Abstract
Feasible peripheral biomarker for Alzheimer’s disease (AD) is lacking. Dysregulation of N-methyl-D-aspartate (NMDA) receptor is implicated in the pathogenesis of AD. D-amino acid oxidase (DAO) and amino acids can regulate the NMDA receptor function. This study aimed to examine whether peripheral DAO and amino acids levels are characteristic of age-related cognitive decline. We enrolled 397 individuals (including amnestic mild cognitive impairment (MCI), mild AD, moderate to severe AD, and healthy elderly). DAO levels in the serum were measured using ELISA. Amino acids levels in serum were measured by high performance liquid chromatography. Severity of the cognitive deficits in subjects was assessed using Clinical Dementia Rating Scale (CDR). The DAO levels increased with the severity of the cognitive deficits. DAO levels were significantly associated with D-glutamate and D-serine levels. The Receiver Operating Characteristics analysis of DAO levels for AD patients vs. healthy controls determined the optimal cutoff value, 30.10, with high sensitivity (0.842) and specificity (0.889) (area under curve = 0.928). This is the first study indicating that the peripheral DAO levels may increase with age-related cognitive decline. The finding supports the hypofunction of NMDA receptor hypothesis in AD. Whether DAO could serve as a potential surrogate biomarker needs further studies.
Similar content being viewed by others
Introduction
Dementia is gaining increasing attention because of the high morbidity and mortality it causes in the population. Although cognitive deterioration is common in old age, the relationship between aging and degenerative dementia such as Alzheimer’s disease (AD) remains unclear. Whereas aging is a risk factor for AD, AD may not be an inevitable part of the aging process. The pathological changes that are detected in the brains of patients with AD, such as the presence of amyloid plaques and neurofibrillary tangles, are considered to appear several years before the development of clinical symptoms1. Therefore, early detection and treatment can help to prevent or slow the progression of AD.
Several mechanisms have been reported to be implicated in the pathogenesis of AD, one of them is dysregulation of glutamate neurotransmission. Glutamate plays a critical role in regulating neurogenesis, neurite outgrowth and synaptogenesis, neuronal survival, and synaptic plasticity2, and its signaling also underlies complex neuronal activities such as learning and memory3. The N-methyl-D-aspartate (NMDA) receptor, a subtype of ionotropic glutamate receptor, plays an important role in synaptic plasticity, learning, memory, and cognition4. In AD patients, glutamate levels were diminished in the cerebrospinal fluid (CSF)5 and in ante mortem brain tissue6, the number of glutamate terminals in the hippocampus were decreased7, and low levels of D-serine (an endogenous full agonist of the glycine site of NMDA receptor) and high levels of L-serine were observed in the serum8. Another study found that D-serine levels were higher in the CSF of probable AD patients than in control subjects9. The density of NMDA receptor also decreases with age10. Therefore, dysfunction in the glutamate neurotransmission via NMDA receptor may contribute substantially to the pathophysiology of AD.
To date, the diagnosing of AD or mild cognitive impairment (MCI, a term to describe a slight impairment in cognitive function that is accompanied with mostly normal function for daily activities)11 relies mainly on clinical manifestations. Favorable laboratory tests, particularly from peripheral approach, are still lacking. Gene expressions associated with modulating NMDA receptor function may be involved in the etiology of MCI and AD. D-amino acid oxidase (DAO) is a flavoenzyme that degrades D-amino acids, mainly D-serine12,13,14. Studies indicate that aging is related with reduced D-serine levels and D-serine treatment decreases the extent of neuron death, suggesting that D-serine has neuroprotective effect against apoptosis15. D-serine is critical for the proliferation and neuronal differentiation of neural stem cells16. A body of evidence suggests that DAO also plays a key role in the process of oxidative stress17,18.
Inhibiting the activity of DAO is one of the avenues to enhance NMDA activation. Our previous study demonstrated that sodium benzoate, a DAO inhibitor, is better than placebo in improving the cognitive function of patients with MCI or mild AD in a randomized, double-blind, placebo-controlled trial19. The aforementioned evidences suggest that DAO and its regulatory function on NMDA receptor may play important roles in the process of aging and its related cognitive decline.
It is difficult to collect sample from brain tissue. Thus, developing accessible peripheral biomarkers becomes more important for mental illness20. Peripheral gene expression may be a useful surrogate for gene expression in the CNS when the relevant gene is expressed in both21. We hypothesize that the level of DAO and related amino acids (including L-glutamate, D-glutamate, L-serine, D-serine, L-alanine, D-alanine, and glycine) in the peripheral blood may be associated with age-related cognitive decline. This study aims to examine whether DAO was over-expressed in people with age-related cognitive deficits, and could serve as a surrogate diagnostic biomarker.
Results
A total of 397 subjects were enrolled: 116 healthy controls, 77 patients with amnestic MCI, 128 patients with mild AD, and 76 patients with moderate to severe AD. There were significantly more male subjects in the healthy control group than the other three groups (p = 0.027). There were significant differences in age distribution and educational levels among the four groups (p < 0.001). The percentage of subjects using anti-dementia agents (including AChEI and memantine) were also significantly different among the amnestic MCI, mild AD, and moderate-severe AD groups (p = 0.001). Among the amino acids that we measured, there were significant inter-groups differences for L-glutamate level, D-glutamate level, L-serine level, D-serine level and D to L-glutamate ratio (p = 0.029, <0.001, 0.049, 0.012, 0.002, respectively). The demographic data of the four groups are summarized in Table 1.
We further matched the four groups in terms of gender, age, and education. In the matched cohort, there were no significant difference among the four groups in gender, age, and education (p = 0.957, 0.666, 0.780, respectively). Among the amnestic MCI, mild AD, and moderate-severe AD groups, there was also no significant difference in the frequence of anti-dementia agents use (p = 0.072). Among the amino acids that we measured, tIhere were significant inter-groups differences for D-glutamate level, glycine level, and D to L-glutamate ratio (p < 0.001, 0.037, 0.043, respectively) in the matched cohort. The demographic data of the matched cohort are summarized in Table 2.
DAO levels were higher in patients with cognitive impairment
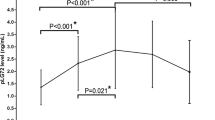
The DAO levels in the healthy controls, amnestic MCI, mild AD, and moderate to severe AD were 23.9 ± 11.2, 32.2 ± 10.8, 38.1 ± 14.4 and 41.4 ± 19.5, respectively (p < 0.001) (Table 1 and Fig. 1). Post-hoc analysis using Bonferroni method showed that the DAO level in healthy controls was significantly lower than that of patients with amnestic MCI, mild AD and moderate to severe AD (p < 0.001, p < 0.001, p < 0.001, respectively). The DAO level in patients with amnestic MCI was significantly lower than that of patients with mild AD and moderate to severe AD (p = 0.023, p < 0.001, respectively). There was no significant difference of DAO level between patients with mild AD and patients with moderate to severe AD (p = 0.642). The DAO level was significantly correlated with CDR score (r = 0.389, p < 0.001). The DAO levels in patients using anti-dementia drugs were very similar to those without anti-dementia drugs (Table 1).
For the matched cohort, the DAO levels in the healthy controls, amnestic MCI, mild AD and moderate to severe AD were 24.1 ± 11.3, 32.7 ± 11.6, 37.0 ± 14.0 and 43.5 ± 19.3, respectively (p < 0.001) (Table 2 and Fig. 2). Post-hoc analysis using Bonferroni method showed that the DAO level in healthy controls was significantly lower than that of patients with amnestic MCI, mild AD and moderate to severe AD (p = 0.022, p < 0.001, p < 0.001, respectively). The DAO level in patients with amnestic MCI was significantly lower than that of patients with moderate to severe AD (p = 0.003). There was no significant difference of DAO level between patients with amnestic MCI and patients with mild AD (p = 0.634). There was also no significant difference of DAO level between patient with mild AD and patients with moderate to severe AD (p = 0.096). The DAO level was also significantly correlated with CDR score in the matched cohort (r = 0.394, p < 0.001). The DAO levels in patients using anti-dementia drugs were also very similar to those without anti-dementia drugs in the matched cohort (Table 2).
DAO level was associated with D-glutamate and D-serine levels
The relationship between DAO and amino acids was testified in multiple linear regression analyses. The regression models were adjusted with age, sex and education. Due to the high co-linearity between amino acids levels and D- to L- form ratios, only amino acids levels were entered in the regression models because amino acids levels were more significantly associated with DAO levels than D- to L- form ratios (data not shown). In the overall cohort, DAO level was significantly associated with D-glutamate level, D-serine level and L-alanine level (adjusted R square = 0.290) (Table 3). In the matched cohort, DAO level was significantly associated with D-glutamate level (p < 0.001) and D-serine level (p = 0.007) (adjusted R square = 0.346) (Table 3).
Factors associated with cognitive function
CDR Score
Multiple linear regression analyses were used to test the potential factors associated with CDR score. The regression models were adjusted with age, sex and education. Similarly, D- to L- forms amino acids ratios were not entered in the regression model due to high co-linearity with amino acids levels. In the overall cohort, CDR score was significantly associated with age, education, DAO level, D-glutamate level and D-serine level (adjusted R square = 0.420) (Table 4). In the matched cohort, CDR was significantly associated with DAO level (p = 0.011), D-glutamate level (p = 0.001) and D-serine level (p = 0.005) (adjusted R square = 0.395) (Table 4).
MMSE Score
Multiple linear regression analyses were used to test the potential factors associated with MMSE score. The regression models were adjusted with age, sex and education. Similarly, D- to L- forms amino acids ratios were not entered in the regression model due to high co-linearity with amino acids levels. In the overall cohort, MMSE score was significantly associated with education, DAO level and D-glutamate level (adjusted R square = 0.344) (Supplementary Table 1). In the matched cohort, MMSE was significantly associated with DAO level (p < 0.001) (adjusted R square = 0.253) (Supplementary Table 1).
DAO, D-glutamate and D-serine levels differentiated between patients with AD and healthy controls
ROC analysis was applied to determine the cutoff value of DAO level and amino acids as potential predictors for AD by plotting the proportion of true-positive results (sensitivity) vs. the proportion of false-positive results (1 - specificity). Patients with amnestic MCI were excluded for this analysis because amnestic MCI has not yet been confirmed as a definite diagnosis. In the overall cohort, the ROC analysis revealed that DAO level for all AD patients vs. healthy controls determined an optimal cutoff value, 29.74, with a good sensitivity (0.823) and modest specificity (0.767) (AUC = 0.868). D-glutamate had the highest AUC (0.798) among all amino acids with an excellent sensitivity (0.967) and modest specificity (0.531) at the optimal cutoff value 975.04. D/L-glutamate ratio had similar AUC (0.784) with a good sensitivity (0.833) and modest specificity (0.708) at the optimal cutoff value 0.18. D-serine level also had good AUC (0.740) with a modest sensitivity (0.635) and good specificity (0.800) at the optimal cutoff value 35.85. We further combined DAO and amino acid levels to generate an equation by logistic regression model. The combination of DAO, D-glutamate and D-serine had good AUC (0.832) with a modest sensitivity (0.645) and excellent specificity (0.967) at the optimal cutoff value 37.00 (Table 5).
For the matched cohort, the ROC analysis revealed that DAO level for all AD patients vs. healthy controls determined an optimal cutoff value, 30.10, with a good sensitivity (0.842) and good specificity (0.889) (AUC = 0.928). D/L-glutamate ratio had the highest AUC (0.834) among all amino acids with a good sensitivity (0.889) and modest specificity (0.737) at the optimal cutoff value 0.18. D-glutamate level had similar AUC (0.813) with a good sensitivity (0.889) and modest specificity (0.632) at the optimal cutoff value 1054.24. D-serine level also had good AUC (0.739) with a modest sensitivity (0.544) and good specificity (0.833) at the optimal cutoff value 39.19. We also combined DAO and amino acid levels to generate an equation by logistic regression model. The combination of DAO and D-serine had excellent AUC (0.940) with a modest sensitivity (0.783) and excellent specificity (1.000) at the optimal cutoff value 80.18 (Table 5).
Discussion
To our knowledge, the current study is the first one demonstrating that the DAO levels in peripheral blood are higher in patients with MCI and AD than healthy individuals, and the peripheral DAO level is positively correlated with the severity of cognitive impairment. DAO can regulate the function of NMDA receptor via metabolizing D-amino acids, particularly D-serine. Our previous clinical trial using a DAO inhibitor, sodium benzoate, has shown beneficial effect for patients with early-phase AD19. The findings of higher DAO levels in patients with MCI and AD are in line with the attenuated NMDA receptor function hypothesis in the aging process and related cognitive decline10. More importantly, developing potential biomarker for cognitive aging from accessible peripheral blood is much more feasible than collecting samples from brain tissue or CSF, which makes early detection and prevention easier.
The findings from peripheral blood in our study support the previous study that D-serine levels were higher in the CSF of AD patients than in control subjects9. Treating neurons with both NMDA and D-serine produced an additive effect than D-serine alone in suppressing neuronal death15. In a 6-wk double-blind, placebo-controlled, crossover trial conducted on Parkinson’s disease patients, treatment with a D-serine adjuvant was shown to reduce the total scores of Unified Parkinson’s Disease Rating Scale, Simpson-Angus Scale for Extrapyramidal Symptoms, and Positive and Negative Syndrome Scale (PANSS)22. An earlier study found that D-serine level declines and DAO increases in the cerebellum of rats during early postnatal development23. A recent study found that the DAO mRNA expression levels were higher in the cerebellum compared to other brain regions, and the DAO mRNA levels were positively correlated with age less than 2 years in the cerebellum and amygdala in normal human post-mortem brain samples24. The above in vitro and in vivo studies suggest that neurotransmission via NMDA receptor is pivotal for regulating the cognitive function in the aging brain.
D-glutamate level was found to decrease with the severity of cognitive impairment in this study. D-glutamate can be detected in blood25, saliva26 and urine27. D-glutamate has been detected in several brain areas, peripheral tissues, plasma and urine of rodents by Hamase’s group28,29. The D-glutamate level and other amino acid levels in this study were similar to those in a previous study in human plasma30. Mangas et al. found that the D-glutamate levels were increased in several brain regions that are important for cognitive and behavioral regulations in rats31. Although L-glutamate is the most abundant excitatory neurotransmitter in the brain32, D-glutamate level is relatively higher in the cortex than other brain regions33, suggesting that D-glutamate may be important for high cortical functions. It is noteworthy to investigate the role of D-glutamate in aging in future studies.
Our finding of increased DAO and D-serine levels as well as decreased D-glutamate levels for people with cognitive aging in the matched cohort were very similar to that of the overall cohort. The findings suggest that the changes of DAO, D-glutamate and D-serine with cognitive deterioration are not affected by the demographic characterics including age, sex and education. This observation supports the viewpoint that cognitive decline may be not necessarily an inevitable part of the normal aging process. However, whether DAO and amino acids levels in patients with cognitive impairment really progress with aging needs further elucidation by prospective study.
Our study has several limitations. First, since this is a cross-sectional study, whether the findings keep constant over cognitive deteriorating process requires further prospective, longitudinal study. Second, although a previous GWAS study has found a suggestive evidence of association for the D-serine plasma-CSF ratio at the DAO gene from the general population34, the peripheral blood-CNS relationship of DAO and amino acids expressions also needs to be examined by studies among individuals with AD. Third, the amino acids levels were not measured for all participants in this study due to limited amounts of blood samples. However, the average sample size with amino acids levels in each group for the matched cohort is around 22, making the results still of some value. Fourth, our findings need to be testified in various racial populations by other groups. Fifth, D-aspartate level was not assayed in this study. D-aspartate can bind to the glutamate site of GluN2 receptor to enhance the NMDA receptor function35. Animal studies found that D-aspartate enhanced NMDA receptor-dependent long-term potentiation in the hippocampus36, and rescued the synaptic plasticity decay in the hippocampus of aged mice35. The role of D-aspartate in cognitive aging deserves further investigation. Lastly, other neurodegenerative diseases (e.g. amyotrophic lateral sclerosis and schizophrenia) that are associated with altered DAO level37,38 could not be entirely ruled out although we had excluded subjects with obvious brain or mental disorders by history, physical examinations and laboratory assessments.
Taken together, peripheral DAO levels are higher in patients with cognitive decline. The findings support the hypofunction of NMDA receptor-mediated neurotransmission hypothesis in MCI and AD39. Since AD is a complex and multifactorial disease, it is reasonable to combine different potential biomarkers for assisting the diagnosis. For example, combining DAO level with other potential tools, e.g. amino acids levels and β-amyloid40, for ascertainment might be a possible approach. In the future, the potential relationship of DAO levels and amino acids levels with treatment response for AD or a subpopulation of AD) requires clarification from larger samples of patients with different severities of cognitive deficits and under various treatments.
Methods
Participants
All subjects were screened and recruited from the following institutes: Department of Psychiatry of Kaohsiung Chang Gung Memorial Hospital, which is a major medical center in southern Taiwan, Department of Psychiatry of China Medical University Hospital, which is a major medical center in central Taiwan, and Department of Psychiatry of Taipei City Municipal Hospital, which is a major medical center in northern Taiwan. This study was approved by the institutional review boards of the aforementioned institutes, and carried out in accordance with the Declaration of Helsinki. After thorough description of the study to the subjects, written informed consents were obtained according to the IRB’s guidelines.
All subjects were Han Chinese, aged 50–100 years, who were physically healthy and had normal laboratory assessments (including blood routine and biochemical tests). Both patients and healthy controls were evaluated by research psychiatrists after a thorough medical workup. Patients were enrolled into this study if they [1] satisfied NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association)41 criteria for probable AD and had a Clinical Dementia Rating (CDR)42 score of 1, or criteria for amnestic MCI43 of a presumably degenerative nature defined as subjective memory complaint corroborated by an informant and insufficient global cognitive and functional impairment to meet NINCDS-ADRDA criteria and had a CDR score of 0.5, [2] had sufficient education to communicate effectively and were capable of completing the assessments of the study, and [3] agreed to participate in the study and provided informed consent. Exclusion criteria included history of significant cerebrovascular disease; Hachinski Ischemic Score >4; major neurological, psychiatric or medical conditions other than AD; substance (including alcohol) abuse or dependence; delusion, hallucination or delirium symptoms; severe visual or hearing loss; and inability to follow protocol.
There was no any Axis I or II psychiatric disorder for the healthy volunteers. All participants had no DSM-IV diagnosis of substance (including alcohol) abuse or dependence to exclude potential confounding effects. Patients with amnestic MCI had a CDR score of 0.5. Patients with mild AD had a CDR score of 1 and patients with moderate to severe AD had a CDR score 2 or greater.
Both anti-dementia drugs free and medicated patients with AD were recruited for examining possible drug effects on the DAO level. Drug-free AD patients had not taken any anti-dementia drug for at least three months. For patients who had already been on anti-dementia drugs therapy, anti-dementia drugs had to be continued for at least three months with unchanged dose before enrollment. Psychotropic use history was ascertained by interviewing the patients and their family members or care givers, contacting other health care providers and reviewing medical records. Healthy individuals had no history of exposure to anti-dementia drugs. All patients with AD were recruited from the outpatient clinic at the aforementioned institutes, and all healthy volunteers were from the communities in northern, central and southern Taiwan.
Among 281 patients with amnestic MCI or AD, 216 were anti-dementia drug free for 3 months or longer and the other 65 were stabilized on anti-dementia drugs (39 donepezil, 14 rivastigmine, 10 galantamine, 2 memantine) for at least 3 months. Among the drug-free patients, 206 patients were drug-naïve and the other 10 were nondrug-naïve.
Cognitive Function Assessments
The participant’s cognitive function was assessed by CDR and Mini-Mental State Examination (MMSE)44. MMSE is a commonly used tool for the measurement of cognitive impairment and the screening for dementia in the elderly people44. However, one of the disadvantages to the utilization of the MMSE is that it is affected by age and education45. Another disadvantage of the MMSE is its lower level of sensitivity for mild degrees of impairment46.
On the other hand, CDR exhibits excellent discriminatory ability in the very mild stages of dementia47. Besides, studies have shown that the CDR appears to be a reliable and valid tool for assessing and staging dementia with moderate to high overall inter-rater reliability48. Therefore, the grouping of subjects and further analysis with blood parameters in this study were based on CDR scores, which can reflect the global cognitive impairment of the subjects and not be affected by age and education level49.
Laboratory Assessments
For both patients and healthy controls, blood sampling was done during 8–12AM after fasting for more than eight hours. Ten ml of blood was collected by personnel trained in phlebotomy using sterile technique. The blood specimens were processed immediately by centrifugation at 1000 x g. After centrifugation, serum was quickly dissected, immediately stored at −80 °C until further measurement.
DAO level measurement
DAO protein concentrations were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacture’s recommended protocol (Cloud-Clone Corp, Houston, TX, USA). Briefly, 100 μL serum samples and the standard were added to each well of a 96-well plate. The solutions were incubated for 1 hour at 37 °C. The liquid was then removed. 100 μL Detection Reagent A was added to each well and incubated for 1 hour at 37 °C. Each well was washed for 3 times. 100 μL of Detection Reagent B was added to each well, and the solutions were incubated for 30 minutes at 37 °C. Each well was washed for 5 times and then incubated with 90 μL substrate solution for 20 min at 37 °C with the protection from light. 50 μL stop solution was added to each well. A Benchmark Plus Microplate Reader (Bio-Rad) was used to read the optical density at 450 nm. The concentrations of DAO in the samples were determined according to a standard curve.
According the instruction manual of the DAO ELISA kit, the assay has high sensitivity (the minimum detectable dose of DAO is typically less than 0.56 ng/mL) and excellent specificity for detection of DAO. To the best of current skills and knowledge, no significant cross-reactivity or interference between DAO and analogues was observed.
Amino acids levels measurement
Serum was firstly extracted by methanol (1:3, by volume), then filtered after 15 min centrifugation (1500 × g) with nylon membranes (0.45 mM, Minisart SRP4, Sartorius, Germany). The filtrate was diluted with proper amount of 20% methanol then derivatized with N-isobutyl-L-cycteine (IBC) and O-phthaldialdehyde mixture for 5 minutes then injected into high performance liquid chromatography (HPLC, L-7100 Pump, L7250 Autosampler, L-7250, with L7480 flourscence Detector, Hitachi, Japan) for analysis. Analytical column (Grom-Sil OPA-2, 5 µm, 250 mm * 4 mm, Part No: GSOP 20512S2504, SAP No: 5113679, Grace, US) with guard column (Grom-Sil OPA-2, 5 µm, 10 mm × 4 mm, Part No: GSOP20512v0104V, Grace, US) were used for the determination. Isocratic elution of mobile phase A (23 mM sodium acetate, pH 6.0) and B (50 mL acetonitrile in 600 mL methanol) were performed under fluorescence detection (excitation 260 nm, emission 455 nm), respectively. Retention time of each amino acid was L-glutamine, 25.5 min; D-glutamine, 27.3 min; L-serine, 33.6 min; D-serine, 35.8 min; glycine, 41.5 min; L-alanine, 47.2 min; D-alanine, 50.3 min, respectively. All amino acids levels were double-checked by performing HPLC analyses for two times in order to confirm that the peaks were not artifact.
Statistical Analysis
All subjects’ clinical characteristics, DAO levels and amino acids levels were presented as mean ± SD or number (percentage). All statistical methods were performed using IBM SPSS Statistics version 22.0 (SPSS inc.). All mean values between groups were compared using independent t test or Mann-Whitney U test for two groups, one-way ANOVA or Kruskal-Wallis test for three groups, and percentages using χ2 test. Multiple linear regression and Receiver Operating Characteristics (ROC) analysis were used to generate predictive models and to evaluate for the significant predictors of AD patients. A P value less than 0.05 was considered statistically significant.
References
Holtzman, D. M., Morris, J. C. & Goate, A. M. Alzheimer’s Disease: The Challenge of the Second Century. Sci. Transl. Med 3 (2011).
Mattson, M. P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci 1144, 97–112, https://doi.org/10.1196/annals.1418.005 (2008).
Tilleux, S. & Hermans, E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res 85, 2059–2070, https://doi.org/10.1002/jnr.21325 (2007).
McDonald, J. W. & Johnston, M. V. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev 15, 41–70 (1990).
Martinez, M., Frank, A., Diez-Tejedor, E. & Hernanz, A. Amino acid concentrations in cerebrospinal fluid and serum in Alzheimer’s disease and vascular dementia. J. Neural Transm. Park. Dis. Dement. Sect. 6, 1–9 (1993).
Lowe, S. L., Bowen, D. M., Francis, P. T. & Neary, D. Ante mortem cerebral amino acid concentrations indicate selective degeneration of glutamate-enriched neurons in Alzheimer’s disease. Neuroscience 38, 571–577 (1990).
Cowburn, R., Hardy, J., Roberts, P. & Briggs, R. Regional distribution of pre- and postsynaptic glutamatergic function in Alzheimer’s disease. Brain Res 452, 403–407 (1988).
Hashimoto, K. et al. Possible role of D-serine in the pathophysiology of Alzheimer’s disease. Prog. Neuropsychopharmacol. B ol. Psychiatry 28, 385–388 (2004).
Madeira, C. et al. d-serine levels in Alzheimer’s disease: implications for novel biomarker development. Translational psychiatry 5, e561, https://doi.org/10.1038/tp.2015.52 (2015).
Segovia, G., Porras, A., Del Arco, A. & Mora, F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev 122, 1–29 (2001).
Levey, A., Lah, J., Goldstein, F., Steenland, K. & Bliwise, D. Mild cognitive impairment: an opportunity to identify patients at high risk for progression to Alzheimer’s disease. Clin. Ther. 28, 991–1001, https://doi.org/10.1016/j.clinthera.2006.07.006 (2006).
Fukui, K. & Miyake, Y. Molecular cloning and chromosomal localization of a human gene encoding D-amino-acid oxidase. The Journal of biological chemistry 267, 18631–18638 (1992).
Vanoni, M. A. et al. Limited proteolysis and X-ray crystallography reveal the origin of substrate specificity and of the rate-limiting product release during oxidation of D-amino acids catalyzed by mammalian D-amino acid oxidase. Biochemistry 36, 5624–5632, https://doi.org/10.1021/bi963023s (1997).
Sasabe, J. et al. D-amino acid oxidase controls motoneuron degeneration through D-serine. Proc Natl Acad Sci USA 109, 627–632, https://doi.org/10.1073/pnas.1114639109 (2012).
Esposito, S. et al. Contribution of serine racemase/d-serine pathway to neuronal apoptosis. Aging Cell, doi:https://doi.org/10.1111/j.1474-9726.2012.00822.x (2012).
Huang, X. et al. D-Serine regulates proliferation and neuronal differentiation of neural stem cells from postnatal mouse forebrain. CNS Neurosci Ther 18, 4–13, https://doi.org/10.1111/j.1755-5949.2011.00276.x (2012).
Lu, J. M., Gong, N., Wang, Y. C. & Wang, Y. X. D-Amino acid oxidase-mediated increase in spinal hydrogen peroxide is mainly responsible for formalin-induced tonic pain. British journal of pharmacology 165, 1941–1955, https://doi.org/10.1111/j.1476-5381.2011.01680.x (2012).
Stegman, L. D. et al. Induction of cytotoxic oxidative stress by D-alanine in brain tumor cells expressing Rhodotorula gracilis D-amino acid oxidase: a cancer gene therapy strategy. Human gene therapy 9, 185–193, https://doi.org/10.1089/hum.1998.9.2-185 (1998).
Lin, C. H. et al. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: a randomized, double-blind, placebo-controlled trial. Biological psychiatry 75, 678–685, https://doi.org/10.1016/j.biopsych.2013.08.010 (2014).
Ilani, T. et al. A peripheral marker for schizophrenia: Increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proceedings of the National Academy of Sciences of the United States of America 98, 625–628 (2001).
Sullivan, P. F., Fan, C. & Perou, C. M. Evaluating the comparability of gene expression in blood and brain. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics 141B, 261–268, https://doi.org/10.1002/ajmg.b.30272 (2006).
Gelfin, E. et al. D-serine adjuvant treatment alleviates behavioural and motor symptoms in Parkinson’s disease. Int J Neuropsychopharmacol 15, 543–549, https://doi.org/10.1017/S1461145711001015 (2012).
Hashimoto, A. Effect of the intracerebroventricular and systemic administration of L-serine on the concentrations of D- and L-serine in several brain areas and periphery of rat. Brain research 955, 214–220 (2002).
Jagannath, V., Marinova, Z., Monoranu, C. M., Walitza, S. & Grunblatt, E. Expression of D-Amino Acid Oxidase (DAO/DAAO) and D-Amino Acid Oxidase Activator (DAOA/G72) during Development and Aging in the Human Post-mortemBrain. Frontiers in neuroanatomy 11, 31, https://doi.org/10.3389/fnana.2017.00031 (2017).
Heresco-Levy, U. et al. High glycine levels are associated with prepulse inhibition deficits in chronic schizophrenia patients. Schizophr Res 91, 14–21, https://doi.org/10.1016/j.schres.2006.12.003 (2007).
Tsuruoka, M. et al. Capillary electrophoresis-mass spectrometry-based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis 34, 2865–2872, https://doi.org/10.1002/elps.201300019 (2013).
Gronwald, W. et al. Detection of autosomal dominant polycystic kidney disease by NMR spectroscopic fingerprinting of urine. Kidney Int 79, 1244–1253, https://doi.org/10.1038/ki.2011.30 (2011).
Han, H. et al. Simultaneous determination of D-aspartic acid and D-glutamic acid in rat tissues and physiological fluids using a multi-loop two-dimensional HPLC procedure. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 879, 3196–3202, https://doi.org/10.1016/j.jchromb.2011.01.023 (2011).
Han, H. et al. Changes in D-aspartic acid and D-glutamic acid levels in the tissues and physiological fluids of mice with various D-aspartate oxidase activities. Journal of pharmaceutical and biomedical analysis 116, 47–52, https://doi.org/10.1016/j.jpba.2015.05.013 (2015).
Grant, S. L., Shulman, Y., Tibbo, P., Hampson, D. R. & Baker, G. B. Determination of d-serine and related neuroactive amino acids in human plasma by high-performance liquid chromatography with fluorimetric detection. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 844, 278–282, https://doi.org/10.1016/j.jchromb.2006.07.022 (2006).
Mangas, A. et al. Immunocytochemical visualization of D-glutamate in the rat brain. Neuroscience 144, 654–664, https://doi.org/10.1016/j.neuroscience.2006.09.045 (2007).
Meldrum, B. S. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 130, 1007S–1015S (2000).
Weatherly, C. A. et al. d-Amino Acid Levels in Perfused Mouse Brain Tissue andBlood: A Comparative Study. ACS chemical neuroscience, doi:https://doi.org/10.1021/acschemneuro.6b00398 (2017).
Luykx, J. J. et al. Genome-wide association study of NMDA receptor coagonists in human cerebrospinal fluid and plasma. Molecular psychiatry 20, 1557–1564, https://doi.org/10.1038/mp.2014.190 (2015).
Errico, F. et al. Increased D-aspartate brain content rescues hippocampal age-related synaptic plasticity deterioration of mice. Neurobiology of aging 32, 2229–2243, https://doi.org/10.1016/j.neurobiolaging.2010.01.002 (2011).
Errico, F. et al. Increased levels of d-aspartate in the hippocampus enhance LTP but do not facilitate cognitive flexibility. Molecular and cellular neurosciences 37, 236–246, https://doi.org/10.1016/j.mcn.2007.09.012 (2008).
Liu, Y. L. et al. Haplotypes of the D-Amino Acid Oxidase Gene Are Significantly Associated with Schizophrenia and Its Neurocognitive Deficits. PloS one 11, e0150435, https://doi.org/10.1371/journal.pone.0150435 (2016).
Paul, P. & de Belleroche, J. Experimental approaches for elucidating co-agonist regulation of NMDA receptor in motor neurons: Therapeutic implications for amyotrophic lateral sclerosis (ALS). Journal of pharmaceutical and biomedical analysis 116, 2–6, https://doi.org/10.1016/j.jpba.2014.12.040 (2015).
Lin, C. H., Huang, Y. J., Lin, C. J., Lane, H. Y. & Tsai, G. E. NMDA neurotransmission dysfunction in mild cognitive impairment and Alzheimer’s disease. Current pharmaceutical design 20, 5169–5179 (2014).
Glenner, G. G. & Wong, C. W. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochemical and biophysical research communications 120, 885–890 (1984).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944 (1984).
Morris, J. C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414 (1993).
Lu, P. H. et al. Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology 72, 2115–2121, https://doi.org/10.1212/WNL.0b013e3181aa52d3 (2009).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 12, 189–198 (1975).
Crum, R. M., Anthony, J. C., Bassett, S. S. & Folstein, M. F. Population-based norms for the Mini-Mental State Examination by age and educational level. Jama 269, 2386–2391 (1993).
Tombaugh, T. N. & McIntyre, N. J. The mini-mental state examination: a comprehensive review. Journal of the American Geriatrics Society 40, 922–935 (1992).
Lim, W. S., Chong, M. S. & Sahadevan, S. Utility of the clinical dementia rating in Asian populations. Clinical medicine & research 5, 61–70, https://doi.org/10.3121/cmr.2007.693 (2007).
Rockwood, K., Strang, D., MacKnight, C., Downer, R. & Morris, J. C. Interrater reliability of the Clinical Dementia Rating in a multicenter trial. Journal of the American Geriatrics Society 48, 558–559 (2000).
Hughes, C. P., Berg, L., Danziger, W. L., Coben, L. A. & Martin, R. L. A new clinical scale for the staging of dementia. The British journal of psychiatry: the journal of mental science 140, 566–572 (1982).
Acknowledgements
This work was funded by Ministry of Science and Technology, Taiwan (NSC 99-3114-B-182A-003-, NSC 101-2314-B-182A-073-MY2, MOST 105-2314-B-182A-059-), Kaohsiung Chang Gung Memorial Hospital, Taiwan (CMRPG8E1041), China Medical University Hospital, Taiwan (DMR-106-099) and Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW106-TDU-B-212-113004). The aforementioned institutes had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
C.H.L. and H.Y.L. involved in conception and design, literature review and interpretation, and manuscript writing; H.T.Y. involved in amino acids levels measurement; C.H.L., C.C.C. and H.Y.L. involved in subjects recruitment; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, CH., Yang, HT., Chiu, CC. et al. Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging. Sci Rep 7, 14849 (2017). https://doi.org/10.1038/s41598-017-13951-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13951-7
This article is cited by
-
Promising applications of D-amino acids in periprosthetic joint infection
Bone Research (2023)
-
Inhibition of CK2 mitigates Alzheimer’s tau pathology by preventing NR2B synaptic mislocalization
Acta Neuropathologica Communications (2022)
-
High performance liquid chromatography determination of l-glutamate, l-glutamine and glycine content in brain, cerebrospinal fluid and blood serum of patients affected by Alzheimer’s disease
Amino Acids (2021)
-
Redox signaling and Alzheimer’s disease: from pathomechanism insights to biomarker discovery and therapy strategy
Biomarker Research (2020)
-
Development of a cognitive function marker based on D-amino acid proportions using new chiral tandem LC-MS/MS systems
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.