Abstract
Dissolved gas analysis (DGA) is widely used in monitoring and diagnosing of power transformer, since the insulation material in the power transformer decomposes gases under abnormal operation condition. Among the gases, acetylene, as a symbol of low energy spark discharge and high energy electrical faults (arc discharge) of power transformer, is an important monitoring parameter. The current gas detection method used by the online DGA equipment suffers from problems such as cross sensitivity, electromagnetic compatibility and reliability. In this paper, an optical gas detection system based on TDLAS technology is proposed to detect acetylene dissolved in transformer oil. We selected a 1530.370 nm laser in the near infrared wavelength range to correspond to the absorption peak of acetylene, while using the wavelength modulation strategy and Herriott cell to improve the detection precision. Results show that the limit of detection reaches 0.49 ppm. The detection system responds quickly to changes of gas concentration and is easily to maintenance while has no electromagnetic interference, cross-sensitivity, or carrier gas. In addition, a complete detection process of the system takes only 8 minutes, implying a practical prospect of online monitoring technology.
Similar content being viewed by others
Introduction
Power transformers are important equipment in the power system, and the stable operation of the transformer is essential for power transmission. In order to prevent the transformer outage due to failure, it is important to monitoring the condition of the transformer1,2.
When specific defects such as overheating, partial discharge, or arc discharge occurs in the transformer, the insulation oil or paper decomposes and produces gases, and the gases are dissolved in insulating oil3,4,5,6. Then, the decomposition products in the oil reduce the insulation properties and change the interfacial tensor3. Thus, aging or defect information can be obtained by analyzing the volume, types, proportions, and rate of production of dissolved gases7,8.
The detection of dissolved gases in liquids has many important applications in different industries, such as water quality assessment (oxygen concentration in water), carbon dioxide concentration in carbonated beverages, and methane detection in crude oil. Besides that, dissolved gas analysis and detection in transformer oil is a useful method for evaluating the transformer condition9,10,11.
Dissolved gas analysis (DGA) is an oil-immersed transformer fault prediction and detection technique, based on the measurement of dissolved gases12. And there are some methods to detect fault gases, such as: gas chromatography (GC)13, thermal conductivity detector (TCD) and optical methods such as: spectral absorption method, photoacoustic spectroscopy14,15. However, these methods have following drawbacks:
-
1)
GC is a widely used technique for quantitatively measuring fault gases concentration. Nevertheless, this analytical technique is not effective for on-line monitoring due to its large size, unstable in field and reliance on carrier gas and columns. After that, the chromatography analysis takes much time16. In addition, physical or chemical detectors are susceptible to cross-sensitivity, while electromagnetic interference and reliability also affect detection accuracy17.
-
2)
The mechanical vibration and noise signals in the substation seriously affect the normal photoacoustic detection.
-
3)
The main drawback of the direct absorption spectroscopy method is that it measures tiny signals at the top of the large background signal. Thus, any noise introduced by the light source or system reduce the detectability of the technology. Therefore, the sensitivity should be improved for field application.
-
4)
Laser calorimetry spectroscopy (LCS), an in-liquid detection technique, will be a promising technology if it can achieve a lower detection limit, but at present, the minimum detection limit for acetylene in transformer oil is 10 ppm18 which does not meet the requirement of DGA.
The direct absorption spectroscopy can be improved in two aspects; to reduce the noise in the signal and to increase the absorption. And modulation techniques can reduce noise. Acetylene is produced in low energy spark discharge and high energy electrical faults (arc discharge). And acetylene detection is of great importance to monitor the incipient faults. Both Ding et al. and He et al. use tunable diode laser absorption spectroscopy (TDLAS) technology to detect acetylene gas, but their limit of detection do not meet the requirements for transformer condition monitoring19,20. Moreover, their experiments lack the discussion of the precision of the test. Thus, there is still a gap from the practical application. Deng et al. used Herriott cell to increase the optical path and detect acetylene gas, but these achievements are made in the gas conditions, unsuitable for transformer oil dissolved gas detection21.
There are some problems need to be solved if we want to use TDLAS technology and Herriott cell for gas detection in transformer oil. For example, if the output laser is directly irradiated in the transformer oil, most of the light intensity will be absorbed by the oil, so the gas cannot be detected. If we increase the optical path to improve the absorption of gas, according to our test results, when the oil sample thickness of 4 cm, only 52.3% of the light intensity through the oil sample, for wavelength of 1557 nm. To solve the problems, a new method is proposed in the paper.
Herriott cell cannot be directly used in oil immersion conditions, since the insulating oil pollutes the mirrors in Herriott cell. Thus, we designed a separation device, using the vacuum degassing method to determine the degassing efficiency, and carried out the calibration test. In the manuscript, the results of the calibration test show that TDLAS technology and Herriott cell can be used to detect dissolved gases in the oil using our designed equipment.
In our case, we modulate the output wavelength of the laser. Near infrared DFB lasers are used in manuscript, for it has an economical advantage over mid-infrared lasers. And the detection sensitivity of the proposed measuring system is also satisfied. The experimental device designed in the manuscript uses Herriott cell and wavelength modulation to improve the monitoring sensitivity, and to meet the requirements of online testing.
Theory of TDLAS technology
When a beam of light passes through an absorption cell with a certain concentration of gas, the light intensity will be attenuated to some extent. This phenomenon reflects the change in gas concentration, and the gas concentration can be determined by measuring the intensity attenuation, which is called Beer-Lambert’s law.
Beer-Lambert Law can be expressed as:
where I (v) and I0 (v) are intensities of incident and emitted laser respectively, V. C is the concentration of gas to be detected, ppm. L is the path length of the beam of light through the material sample, m. α(v) is the absorption coefficient per unit distance and per unit concentration of the gas, cm/mol. A is absorbance.
The derivative of A shows a linear relationship between the amplitude and the concentration C as other conditions do not change. When n = 2, the 2 f signal is
The different order derivatives of A are shown in Fig. 1.
According to HITRAN database, acetylene has 5560 absorption lines in the near infrared band (780 nm ~ 2526 nm. When it comes to absorption lines, the high absorption intensity and the avoidance of interference are the main concerns. For these considerations, we chose the “P9 peak” of acetylene (1530.370 nm) as the central wavelength of the DFB laser. The molecule absorption intensity distribution of acetylene is shown in Fig. 2, available from website http://hitran.org/.
TDLAS uses a laser with a line width much smaller than the gas absorption line width, and a single absorption line of the gas is scanned by wavelength modulation22,23. Then, we can obtain the harmonic component of the absorption signal through the lock-in amplifier, and the concentration of the test gas is proportional to the amplitude of the harmonic signal.
The coefficient of the odd harmonic component is zero at the center wavelength and the even harmonic is the maximum. Since the amplitude of the harmonic signal decreases with increasing order, we choose the second harmonic as the actual detection signal. As a calibration experiment is carried out to determine the relationship between the gas concentration and the second harmonic signal amplitude, we can measure the harmonic signal to obtain gas concentration in field application24.
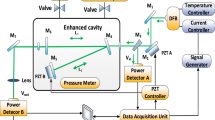
Experimental setup
The test platform is mainly composed of oil chamber, gas detection system and vacuum device. Among them, the oil chamber is designed to complete the oil and gas separation. The gas to be measured then passed into the air chamber and the acetylene concentration information is detected by the TDLAS controller and the acquisition system. The vacuum pump is used to make the vacuum of the gas detection system and to remove the impurity gas (including the last test residual gas).
Gas preparation method is as follows: Use a syringe to take 40 mL of oil, inject 5 mL of acetylene gas and seal it. Place the syringes on a vibrating and heating platform for oil-gas mixing. The heating temperature is set to be 50 degrees Celsius while the vibration lasts half hour. Take a few drops from the syringes to the beaker, then add different volume pure transformer oil to obtain the different concentrations test oil samples.
Seven groups of different concentrations of oil were prepared in the test, about 500 mL in each group, of which 300 mL was used for oil-gas separation and TDLAS testing, and 40 mL was used for gas chromatography. The gas detection system uses a multi-pass Herriott cell, which has the advantages of small size (34 cm), long optical path length (10.1 m), strong absorbance and high sensitivity. The experimental setup is shown in Fig. 3. The photodetector used by measuring system was encapsulated in metal shield, which has good electromagnetic compatibility.
The test process involves two types of oil samples: pure oil and dissolved acetylene oil. The test should be in accordance with the following steps:
-
Test sequence: pure oil → dissolved acetylene oil (from low to high concentration).
-
Ensure the oil chamber and air chamber air tightness.
-
When the transformer oil enters and avoid polluting the air chamber, slowly open the exhaust valve within 1 minute.
-
Before vacuum extraction, oil sample is prepared for offline DGA test in the lab for 3 times and the average value is recorded.
The main procedure of TDLAS detection includes oil sample preparation, vacuum extraction, oil injection, oil and gas separation, acetylene detection and data recording and processing, as shown in Fig. 4. Detection process does not require carrier gas. After oil and gas separation, the transformer oil by degassing, filtration can be injected back to the transformer.
It should be noted that the operating time of the TDLAS gas detection system includes the following steps: vacuum extraction, 2 minute; oil injection, 1 minute; oil and gas separation, 2 min; gas detection and data processing, 1 minute; emptying the oil chamber, 2 min. Therefore, the entire detection process is less than 8 minutes which can meet the needs of on-line monitoring and rapid measurement.
The detection system can respond quickly to changes in gas concentration. When the oil and gas separation is complete, the detection system can get the gas signal within 5 seconds.
Results
Cross-sensitivity
Typically, there are 7 fault gases produced by oxidation, insulation decomposition, and oil breakdown: H2, CH4, C2H2, C2H4, C2H6, CO, CO2. H2O is also included in the oil.
In order to determine whether there is a multi-gas cross-sensitive problem in the selected wavelength range, we query the HITRAN database and perform cross-sensitivity testing. The absence of absorption lines of CH4, C2H2, H2O, and CO2 from HITRAN in the vicinity of 1530.370 nm are shown in Fig. 5(a). In the region, water (other impurities absorption intensity is weak) has absorption line, but the absorption intensity of water is four orders of magnitude lower than that of acetylene. Besides that, the water content in the oil is strictly controlled to very small for insulation strength. The impact of these impurities on the detection of acetylene is minimal. The gas listed in Fig. 5(b) is the fault gas that characterizes the transformer failure. It can be seen that these impurities do not affect acetylene detection.
Therefore, the selection of wavelengths of 1530.370 nm absorption lines can avoid H2, CH4, C2H4, C2H6, CO, CO2 gas cross-interference.
In the same environment, different concentrations of C2H2, H2, CO2, CH4, C2H6, C2H4 and CO, ranging from 200 to 1000 ppm were mixed and measured, and 2 f signals were shown in Fig. 5(b), where only acetylene gas has a significant absorption signal, the absorption of other gases does not exceed 2% of acetylene.
The results of cross sensitivity test showed that H2, CH4, C2H4, C2H6, CO, and CO2 do not change with the increase of concentration in the selected wavelength range. Only the acetylene gas concentration and the second harmonic signal showed a significant positive correlation. Concluded from the absorption intensity in the HITRAN database and the result of multi gases interference tests, the detection of acetylene in this wavelength range is not affected by the concentration of other gases.
Result of calibration
According to the procedure mentioned in Fig. 4, the prepared oil samples were injected into the oil chamber. The detection performance indexes of the TDLAS system are obtained with experiment, including goodness of fit, repeatability, limit of detection.
There were seven different concentrations acetylene of oil in the test. Six oil samples were prepared for each concentration. We tested a total of 42 oil samples and shown the test result at Fig. 6. Equation (4) is calculated based on the actual measurement of all 42 oil samples.
Goodness of fit
The test results show that 2 f signal is proportional to the concentration of acetylene gas dissolved in the oil as shown in Fig. 6(c). According to 2 f signal and gas concentration to fit, the expression is:
x is the concentration of gas to be detected, ppm. y is the 2 f signal amplitude, V.
The coefficient of determination R2 is 0.9965. A linear relationship between peak-peak value of 2 f signal and acetylene concentration can be obtained. And the sensitivity can be calculated as 7.1 mV/ppm.
To investigate the goodness of fitting, the amplitude of 2 f signal was substituted into fitting equation, and residual errors were obtained by subtracting calculated value from conventional DGA results, as shown in Fig. 6(d).
Repeatability
In the test, all seven different concentrations oil samples were tested for six times.
No. 6 concentration oil samples are the most dispersed. No.6 oil samples concentrations is 186.9 ppm, measured by GC (gas chromatography). The average concentration of the six tests is 176.6 ppm. The deviation is 5.6% of the value measured by GC.
The system has better performance in lower concentration range (below 50 ppm).The maximum concentration deviation is 1.4 ppm.
The results show that the trace gas measurement system has good repeatability.
Limit of detection
In order to obtain limit of detection, a signal-noise ratio (SNR) method is commonly used. It is generally considered that the signal-to-noise ratio at 3: 1 is acceptable for the limit of detection. The initial noise data that not been pre-processed has a large effect on the detection limit, so we use Savitzky–Golay filter25,26 to reduce the effect of high frequency noise on the calculation results. The results of the filtering are shown in Fig. 7.
Then the blank noise is 0.0163 V, and the limit of detection is:
Test results of operational transformer
We obtained four oil samples from two operational transformers in the 220 kV substation. The test results are shown in Table 1. Oil samples were taken from Gukeng substation 220 kV transformer, Dongguan City, Guangdong Province, China. For the transformer, high concentration of acetylene indicates that the transformer has some defects that may induce transformer failure, and low concentration we measured indicates that the transformer is operating in good condition.
The maximum measurement deviation of the test results is 0.61 ppm. These results indicate that the detection system designed in this manuscript can be used for substation monitoring.
According to standard IEC 60599 and IEEE Guide for the Interpretation of Gases Generated in Oil-Immersed Transformers, acetylene ranges of 90% typical gas concentration values observed in power transformers, from about 25 electrical networks worldwide and including more than 20000 transformers, is 2–20ppm. And a four-level criterion has been developed to classify risks to transformers. For condition 4, when acetylene > 35ppm, continued operation could result in failure of the transformer. Thus, the detection range of the system in the experiment, 0–250 ppm, is enough to evaluate the system performance.
Conclusion
In this paper, the following conclusions have been drawn
-
I.
A new optical method based on the tunable diode laser absorption spectroscopy (TDLAS) technique is proposed to detect the dissolved acetylene gas in transformer oil. The wavelength modulation strategy with center wavelength of 1530.370 nm and Herriott cell were selected to ensure high sensitivity detection. The TDLAS acetylene detection system developed in this manuscript has excellent performance: sensitivity of 7.1 mV /ppm; limit of detection is 0.49 ppm, lower than the online monitoring requirement.
-
II.
The proposed detection system has tremendous advantages, such as: immediate response to concentration changes; eliminating carrier gas; no cross interference from background gases; wide range of measurable gases. And the system can save the time of detection, with the detection process less than eight minutes, proved to be a promising technique instead of conventional DGA equipment.
Data availability statement
All data generated or analyzed during this study are included in this published article.
References
Singh, J., Sood, Y. R., Jarial, R. K. & Verma, P. Condition monitoring of power transformers-bibliography survey. IEEE Electrical Insulation Magazine 24, 11, https://doi.org/10.1109/MEI.2008.4591431 (2008).
Duval, M. A review of faults detectable by gas-in-oil analysis in transformers. IEEE electrical Insulation magazine 18, 8–17, https://doi.org/10.1109/MEI.2002.1014963 (2002).
Baka, N. A., Abu-Siada, A., Islam, S. & El-Naggar, M. A new technique to measure interfacial tension of transformer oil using UV-Vis spectroscopy. IEEE Transactions on Dielectrics and Electrical Insulation 22, 1275–1282, https://doi.org/10.1109/TDEI.2015.7076831 (2015).
Kim, S.-w. et al. New methods of DGA diagnosis using IEC TC 10 and related databases Part 1: application of gas-ratio combinations. IEEE Transactions on Dielectrics and Electrical Insulation 20, 685–690, https://doi.org/10.1109/TDEI.2013.6508773 (2013).
Duval, M. & Dukarm, J. Improving the reliability of transformer gas-in-oil diagnosis. IEEE Electrical Insulation Magazine 21, 21–27, https://doi.org/10.1109/MEI.2005.1489986 (2005).
Kan, H. & Miyamoto, T. Proposals for an improvement in transformer diagnosis using dissolved gas analysis (DGA). IEEE Electrical Insulation Magazine 11, 15–21, https://doi.org/10.1109/57.475904 (1995).
Haema, J. & Phadungthin, R. A prediction technique of power transformer condition assessment via DGA parameters. Proceedings of the IEEE PES Asia-Pacific Power and Energy Engineering Conference, https://doi.org/10.1109/APPEEC.2013.6837171 (2013).
Muhamad, N., Phung, B., Blackburn, T. & Lai, K. Comparative study and analysis of DGA methods for transformer mineral oil. Proceedings of The PowerTech Conference. 45-50, https://doi.org/10.1109/PCT.2007.4538290 (2007).
Kannel, P. R., Lee, S., Lee, Y.-S., Kanel, S. R. & Khan, S. P. Application of Water Quality Indices and Dissolved Oxygen as Indicators for River Water Classification and Urban Impact Assessment. Environmental Monitoring and Assessment 132, 93–110, https://doi.org/10.1007/s10661-006-9505-1 (2007).
Teerasong, S. et al. A reagent-free SIA module for monitoring of sugar, color and dissolved CO2 content in soft drinks. Analytica Chimica Acta 668, 47–53, https://doi.org/10.1016/j.aca.2010.01.021 (2010).
Mullins, O. C., Daigle, T., Crowell, C., Groenzin, H. & Joshi, N. B. Gas-Oil Ratio of Live Crude Oils Determined by Near-Infrared Spectroscopy. Appl. Spectrosc. 55, 197–201, https://doi.org/10.1366/0003702011951506 (2001).
Kelly, J. J. Transformer fault diagnosis by dissolved-gas analysis. IEEE Transactions on Industry Applications 6, 777–782, https://doi.org/10.1109/TIA.1980.4503871 (1980).
Wan, F. et al. Using a sensitive optical system to analyze gases dissolved in samples extracted from transformer oil. IEEE Electrical Insulation Magazine 30, 15–22, https://doi.org/10.1109/MEI.2014.6882596 (2014).
Ding, J. et al. New sensor for gases dissolved in transformer oil based on solid oxide fuel cell. Sensors and Actuators B: Chemical 202, 232–239, https://doi.org/10.1016/j.snb.2014.05.061 (2014).
Wu, Z., gong, Y. & Yu, Q. Photoacoustic spectroscopy detection and extraction of discharge feature gases in transformer oil based on 1.5μ tunable fiber laser. Infrared Physics & Technology 58, 86–90, https://doi.org/10.1016/j.infrared.2013.01.002 (2013).
Bakar, N., Abu-Siada, A. & Islam, S. A review of dissolved gas analysis measurement and interpretation techniques. IEEE Electrical Insulation Magazine 30, 39–49, https://doi.org/10.1109/MEI.2014.6804740 (2014).
Wang, Q. An all-Optical photoacoustic spectrometer for remote detection of acetyelene gas in power transformer. Proceedings of The International Symposium on Photonics and Optoelectronics. 1–4, https://doi.org/10.1109/SOPO.2011.5780682 (2011).
Nagapriya, K. et al. Laser calorimetry spectroscopy for ppm-level dissolved gas detection and analysis. Scientific Reports 7, 42917, https://doi.org/10.1038/srep42917 (2017).
He, Q., Zheng, C., Liu, H. & Wang, Y. A near-infrared gas sensor system based on tunable laser absorption spectroscopy and its application to CH4/C2H2 detection. Proceedings of SPIE 10111, Quantum Sensing and Nano Electronics and Photonics XIV. https://doi.org/10.1117/12.2250965 (2017)
Zhiqun, D., Jilong, B., Xiaohui, F., Hongxia, Z. & Hui, J. A novel gas sensor used for C2H2 trace detection in power transformer. Proceedings of SPIE 7656, 5th International Symposium on Advanced Optical Manufacturing and Testing Technologies: Optical Test and Measurement Technology and Equipment. https://doi.org/10.1117/12.865966 (2010).
Deng, H. et al. Sensitive detection of acetylene by second derivative spectra with tunable diode laser absorption spectroscopy. Optica Applicata 46, 353–363, https://doi.org/10.5277/oa160303 (2016).
Werle, P. et al. Near-and mid-infrared laser-optical sensors for gas analysis. Optics and lasers in engineering 37, 101–114, https://doi.org/10.1016/S0143-8166(01)00092-6 (2002).
Durry, G. et al. Near infrared diode laser spectroscopy of C 2 H 2, H 2 O, CO 2 and their isotopologues and the application to TDLAS, a tunable diode laser spectrometer for the martian PHOBOS-GRUNT space mission. Applied Physics B: Lasers and Optics 99, 339–351, https://doi.org/10.1007/s00340-010-3924-y (2010).
Schilt, S., Thevenaz, L. & Robert, P. Wavelength modulation spectroscopy: combined frequency and intensity laser modulation. Applied optics 42, 6728–6738, https://doi.org/10.1364/AO.42.006728 (2003).
Krishnan, S. R. & Seelamantula, C. S. On the selection of optimum Savitzky-Golay filters. IEEE transactions on signal processing 61, 380–391, https://doi.org/10.1109/TSP.2012.2225055 (2013).
Jiang, J. et al. Tracing methane dissolved in transformer oil by tunable diode laser absorption spectrum. IEEE Transactions on Dielectrics and Electrical Insulation 23, 3435–3442, https://doi.org/10.1109/TDEI.2016.005810 (2016).
Acknowledgements
This work was supported in part by Fundamental Research Funds for the Central Universities (JB2015RCY02), National Natural Science Foundation of China (Grant No. 51677070), Young Elite Scientists Sponsorship Program by CAST, and the 111 Project.
Author information
Authors and Affiliations
Contributions
Shu-jing ZHAO, Hong-tu SONG and Jun JIANG designed the test.Shu-jing ZHAO, Hong-tu SONG completed the test and data collection. Guo-ming MA proposed the concept, design of the work and rewrote the manuscript.Cheng-rong LI guided the test process. Ying-ting LUO and Hao WU provided field data and analyzed two different method results. Guo-ming MA is the corresponding author.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, Gm., Zhao, Sj., Jiang, J. et al. Tracing Acetylene Dissolved in Transformer Oil by Tunable Diode Laser Absorption Spectrum. Sci Rep 7, 14961 (2017). https://doi.org/10.1038/s41598-017-13823-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13823-0
This article is cited by
-
Mid-infrared-scanning cavity ring-down CH2F2 detection using electronically tuned Cr:ZnSe laser
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.