Abstract
Postoperative neutrophil-to-lymphocyte ratio change (NLRc) reflects the dynamic change of balance between host inflammatory response and immune response after treatment. In gastric cancer, an elevated initial NLR (iNLR) is reported to be a prognostic predictor, but the clinical application of the NLRc remains unclear. The NLRc was assessed in 734 patients undergoing total/subtotal gastrectomy and endoscopic submucosal dissection for gastric adenocarcinoma. The iNLR and NLRc were recorded within 10 days of the first diagnosis and 3–6 months after surgery, respectively. Using receiver operating characteristic (ROC) curves, we investigated the relationship between NLRc or iNLR and patient survival. The analysis revealed a higher predictive power for correlating patient survival with the NLRc compared with iNLR. NLRc was defined as negative (lower than iNLR) and positive (higher than iNLR). A positive NLRc was frequently observed in patients with advanced AJCC stage, local recurrence, distant metastasis, perineural invasion, and adjuvant chemotherapy (all p < 0.05). Univariate and multivariate analyses revealed a significant relationship between patient survival and NLRc (all p < 0.05) but no association between survival and iNLR. The NLRc could be a better indicator than iNLR for predicting survival in patients with gastric cancer.
Similar content being viewed by others
Introduction
Gastric cancer is a complex, heterogeneous disease with a global variation in its aetiology, incidence, natural course, and management. The tumourigenic conditions of gastric cancers are associated with infectious agents (Helicobacter pylori or Epstein-Barr virus)1,2, lecular subset (CDH1 mutation)3, and different levels of susceptibility and aggressiveness of the tumours. The clinical factors for determining a treatment plan are location of the primary tumour (proximal and distal), subtypes of adenocarcinoma (diffuse, intestinal, or mixed)4, tumour susceptibility based on ethnicity, and predictive biomarkers (HER2)5. Generally, the following prognostic indicators are considered in clinical management: American joint Committee on Cancer (AJCC) stage and size, histological type and grade, and lymphovascular and perineural invasion. A study by the Cancer Genome Atlas Research Network indicated that gastric cancers can be classified into four subtypes based on molecular characteristics: Epstein-Barr virus positivity, microsatellite instability, genomic stability, and chromosomal instability6. However, these molecular analyses might be limited in general hospitals that lack facilities. Moreover, molecular markers are expensive and often time-consuming to measure. When there are sufficient technologists with an appropriate laboratory setting, genetic analyses to determine the new subtypes can be easily used in clinical application.
Systemic inflammation is associated with the progression of various cancers by induction of angiogenesis, metastasis and malignant cell proliferation, and alteration of the response to systemic therapy7. Neutrophils are known to be key mediators of tumour inflammation8 and angiogenesis9. Lymphocytes have been suggested to play vital roles in cytotoxic tumour cell death linked to tumour-infiltrating lymphocytes, especially T lymphocytes10. The interaction between the cancer and immune system might also extend beyond the local tissue environment. Other systemic inflammatory markers such as C-reactive protein and interleukin-6, have been associated with worse survival in patients with gastric cancer11. The imbalance between neutrophils and lymphocytes is thought to be secondary to tumour hypoxia or necrosis and associated with anti-apoptotic effects. Published data demonstrated that the initial neutrophil-to-lymphocyte ratio (iNLR) at that time of first diagnosis is linked to prognosis of different types of cancer12,13,14,15 as well as of other conditions such as cardiovascular disease16 and bacterial infection17. Accordingly, the iNLR might represent the balance between pro-tumour inflammatory status and anti-tumour immune status.
Several studies focused primarily on iNLR, while the dynamic change of neutrophil-to-lymphocyte ratio after treatment was not considered. The postoperative neutrophil-to-lymphocyte ratio change (NLRc) might be a meaningful factor to assess prognosis after treatment, because there has been a change in the treatment approach including tumour removal and chemotherapy. However, although the NLRc might dynamically reflect the alteration of balance between host inflammatory response and immune response against cancer after treatment, its significance is largely unclear.
The aim of this study was to investigate the relationships between iNLR and NLRc and clinicopathological parameters, and to evaluate the prognostic value of the NLRc in patients with gastric cancer.
Results
Patient characteristics
The median age of the 734 patients was 66 (range: 23–87) years. The following clinicopathological variables were recorded: Sex; age; 8th AJCC stage; location; local recurrence; distant metastasis; size; Lauren type; histological grade; lymphatic, vascular, and perineural invasion; presence of adjuvant chemotherapy; margin involvement, and patient survival.
EGC was present in 395 patients and advanced gastric cancer (AGC) in 339. In early gastric cancer (EGC), the predominant gross type was classified as protruding (type I) in 21 patients, slightly elevated (type IIa) in 28, flat (type IIb) in 113, slightly depressed (type IIb) in 196, and excavated (type III) in 37. In AGC, the predominant gross type was classified as fungating (Borrmann type 1) in 11 patients, ulcerative (Borrmann type 2) in 66, ulcero-infiltrative (Borrmann type 3) in 221, and linitis plastica (Borrmann type 4) in 41. According to the Lauren classification, 396 patients had intestinal type, followed by 217 with a diffuse type and 121 with a mixed type. The surgical procedures included subtotal gastrectomy in 567, total gastrectomy in 106, endoscopic submucosal dissection in 54, and endoscopic mucosal resection in seven. Two hundred thirty-one patients received adjuvant chemotherapy such as tegafur-uracil. After surgery, 142 patients developed local recurrence and/or new distant metastases, and 172 patients died during the median follow-up period of 49.41 months.
Evaluation of the neutrophil-to-lymphocyte ratio
In comparison of systemic inflammation response markers, there was a negative association between absolute neutrophil counts and absolute lymphocyte counts (Fig. 1).
We subsequently analysed survival using receiver operating characteristic (ROC) curves to evaluate the probability of iNLR and NLRc according to patient survival. The ROC curve revealed that the NLRc (area under the ROC curve, 0.674; sensitivity, 59.9%; specificity, 75.4%; positive predictive value, 14%, negative predictive value, 57.3%) had a greater predictive power for determining OS than the iNLR (area under the ROC curve, 0.584; sensitivity, 50%; specificity, 66.9%; positive predictive value, 18.6%, negative predictive value, 68.4%). Using DFS as the end point, the area under the ROC curve for the NLRc (area under the ROC curve, 0.637; sensitivity, 50%; specificity, 82.3%; positive predictive value, 12.7%, negative predictive value, 59.7%) was higher than that for iNLR (area under the ROC curve, 0.560; sensitivity, 47.2%; specificity, 65.4%; positive predictive value, 16.2%, negative predictive value, 75.4%) (Fig. 2).
The NLRc was defined as negative (NLRc ≤ 0) when the postoperative NLR was lower than the preoperative NLR. The NLRc was defined as positive (NLRc > 0) when the postoperative NLR was higher than the preoperative NLR.
Associations between the neutrophil-to-lymphocyte ratio change and clinicopathological parameters
We analysed clinicopathological differences between patients with a negative and positive NLRc. The positive NLRc correlated to advanced AJCC stage (I, II vs. III; OR, 1.57; 95% CI: 1.12–2.2; P = 0.008), high T stage (1 vs. 2, 3, 4; OR, 1.39; 95% CI, 1.02–1.88; P = 0.037), high N stage (0, 1 vs. 2, 3; OR, 1.69; 95% CI, 1.21–2.36; P = 0.002), local recurrence (OR: 3.44, 95% CI: 1.55–7.62, P = 0.001), distant metastasis (OR: 2.79, 95% CI: 1.83–4.05, P < 0.001), perineural invasion (OR: 1.57, 95% CI: 1.08–2.29, P = 0.019), and administration of adjuvant chemotherapy (OR: 1.55, 95% CI: 1.125–2.15, P = 0.007) (Table 1). In addition, a positive NLRc was frequently observed in patients with AGC than in those with EGC (OR, 1.41; 95% CI, 1.04–1.91; P = 0.028). In comparisons of clinicopathological parameters between AGC and EGC, AGC was associated with high N stage (0, 1 vs. 2, 3; OR, 46.7; 95% CI, 24.7–88.29; P < 0.001), large tumour size (≤3 vs. >3 cm; OR, 10.9; 95% CI, 7.62–15.59; P < 0.001), tumour location (cardia fundus, body vs. antrum, pylorus; OR, 0.68; 95% CI, 0.51–0.91; P = 0.009), advanced histological grade (well, moderately vs. poorly, signet ring; OR, 2.52; 95% CI, 1.87–3.39; P < 0.001), diffuse type (OR, 2.26; 95% CI, 1.63–3.13; P < 0.001), lymphovascular (OR, 9.41; 95% CI, 6.68–13.27; P < 0.001) and perineural invasion (OR, 34.9; 95% CI, 16.02–76.06; P < 0.001), and administration of adjuvant chemotherapy (OR, 2.55; 95% CI, 1.85–3.5; P < 0.001).
Differences in disease-free and overall survival according to the neutrophil-to-lymphocyte ratio change
Based on previous studies18,19, the cut-off values of iNLR were <3 and ≥3. The iNLR was significantly related to overall survival (OS) (HR, 1.74; 95% CI, 1.22–2.48; P = 0.002), but not to disease-free survival (DFS) (HR, 1.38; 95% CI, 0.91–2.09; p = 0.128). After adjustment for confounders including age, T and N criteria, lymphovascular and perineural invasion, and margin involvement, there was no significant relationship between iNLR and DFS or OS (multivariate DFS HR, 0.8; 95% CI, 0.52–1.23; p = 0.306 and OS HR, 1.07; 95% CI, 0.74–1.54; p = 0.732) (Table 2).
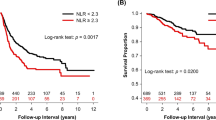
In univariate analyses of the NLRc, there was a significant difference in DFS (HR, 2.97; 95% CI, 2.13–4.14; p < 0.001) and OS (HR, 3.55; 95% CI, 2.61–4.81; p < 0.001) between the negative and positive groups. In multivariate analyses, positive NLRc was associated with worse DFS (HR, 2.53; 95% CI, 1.8–3.56; p < 0.001) and OS (HR, 1.45; 95% CI, 1.06–1.97; p < 0.001) (Fig. 3).
In univariate and multivariate analyses according to the Lauren classification, a positive NLRc was associated with poor DFS and OS in patients with intestinal (univariate DFS HR, 3.4; 95% CI, 2.04–5.65; p < 0.001 and OS HR, 4.34; 95% CI, 2.72–6.95; p < 0.001; multivariate DFS HR, 3.25; 95% CI, 1.91–5.52; p < 0.001 and OS HR, 4.56; 95% CI, 2.81–7.4; p < 0.001), diffuse (univariate DFS HR, 2.78; 95% CI, 1.62–4.79; p < 0.001 and OS HR, 3.53; 95% CI, 2.1–6.01; p < 0.001; multivariate DFS HR, 2.52; 95% CI, 1.42–4.49; p < 0.001 and OS HR, 2.92; 95% CI, 1.67–5.1; p = 0.002), or mixed type (univariate DFS HR, 2.28; 95% CI, 1.1–4.86; p = 0.028 and OS HR, 2.39; 95% CI, 1.25–4.56; p = 0.007; multivariate DFS HR, 2.52; 95% CI, 1.1–5.79; p = 0.013 and OS HR, 2.45; 95% CI, 1.21–4.97; p = 0.029) (Fig. 4).
In univariate and multivariate analyses according to histological grade, there were significant associations between NLRc and DFS and OS in patients with well (univariate DFS HR, 4.84; 95% CI, 1.72–13.64; p = 0.001 and OS HR, 4.01; 95% CI, 1.49–10.77; p = 0.003; multivariate DFS HR, 11.55; 95% CI, 2.89–46.15; p = 0.001 and OS HR, 5.82; 95% CI, 1.76–19.22; p = 0.004), poorly differentiated (univariate DFS HR, 2.52; 95% CI, 1.57–4.04; p < 0.001 and OS HR, 3.42; 95% CI, 2.2–5.29; p < 0.001; multivariate DFS HR, 2.36; 95% CI, 1.45–3.85; p = 0.001 and OS HR, 2.91; 95% CI, 1.86–4.56; p < 0.001), or signet ring cell types (univariate DFS HR, 3.83; 95% CI, 1.56–9.38; p = 0.002 and OS HR, 2.73; 95% CI, 1.05–7.08; p = 0.031; multivariate DFS HR, 3.94; 95% CI, 1.53–10.1; p = 0.004 and OS HR, 2.74; 95% CI, 1.01–7.42; p = 0.048). In univariate analyses of moderate histological grade, the NLRc was statistically related to DFS and OS (DFS HR, 2.48; 95% CI, 1.3–4.74; p = 0.004 and OS HR, 3.39; 95% CI, 1.94–5.91; p < 0.001). In multivariate analyses of moderate histological grade, the NLRc was statistically related to OS (HR, 3.84; 95% CI, 2.14–6.88; p < 0.001) but not to DFS (HR, 1.94; 95% CI, 0.98–3.83; p = 0.057) (Fig. 5).
In univariate and multivariate analyses according to adjuvant chemotherapy, there were significant associations between the NLRc and DFS or OS in patients receiving adjuvant chemotherapy (univariate DFS HR, 5.93; 95% CI, 3.3–10.64; p < 0.001 and OS HR, 4.62; 95% CI, 2.74–7.79; p < 0.001; multivariate DFS HR, 6.36; 95% CI, 3.47–11.67; p < 0.001 and OS HR, 4.93; 95% CI, 2.87–8.47; p < 0.001). In univariate analyses of patients not receiving adjuvant chemotherapy, the NLRc was linked to poor DFS and OS (DFS HR, 1.77; 95% CI, 1.14–2.76; p = 0.011 and OS HR, 2.9; 95% CI, 1.97–4.27; p < 0.001). In multivariate analyses, a positive NLRc was associated with poor OS (HR, 4.93; 95% CI, 2.87–8.47; p < 0.001) but not with worse DFS, (HR, 1.13; 95% CI, 0.71–1.8; p = 0.602) (Table 3).
Discussion
The systemic inflammatory reaction in the tumour microenvironment is essential for cancer growth and development. The concept of inflammation-based scores has been applied in many types of cancer. Neutrophils are thought to play a key role in normal physiological angiogenesis and tumour angiogenesis20,20. Activated neutrophils can release matrix metalloproteinases (MMPs), in particular MMP-9, which activate potent angiogenic factors (vascular endothelial growth factor, fibroblast growth factor-2)21,22. The increase in neutrophil count leads to induction of tumour progression and development of metastases via secretion of cytokines (tumor necrosis factor, IL-1, IL-6)23,24. In contrast, lymphocytes are a major factor for the suppression of cancer progression. Cytotoxic lymphocytes, which are ultimately responsible for killing tumour cells and eradicating the tumour, are applicable to cancer immunotherapy25. Based on the opposite functions of neutrophils and lymphocytes, the NLR might provide improved prognostic information regarding cancer progression.
The NLRc is a readily available and inexpensive biomarker for differential types of cancer. Nevertheless, the precise mechanisms by which the relationship between neutrophils and lymphocytes might predict clinical outcomes are not fully understood. A study by El-Hag et al. demonstrated that neutrophils lead to suppression of the cytolytic activity of immune cells such as lymphocytes, natural killer cells, and activated T cells26. In our results, a negative correlation between neutrophils and lymphocytes supports the above concept. Other published data have described several mechanisms in detail as follows: First, the anticancer responses of natural killer cells and activated T cells might be suppressed by marked neutrophil infiltration around the tumour27. Therefore, a high NLR might decrease the effects of the lymphocyte-mediated cellular immune response and promote cancer progression. Second, circulating neutrophils contribute to tumour growth and progression by producing cytokines. Considering the complexity and heterogeneity of cancer, the neutrophil count alone might not reflect a decreased lymphocyte-mediated immune response, and a low lymphocyte count alone might not reflect the neutrophil-driven tumour growth process. In addition, a study by Kobayashi et al. suggested that the NLR represents the relative extent of inflammation and host immunity against cancer, as its value is directly affected by the total count of neutrophils and lymphocytes. The combined effects of neutrophilia and lymphocytopenia might be better than the effect of a single marker for predicting clinical outcome.
In gastric cancer, a previous study reported the role of the iNLR without evaluation of the NLRc14,18,19. These studies focused only on the iNLR, not on the dynamic changes in the NLR after treatment. In different types of cancer, few studies have evaluated dynamic changes in the NLR after treatment. In studies of renal cell carcinoma, a negative NLRc was associated with favourable outcomes28. Another study demonstrated that the NLRc is related to clinicopathological parameters such as recurrence, tumour size, and stage29. In studies of non-small cell lung cancer and urothelial carcinoma, the NLRc had a greater statistical power than the iNLR in evaluating patient survival30,31. A positive NLRc indicates that the microenvironment supporting cancer growth is persistent, despite removing the causal risk factor related to inflammation after cancer treatment. Therefore, the NLRc after treatment is more meaningful than the iNLR at the time of the first diagnosis, because the NLRc reflects the dynamic change of the immune response after treatment. In our study, a positive NLRc had more statistical power than iNLR in predicting DFS and OS.
In summary, the NLRc is statistically associated with increased mortality rates in patients with gastric cancer and has better statistical power than the iNLR. Compared to molecular markers, the NLRc seems to be a convenient, easily obtainable and repeatable, low cost, and reliable predictor for patients with gastric cancer. Practically, this concept of the NLRc could provide clear, concise, and easily applicable information for evaluating prognosis in gastric cancer, which comprises a heterogeneous cancer cell population. Larger scale studies are required to better assess the relationships between the NLRc and prognosis of other types of cancer.
Materials and Methods
Patient selection
This retrospective study included 734 patients diagnosed with gastric adenocarcinoma at two hospitals between 2000 and 2013. The Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria were followed throughout this study. The inclusion criteria were: 1) patients with histopathological evidence of primary adenocarcinoma confirmed by pathologists, and known clinical outcome; and 2) patients with postoperative follow-up blood samples, which were collected in ethylenediaminetetraacetic acid-containing tubes according to other published studies32. The exclusion criteria were: patients diagnosed with adenocarcinoma but with inadequate clinical history and/or no available microscopic slides.
Interpretation of the neutrophil-to-lymphocyte ratio
Total white blood cells (WBCs), neutrophils, and lymphocytes were obtained by complete blood counts before surgery. A follow-up blood sample was obtained at 3–6 months after surgery.
The absolute neutrophil and lymphocyte counts were calculated by multiplying the percentage of each component by number of WBCs. The neutrophil-to-lymphocyte ratio was determined from the differential count by dividing the absolute neutrophil count by the absolute lymphocyte count. To evaluate the NLRc, we subtracted the postoperative NLR from the preoperative NLR: postoperative NLR – preoperative NLR = NLR change.
Statistical analysis
The associations between clinicopathological parameters and the NLRc were analysed by the chi-square test. The DFS time was defined as the time from the date of diagnosis to the date of local recurrence or new distant metastasis. The OS time was defined as the time from the date of diagnosis to all-cause death. Survival curves were generated using the Kaplan-Meier method and were compared by the log rank test. A Cox regression model was used for multivariate analysis. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistics (version 20.0, Chicago, IL, USA) and R packages (http://www.r-project.org/).
Ethics approval
This study (involving human participants) was approved by the Ethics Committee of the Eulji Hospital (EuljiIRB 15–62), and performed with respect to the ethical standards of the Declaration of Helsinki, as revised in 2008. The IRB review confirmed that the informed consent is not necessary in this study.
References
Uemura, N. et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345, 784–789 (2001).
Ma, C. et al. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability. Am J Surg Pathol 40, 1496–1506 (2016).
Lynch, H. T., Grady, W., Suriano, G. & Huntsman, D. Gastric cancer: new genetic developments. J Surg Oncol 90, 114–33; discussion 133 (2005).
Lauren, P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64, 31–49 (1965).
Jomrich, G. & Schoppmann, S. F. Targeted therapy in gastric cancer. European surgery: ACA: Acta chirurgica Austriaca 48, 278–284 (2016).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Benarafa, C. Tumor-induced inflammation alters neutrophil phenotype and disease progression. Breast Cancer Res 17, 135 (2015).
Liang, W. & Ferrara, N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunol Res 4, 83–91 (2016).
Baker, K. et al. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology 58, 1107–1116 (2011).
Kim, D.-K. et al. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer 9, 155 (2009).
Chen, F. et al. Preoperative Neutrophil-to-Lymphocyte Ratio Predicts the Prognosis of Oral Squamous Cell Carcinoma: A Large-Sample Prospective Study. J Oral Maxillofac Surg. https://doi.org/10.1016/j.joms.2016.11.022 (2016).
Oh, S. Y., Kim, Y. B. & Suh, K. W. Prognostic significance of systemic inflammatory response in stage II colorectal cancer. J Surg Res 208, 158–165 (2017).
Zhou, X. et al. The hematologic markers as prognostic factors in patients with resectable gastric cancer. Cancer Biomark 17, 359–367 (2016).
Kijima, T. et al. Combined Fibrinogen and Neutrophil-Lymphocyte Ratio as a Prognostic Marker of Advanced Esophageal Squamous Cell Carcinoma. Cancer Sci, https://doi.org/10.1111/cas.13127 (2016)
Ozal, E., Belen, E., Ozgun Cakmak, E., Durmus, G. & Pusuroglu, H. The Presence of Left Atrial Thrombus is Associated with the Neutrophil-to-Lymphocyte Ratio in Patients with Rheumatic Mitral Valve Stenosis. J Heart Valve Dis 25, 198–202 (2016).
Naess, A., Nilssen, S. S., Mo, R., Eide, G. E. & Sjursen, H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection, https://doi.org/10.1007/s15010-016-0972-1 (2016)
Jin, H. et al. Blood neutrophil-lymphocyte ratio predicts survival for stages III-IV gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol 11, 112 (2013).
Lee, S. et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 13, 350 (2013).
Tazzyman, S., Lewis, C. E. & Murdoch, C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol 90, 222–231 (2009).
Bergers, G. & Benjamin, L. E. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3, 401–410 (2003).
An, X. et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 15, 516–522 (2010).
Qiu, M. et al. Incidence of anemia, leukocytosis, and thrombocytosis in patients with solid tumors in China. Tumour Biol 31, 633–641 (2010).
Takahashi, R. et al. Prognostic significance of systemic neutrophil and leukocyte alterations in surgically treated endometrial cancer patients: a monoinstitutional study. Gynecol Oncol 137, 112–118 (2015).
Martínez-Lostao, L., Anel, A. & Pardo, J. How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res 21, 5047–5056 (2015).
el-Hag, A. & Clark, R. A. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol 139, 2406–2413 (1987).
Shau, H. Y. & Kim, A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol 141, 4395–4402 (1988).
Templeton, A. J. et al. Change in Neutrophil-to-lymphocyte Ratio in Response to Targeted Therapy for Metastatic Renal Cell Carcinoma as a Prognosticator and Biomarker of Efficacy. Eur Urol 70, 358–364 (2016).
Ohno, Y. et al. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol 187, 411–417 (2012).
Jin, F., Han, A., Shi, F., Kong, L. & Yu, J. The postoperative neutrophil-to-lymphocyte ratio and changes in this ratio predict survival after the complete resection of stage I non-small cell lung cancer. Onco Targets Ther 9, 6529–6537 (2016).
Kang, M., Jeong, C. W., Kwak, C., Kim, H. H. & Ku, J. H. The Prognostic Significance of the Early Postoperative Neutrophil-to-Lymphocyte Ratio in Patients with Urothelial Carcinoma of the Bladder Undergoing Radical Cystectomy. Ann Surg Oncol 23, 335–342 (2016).
Setälä, L. P. et al. Prognostic factors in gastric cancer: the value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br J Cancer 74, 766–772 (1996).
Acknowledgements
This work was supported by the research fund of Hanyang University (HY-2017). We are grateful to English teacher, Ina Jeong, for editing this manuscript.
Author information
Authors and Affiliations
Contributions
K.-W.M. contributed to acquisition and analysis of clinicopathological data, statistical analyses and manuscript preparation. M.J.K., D.H.K., B.K.S., E.K.K., Y.H.O. and Y.C.W. contributed to collect the data. D.H.K. and B.K.S. contributed to study design and data interpretation, and supervised the study.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Min, KW., Kwon, M.J., Kim, DH. et al. Persistent elevation of postoperative neutrophil-to-lymphocyte ratio: A better predictor of survival in gastric cancer than elevated preoperative neutrophil-to-lymphocyte ratio. Sci Rep 7, 13967 (2017). https://doi.org/10.1038/s41598-017-13969-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13969-x
This article is cited by
-
Changes in neutrophile-to-lymphocyte ratio as predictive and prognostic biomarker in metastatic prostate cancer treated with taxane-based chemotherapy
Discover Oncology (2022)
-
High postoperative neutrophil–lymphocyte ratio and low preoperative lymphocyte-monocyte ratio predict poor prognosis in gastric cancer patients receiving gastrectomy with positive lavage cytology: a retrospective cohort study
Langenbeck's Archives of Surgery (2021)
-
Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer?
Military Medical Research (2020)
-
The preoperative and the postoperative neutrophil-to-lymphocyte ratios both predict prognosis in gastric cancer patients
World Journal of Surgical Oncology (2020)
-
An increase in the neutrophil-to-lymphocyte ratio during adjuvant chemotherapy indicates a poor prognosis in patients with stage II or III gastric cancer
BMC Cancer (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.