mortality. Neurosci Biobehav Rev. 56, 139–50 (2015).
Radley, J., Morilak, D., Viau, V. & Campeau, S. Chronic stress and brain plasticity: Mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci Biobehav Rev. 58, 79–91 (2015).
Pinheiro, C. R. et al. Developmental plasticity in adrenal function and leptin production primed by nicotine exposure during lactation: gender differences in rats. Horm Metab Res. 43, 693–701 (2011).
Arnetz, L., Rajamand Ekberg, N., Brismar, K. & Alvarsson, M. Gender difference in adrenal sensitivity to ACTH is abolished in type 2 diabetes. Endocr Connect. 4, 92–9 (2015).
Cheong, J. N. et al. Sex-Specific Metabolic Outcomes in Offspring of Female Rats Born Small or Exposed to Stress During Pregnancy. Endocrinology. 157, 4104–20 (2016).
Li, J. et al. Gender-specific increase in susceptibility to metabolic syndrome of offspring rats after prenatal caffeine exposure with post-weaning high-fat diet. Toxicol Appl Pharmacol. 284, 345–53 (2015).
Zhang, C. et al. Prenatal xenobiotic exposure and intrauterine hypothalamus-pituitary-adrenal axis programming alteration. Toxicology. 325, 74–84 (2014).
Meaney, M. J., Szyf, M. & Seckl, J. R. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 13, 269–77 (2007).
Cuffe, J. S., Turton, E. L., Akison, L. K., Bielefeldt-Ohmann, H. & Moritz, K. M. Prenatal corticosterone exposure programs sex-specific adrenal adaptations in mouse offspring. The Journal of endocrinology. 232, 37–48 (2017).
Legendre, A. & Harris, R. B. Exaggerated response to mild stress in rats fed high-fat diet. Am J Physiol Regul Integr Comp Physiol. 291, R1288–1294 (2006).
Mitra, A., Crump, E. M., Alvers, K. M., Robertson, K. L. & Rowland, N. E. Effect of high-fat diet on stress responsiveness in borderline hypertensive rats. Stress. 14, 42–52 (2011).
Auvinen, H. E. et al. The effects of high fat diet on the basal activity of the hypothalamus-pituitary-adrenal axis in mice. J Endocrinol. 214, 191–7 (2012).
Schreier, H. M. et al. Mercury and psychosocial stress exposure interact to predict maternal diurnal cortisol during pregnancy. Environ Health. 14, 28–39 (2015).
Li, C. et al. Long-term effects of early adolescent stress: dysregulation of hypothalamic-pituitary-adrenal axis and central corticotropin releasing factor receptor 1 expression in adult male rats. Behav Brain Res. 288, 39–49 (2015).
Uetake, Y. et al. High-salt in addition to high-fat diet may enhance inflammation and fibrosis in liver steatosis induced by oxidative stress and dyslipidemia in mice. Lipids Health Dis. 14, 6–19 (2015).
Bouazza, A. et al. Effect of fruit vinegars on liver damage and oxidative stress in high-fat-fed rats. Pharm Biol. 54(2), 260–5 (2016).
Paglialunga, S., Ludzki, A., Root-McCaig, J. & Holloway, G. P. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia. 58, 1071–80 (2015).
Xia, L. P. et al. Prenatal ethanol exposure enhances the susceptibility to metabolic syndrome in offspring rats by HPA axis-associated neuroendocrine metabolic programming. Toxicol Lett. 226, 98–105 (2014).
Zhang, Y. P., Gong, Y., Zeng, Q. Y., Hou, Z. D. & Xiao, Z. Y. A long-term, observational cohort study on the safety of low-dose glucocorticoids in ankylosing spondylitis: adverse events and effects on bone mineral density, blood lipid and glucose levels and body mass index. BMJ Open 5, e006957 (2015).
Magomedova, L. & Cummins, C. L. Glucocorticoids and Metabolic Control. Handb Exp Pharmacol. 233, 73–93 (2015).
Keller-Wood, M. et al. Elevated maternal cortisol leads to relative maternal hyperglycemia and increased stillbirth in ovine pregnancy. Am J Physiol Regul Integr Comp Physiol. 307, R405–13 (2014).
Waddell, B. J., Bollen, M., Wyrwoll, C. S., Mori, T. A. & Mark, P. J. Developmental programming of adult adrenal structure and steroidogenesis: effects of fetal glucocorticoid excess and postnatal dietary omega-3 fatty acids. The Journal of endocrinology. 205, 171–8 (2010).
Manuel-Apolinar, L., Zarate, A., Rocha, L. & Hernandez, M. Fetal malnutrition affects hypothalamic leptin receptor expression after birth in male mice. Arch Med Res. 41, 240–5 (2010).
Zhang, L. et al. Prenatal food restriction induces a hypothalamic-pituitary-adrenocortical axis-associated neuroendocrine metabolic programmed alteration in adult offspring rats. Arch Med Res. 44, 335–45 (2013).
Willner, P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 52, 90–110 (2005).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Nos 81220108026, 81430089, 81673524), the National Key Research and Development Program of China (2017YFC1001300), and Hubei Province Health and Family Planning Scientific Research Project (No. WJ2017C0003).
Author information
Authors and Affiliations
Contributions
Z.H., F.L., H.H. and C.Z. contributed to the acquisition of animal data. Z.H., F.L., L.L. and H.H. performed the total RNA isolation and participated in the experimental design, interpretation and manuscript writing. Y.D., L.Z. and Y.L. assisted with data analysis and statistical methods. H.W. and C.S. provided the experimental design, interpretation and critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, Z., Lv, F., Ding, Y. et al. High-fat diet and chronic stress aggravate adrenal function abnormality induced by prenatal caffeine exposure in male offspring rats. Sci Rep 7, 14825 (2017). https://doi.org/10.1038/s41598-017-14881-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-14881-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.