Abstract
Cardiac hypertrophy is an adaptive response triggered by pathological stimuli. Regulation of the synthesis and the degradation of the Ca2+ channel inositol 1,4,5-trisphosphate receptor (IP3R) affects progression to cardiac hypertrophy. Herpud1, a component of the endoplasmic reticulum-associated degradation (ERAD) complex, participates in IP3R1 degradation and Ca2+ signaling, but the cardiac function of Herpud1 remains unknown. We hypothesize that Herpud1 acts as a negative regulator of cardiac hypertrophy by regulating IP3R protein levels. Our results show that Herpud1-knockout mice exhibit cardiac hypertrophy and dysfunction and that decreased Herpud1 protein levels lead to elevated levels of hypertrophic markers in cultured rat cardiomyocytes. In addition, IP3R levels were elevated both in Herpud1-knockout mice and Herpud1 siRNA-treated rat cardiomyocytes. The latter treatment also led to elevated cytosolic and nuclear Ca2+ levels. In summary, the absence of Herpud1 generates a pathological hypertrophic phenotype by regulating IP3R protein levels. Herpud1 is a novel negative regulator of pathological cardiac hypertrophy.
Similar content being viewed by others
Introduction
Pathological cardiac hypertrophy is a phenotypic alteration of the heart to compensate for loss of function after myocardial infarction or in association with chronic stress, as in hypertension1,2,3. Cardiomyocytes are terminally-differentiated cells that are responsible for myocardial contraction. In response to pro-hypertrophic stimuli, cardiomyocytes activate signaling pathways such as calcineurin/NFAT that favor their growth and increase their contractile function4. Activation of these pathways triggers reprogramming of gene expression in cardiomyocytes, including fetal genes such as the beta-myosin heavy chain (Myhβ)5. These processes initially serve to enhance cardiomyocyte performance but eventually lead to contractile dysfunction and cell death by apoptosis6. At the tissue level, these events may also lead to interstitial fibrosis7 and decreased vascular density8. Therefore, although hypertrophy is initially an adaptive response to increased demand, sustained hypertrophy may progress into heart failure9. Developing therapies that might prevent or reverse pathological hypertrophy, thereby avoiding progression to heart failure, requires an improved understanding of the mechanisms that lead to cardiomyocyte hypertrophy and identification of novel therapeutic targets.
Intracellular Ca2+-signaling pathways play a key role in the cardiac hypertrophy10, along with proteins that regulate Ca2+, including the inositol trisphosphate receptor (IP3R)11,12. IP3R is a tetrameric Ca2+ channel found in the endoplasmic reticulum (ER) membrane and nuclear envelope13,14. Three isoforms of IP3R have been described, IP3R1, IP3R2, and IP3R3, each of which forms homo- or hetero-tetramers with different properties and tissue distributions. IP3R2 is the predominant isoform in the heart15. Overexpression of IP3R2 induces cardiomyocyte hypertrophy, favoring a release of Ca2+ from the ER11,12 that is independent of the Ca2+ release required for contraction. This Ca2+ release activates pro-hypertrophic signaling pathways such as calcineurin/NFAT4,11,16. Thus, regulation of both the synthesis and the degradation of IP3R may be relevant to the genesis and progression of cardiac hypertrophy.
Herpud1 is an ER membrane protein, originally described as a target in the unfolded protein response (UPR), as its levels rise significantly in response to various stressors17. Herpud1 was subsequently reported to facilitate the retro-translocation of proteins from the ER to the 26 S proteasome in the cytosol for proteolytic elimination18,19,20. IP3R1 has been shown to be a Herpud1 target20 with the ability to regulate intracellular Ca2+ levels in neuron and HeLa cell lines20,21. This ability suggests a potential role in cardiac IP3R degradation, which occurs via the proteasome pathway22. Although the presence of Herpud1 mRNA has been described in cardiac tissue17, the expression and physiological function of the Herpud1 protein in the heart are unclear, and its potential role in cardiac hypertrophy remains unexplored.
Given the above, we hypothesized that Herpud1 regulates IP3R degradation in the cardiomyocyte, acting as a negative regulator of cardiac hypertrophy. The goal of this study was to elucidate the role of Herpud1 in cardiac pathophysiology and to assess whether Herpud1 might serve as a novel therapeutic target in pathological cardiac hypertrophy.
Results
Herpud1 is present in the mouse heart
Cardiac hypertrophy is a pathological process favored by changes in Ca2+ signaling. Cytoprotective proteins such as Herpud1 may regulate these signals, mainly by inducing degradation of ER proteins and maintaining Ca2+ homeostasis20,21. To date, the presence of Herpud1 mRNA has only been reported in human cardiac tissue17. To elucidate whether the Herpud1 protein is present in mouse cardiac tissue, we studied 10-to-12-week-old C57BL/6 wild-type (WT) and Herpud1-knockout (KO)23 male mice (Supplementary Fig. 1). Samples were obtained from the heart, brain, liver, and skeletal muscle of the mice. The Herpud1 protein was found in these tissues in WT mice but was completely absent in the tissues from Herpud1-KO mice (Fig. 1). The distribution of Herpud1 in the adult heart in WT and KO mice as well as in NRVM was studied by immunohistochemistry and immunocytochemistry (Supplementary Fig. 2), These results provide the first evidence of the presence and distribution of Herpud1 in the mouse heart. The relative expression of Herpud1 mRNA was also assessed in the cardiac tissue samples and was found to be expressed in WT animals but not Herpud1-KO mice (Supplementary Table 1).
Herpud1-KO mice develop cardiac hypertrophy and systolic dysfunction
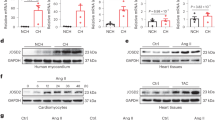
Morphological studies showed significantly elevated heart mass in Herpud1-KO mice as compared to their WT counterparts, as measured by heart weight/total body weight (Fig. 2a–c) and heart weight/tibia length (Fig. 2d) ratios. Similarly, the left ventricular + septum weight/tibia length (Fig. 2e) and atrial weight/tibia length (Fig. 2f) ratios were elevated in Herpud1-KO group as compared to WT group. In agreement with the development of cardiac hypertrophy, cardiomyocyte cross-sectional area was significantly increased in Herpud1 silenced mice (Fig. 2b). We also assessed the relative levels of genes expressed during the development of pathological cardiac hypertrophy, such as atrial natriuretic peptide (Nppa)24, brain natriuretic peptide (Nppb)24, regulator of calcineurin 1 (Rcan1)25 and the fibrosis marker collagen type I alpha (Col1-α)26. Results show that Nppb expression was significantly increased in the KO mice while the other three genes showed a slight, although not significant, increment (Supplementary Table I). Cardiac hypertrophy is a phenotypic change in response to a physiological or pathological stimulus1; in pathological cases, an irreversible tissue remodeling occurs, gradually leading to heart failure. To characterize the myocardial function of Herpud1-KO mice, various echocardiographic parameters were measured in M-mode (Fig. 3a). The Herpud1-KO animals showed significant decreases in fractional shortening (FS), ejection fraction (EF), and stroke volume (Fig. 3b–d) as compared to WT mice, confirming the importance of Herpud1 in myocardial function. Additionally, both left-ventricular end-systolic diameter (LVESd) and volume (LVESv) were significantly higher in Herpud1-KO mice as compared to their WT counterparts, while left-ventricular end-systolic parameters (LVEDd and LVEDv) showed no significant between-group differences (Table 1). The latter finding suggests that the left ventricular cavity is not affected in Herpud1-KO mice during relaxation but does exhibit impaired contractile capacity during systole, reflected as a reduced ejection volume. Taken together, the results show that a Herpud1 deficiency leads to the development of cardiac hypertrophy and impaired myocardial function.
Herpud1-KO mice develop cardiac hypertrophy. (a) Four-chamber image, 40X, with hematoxylin and eosin staining (n = 3). (b) Representative images (400X) of paraffin embedded cardiac tissue stained with wheat germ agglutinin (WGA) and quantification of cardiomyocyte cross-sectional area (n = 6). (c) Heart weight/body weight (mg g−1, n = 12). (d) Heart weight/tibia length (mg cm−1, n = 12). (e) Left and right ventricular plus septum weight/tibia length (mg cm−1, n = 6). (f) Left and right atrial weight/tibia length−1 (mg cm−1, n = 6) in wild-type (WT) and Herpud1-knockout (Herpud1-KO) 10-to-12-week-old mice. Mean ± SEM, analyzed using a t-test. *p < 0.05 vs. WT.
Herpud1-KO mice develop myocardial dysfunction. (a) Representative long-axis echocardiography images, 2D and M-mode, of the left ventricle. LV: Left ventricle, Pap: papillary muscle. (b) % Fractional shortening (n = 12). (c) % Ejection fraction (n = 12). (d) Stroke volume (n = 12) in wild-type (WT) and Herpud1-knockout (Herpud1 KO) 10-to-12-week-old mice. Mean ± SEM, analyzed using a t-test. *p < 0.05 vs. WT.
In vitro Herpud1 deficiency induces cardiomyocyte hypertrophy
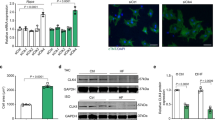
We assessed the presence of the Herpud1 protein in the two main cardiac cell types, cardiomyocytes and fibroblasts, under basal and ER stress-induced conditions, where Herpud1 overexpression has been widely documented17,27. Neonatal rat ventricular myocytes (NRVM) and cardiac fibroblasts and adult rat cardiomyocytes were used in this analysis. Herpud1 was present in all three cell types and overexpressed after treatment with the ER stressor tunicamycin28 (Fig. 4a). To assess the effect of Herpud1 silencing on cardiomyocytes, NRVM were transfected with one of two different Herpud1 siRNAs (#1 and #2). In both cases, Herpud1 protein levels were decreased to approximately 50% after 48 h with a siRNA concentration of 200 nM (Supplementary Fig. 3). The same conditions were used in all subsequent Herpud1 knockdown experiments. Morphological studies were also performed in Herpud1-knockdown NRVM. F-actin was stained with rhodamine-phalloidin (Fig. 4b), allowing for quantification of the cell area, cell perimeter, and percentage of sarcomerized cells, defined as cells with an ordered, stair-like fluorescence pattern (Fig. 4c). Results show that Herpud1 knockdown induced significant increases in cell area, perimeter and sarcomerization in cardiomyocytes (Fig. 4d). In addition, the levels of hypertrophy markers beta-myosin heavy chain (β-MHC) and calcineurin regulator 1.4 (Rcan 1.4) were assessed in Herpud1-silenced NRVM. β-MHC is considered a general marker of hypertrophy5, and Rcan1.4 is a physiological regulator of calcineurin whose expression is induced by a Ca2+-dependent calcineurin activation, thus accounting for the activation of the pro-pathological hypertrophic Ca2+/calcineurin/NFAT axis4. Both β-MHC and Rcan1.4 levels were increased in Herpud1-knockdown NRVM (Fig. 4e). Taken together, the above results suggest that reduced Herpud1 protein levels alone are sufficient to trigger an intrinsic hypertrophic phenotype in NRVM, with pathological features such as elevated Rcan 1.4 levels.
Herpud1 siRNA triggers cardiomyocyte hypertrophy. (a) Representative Western blot of Herpud1 protein levels in isolated neonatal (Neo) and adult (Adu) cardiomyocytes (Cardio) and neonatal fibroblasts (Fibro) cultured under basal conditions or treated with tunicamycin for 4 h (Tn, 1.2 mM). Ponceau red shows the loading of each sample (n = 3). (b) Representative images (400X) of fixed NRVM stained with the fluorescent probe rhodamine-phalloidin after incubation with unrelated siRNA (UNR, 200 nM) or anti-Herpud1 siRNA (siHerpud1 #1) (200 nM) for 48 h (n = 3). (c) White arrows show analyzed sarcomeres in the fluorescent profiles of sarcomeres from the previous images. (d) Results for area, perimeter, and percentage of sarcomerized cells for fixed NRVM stained with rhodamine-phalloidin after incubation with unrelated siRNA (UNR) or anti-Herpud1 siRNA (siHerpud1 #1) for 48 h (n = 3). (e) Representative Western blots for Herpud1 (n = 6), β-MHC (n = 6), and Rcan1.4 (n = 4) for NRVM incubated with unrelated siRNA (UNR) or anti- Herpud1 siRNA (siHerpud1#2) for 48 h. Graphs represent the measurements for each protein using β-tubulin as the loading control. Mean ± SEM, analyzed using a t-test. *p < 0.05 vs. UNR.
Herpud1 deficiency leads to decreased IP3R degradation and increased intracellular calcium
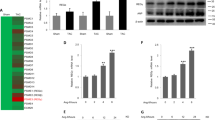
Ca2+ is a key second messenger in the generation of cardiac hypertrophy10. The release of Ca2+ from the ER into the cytosol or nucleus plays a major role in the activation of pro-hypertrophic pathways29. Therefore, IP3Rs may be of paramount relevance in the Ca2+ signaling that leads to cardiac hypertrophy. IP3R degradation occurs through the proteasome22, and the degradation of isoform 1 depends on Herpud1 in PC12 cells20. To investigate whether this dependence is also observed in cardiac tissue, IP3R levels were evaluated in Herpud1-knockdown NRVM and cardiac samples from Herpud1-KO mice. IP3R protein levels were elevated in both models as compared to control conditions (Fig. 5a). This increase could be attributed to two different processes: increased IP3R synthesis and/or decreased IP3R degradation. To shed more light on this process, therefore, IP3R mRNA content was quantified in the samples. Despite the increased IP3R protein levels observed, the Herpud1-knockdown NRVM did not show significantly increased mRNA levels for any of the IP3R isoforms. In fact, decreased levels of isoform 2 transcripts were seen in Herpud1-KO cardiac tissue as compared to WT tissue (Fig. 5b). Taken together, these results suggest that the changes in IP3R content associated with a Herpud1 deficiency are likely attributable to decreased IP3R degradation rather than increased de novo synthesis of IP3R. These results also suggest that Herpud1 may participate in IP3R degradation, as Herpud1 has known degradative functions30. For instance, Herpud1 ubiquitin-like domain (ULD) allows for the polyubiquitination of ER proteins, driving them towards degradation30. Since no reports have addressed this potential function of Herpud1 in cardiomyocytes, the proteasomal degradation pathway was analyzed in Herpud1-knockdown NRVM. Lysine 48-linked polyubiquitin content was measured in the presence and absence of MG-132, an inhibitor of proteasomal activity. Levels of polyubiquitin aggregates were significantly lower in MG-132-treated cells after Herpud1 silencing (Fig. 6a), suggesting that Herpud1 indeed participates in the polyubiquitination of a relevant number of proteins in cardiomyocytes, possibly including IP3R.
IP3R protein and mRNA levels in cardiomyocytes treated with Herpud1 siRNA and in hearts from Herpud1-KO mice. (a) Representative Western blot of IP3R protein levels in Left: control (UNR) and Herpud1-knockdown NRVM (siHerpud1 #2, 48 h) (n = 6) and Right: samples from wild-type (WT) and Herpud1-knockout (Herpud1 KO) mice (n = 3). (b) Graphs show the relative expression of IP3R1, IP3R2, and IP3R3 mRNA in control (UNR) and Herpud1-knockdown NRVM (siHerpud1 #2, for 48 h) (n = 4) and IP3R2 mRNA in cardiac samples from wild-type (WT) and Herpud1-knockout (Herpud1 KO) 10-to-12-week-old mice (n = 3). Pabpn1 was used as a housekeeping messenger in isolated NRVM, and the 18 S ribosomal subunit was used in the cardiac tissue samples. Mean ± SEM, analyzed using a t-test. *p < 0.05 vs. UNR.
Effect of Herpud1 siRNA on polyubiquitin levels and cytosolic and nuclear Ca2+ levels in cultured NRVM. (a) Representative Western blots for lysine 48-linked polyubiquitin (Poly-Ub) in control (UNR) and Herpud1-knockdown NRVM (siHerpud1 #2, 200 nM, 48 h) in the presence or absence of the proteasome inhibitor MG-132 (4 μM, 24 h). Graphs correspond to the quantification of each lane, using β-tubulin as a loading control (n = 6). (b) Graph of cytosolic Ca2+ concentration kinetics and quantification of the area under the curve (n = 3). (c) Graph of nuclear Ca2+ kinetics and quantification of the area under the curve (n = 3). Both (b) and (c) correspond to control (UNR) and Herpud1-knockdown NRVM (siHerpud1 #2, 48 h). (d) Graph of cytosolic Ca2+ concentration kinetics and quantification of the area under the curve, in control (UNR) and Herpud1-knockdown NRVM (siHerpud1 #2, 48 h) in the absence or presence of xestospongin C (XeC, 100 μM, 30 min) (n = 3). The black arrow shows the time of addition of histamine (100 mM). Mean ± SEM analyzed using a t-test or one-way ANOVA followed by Bonferroni’s post-test. *p < 0.05 vs. UNR, † p < 0.05 vs. UNR + MG-132; # p < 0.05 vs. same condition without MG-132, ‡p < 0.05 vs siHerpud1.
Herpud1 has also been proposed as a regulator of Ca2+ based on its capacity to modulate the release of this cation from the ER20,21. As mentioned, it remains unknown whether Herpud1 exercises this function in cardiomyocytes, where Ca2+ signaling is crucial to a wide variety of intracellular processes including contraction, signal transduction, hypertrophy development, electrical signaling, gene transcription, and cell death31. To evaluate whether the observed increase in IP3R protein levels also drives an increase in cytoplasmic Ca2+, cytoplasmic and nuclear Ca2+ levels were measured, using the ratiometric fluorescent probe Fura-2AM. Ca2+ levels were measured in the presence of histamine, which induces the generation of IP3 and the release of Ca2+ from the ER through IP3R activation, and in the absence of external Ca2+. Both cytoplasmic (Fig. 6b) and nuclear (Fig. 6c) Ca2+ levels were elevated in Herpud1-silenced NRVM as compared to control cells. An increase in cytosolic Ca2+was also seen in NRVM preincubated with MG-132 (1 μM for 3 h, Supplementary Fig. 4). Interestingly, the IP3R antagonist xestospongin C (XeC. 100 μM for 30 min, Fig. 6d) prevents histamine-induced Ca2+ signal in Herpud1-knockdown NRVM. This result supports the notion that the increased level of IP3R protein observed upon Herpud1 silencing stimulates the release of Ca2+ from the ER into other intracellular compartments.
Discussion
Diverse proteins and cellular processes are involved in the genesis and progression of pathological cardiac hypertrophy. In the present study, we evaluated the potential of Herpud1 as a novel negative regulator of cardiomyocyte hypertrophy and therefore as a putative new pharmacological target.
Our results show for the first time that the Herpud1 protein is present in the heart, specifically in cardiomyocytes and fibroblasts. Deletion or reduction of this protein induces cardiac hypertrophy both in vivo and in vitro, indicating the importance of Herpud1 in maintaining basal homeostasis as well as suggesting a potential role in anti-hypertrophic mechanisms. Furthermore, Herpud1-knockout mice suffered a significant decrease in myocardial function, including decreased ejection fraction, fractional shortening, and stroke volume. We also found that IP3R protein levels were elevated and that Ca2+ release from intracellular reservoirs was increased when this receptor was activated by histamine, a phenomenon that may facilitate activation of pro-hypertrophic pathways. We propose a new model for the generation of pathological hypertrophy in vivo and in vitro based on our findings of decreased cardiac contractile function in isolated NRVM, as well as increased protein levels for a negative regulator of calcineurin (Fig. 7).
Proposed model for hypertrophy induced by Herpud1 silencing. Herpud1 plays a role in the regulation of Ca2+ levels in cardiomyocytes, possibly by modulating IP3R degradation. Reduction in Herpud1 protein levels allows for activation of pro-hypertrophic signaling cascades such as the calcineurin/NFAT pathway. This new evidence suggests an alternative model for the development of pathological cardiac hypertrophy and a novel putative pharmacological treatment target.
Because complete Herpud1-knockout mice were used in the present study, it is difficult to rule out the participation of other organs or extra-cardiac processes, such as increased blood pressure or renal failure, in triggering the observed hypertrophic phenotype. However, the hypertrophic phenotype and the loss of cardiac function observed in vivo was correlated with the cardiomyocyte hypertrophy observed upon Herpud1 silencing in vitro, supporting the contention that the pathological phenomenon is likely caused by an intrinsic process of the contractile cells. Moreover, while it has been widely described that pressure or volume overload triggers left ventricular hypertrophy32 and that increased pulmonary pressure causes right ventricular hypertrophy33, there was no specific increase in right or left ventricular weight (Supplementary Table 2), although there was a general increase in organ size involving both the atria and ventricles and a significant increase in cardiomyocyte cross-sectional area (Fig. 2). Furthermore, it would seem that the process triggered by Herpud1 silencing is not attributable to hemodynamic changes or increased sympathetic tone as there were no significant differences in heart rate between WT and Herpud1-knockout mice (Supplementary Table 2), although the latter group developed cardiac hypertrophy and dysfunction. Finally, it has been described that cardiac hypertrophy accompanied by decreased ejection fraction is evidence of intrinsic cardiomyocyte dysfunction, which is associated with extrinsic heart growth and therefore increased risk of progression into heart failure34. However, the long-term effects of the phenotypic change observed in the knockout mice, such as increased prevalence of heart failure at older ages, remain unknown.
The activity of Ca2+-dependent signaling pathways such as calcineurin/NFAT increases during the progression to pathological hypertrophy, and the perpetuation of these signals depends on overexpression of IP3R12. Evidence regarding the role of these pathways during later stages of heart failure is controversial, however, as some reports suggest that IP3R and other Ca2+-handling proteins are downregulated at these stages35, while others have shown a redistribution of ryanodine receptor type 2 and sarco/endoplasmic reticulum Ca2+-ATPase type 2a as well as increased IP3R2 levels14. In the present work, the IP3R protein levels increased during the hypertrophy induced by Herpud1 silencing, possibly triggering a Ca2+-dependent induction of the hypertrophic phenotype. However, the Herpud1-knockout mice and knockdown cells, respectively, showed decreased or unchanged cardiac IP3R mRNA levels, indicating that the increased IP3R levels were due to decreased degradation rather than increased IP3R transcription.
As mentioned above, Herpud1 participates in the degradation of ER proteins through the proteasome, facilitating their ubiquitination and transport20,30. Therefore, lysine 48-linked polyubiquitin aggregates were measured in control and Herpud1-silenced NRVM to confirm the degradative function of the latter protein in cardiac cells. In agreement with previous reports18,19,20, our results showed that Herpud1 mediates the proteasomal degradation of a significant number of proteins, possibly including IP3R, as its silencing significantly decreased the polyubiquitin aggregate content.
One of the questions arising from this work is how the hypertrophic process is triggered under basal conditions, without exogenous stimuli such as neurohumoral factors and/or mechanical stress. Even if IP3R protein levels were elevated due to a partial or complete absence of Herpud1, intracellular Ca2+ levels would not necessarily increase, since IP3 must bind to its receptor to allow for channel opening. Therefore, it remains unclear how the increase in Ca2+ and the hypertrophic signaling are triggered. It is important to note that a cell-signaling pathway has a basal level and is gradually activated. In the present case, the pathway may be triggered to induce the hypertrophic phenotype by increased IP3R protein levels. Each of the three IP3R isoforms has a different sensitivity to IP3-induced activation and Ca2+-induced deactivation36,37,38. Isoform 2, which is the isoform primarily expressed in cardiac tissue, may be activated by basal IP3 concentrations, and its deactivation may require higher Ca2+ concentrations than the other isoforms15,39. As a result, cardiomyocytes have a more sensitive and complex Ca2+ signaling profile than other cell types. Therefore, it is possible that elevated IP3R protein levels alone may trigger pathological hypertrophy in cardiomyocytes, both in isolated cells and whole hearts. However, because the IP3R antibody used in this study recognizes all three isoforms, a future study to measure the protein levels of each variant may shed more light on the role of IP3R2 in the Herpud1-dependent hypertrophic process.
Diverse evidence suggests that intracellular Ca2+ is a critical signal for the development of cardiac hypertrophy10. The two major pathways linked to the genesis of cardiac hypertrophy, calcineurin/NFAT and histone deacetylase, are Ca2+-dependent4,40. As mentioned before, elevated cytosolic and nuclear levels of Ca2+ were observed in Herpud1-knockdown NRVM (Fig. 6b–c). A similar effect was seen upon the addition of the proteasomal inhibitor MG-132 (Supplementary Fig. 4). This effect may be associated with the ability of Herpud1 to regulate the degradation of proteins involved in the Ca2+ release from the ER, as shown in other cellular models20,21,41. Furthermore, the increment on cytosolic Ca2+ levels induced by histamine in Herpud1-knockdown was completely inhibited by the antagonist of IP3R XeC (Fig. 6d). These results support the notion that the Ca2+ increasing effect of the silencing of Herpud1 is caused by an increase in the levels of IP3R. The development of cardiac hypertrophy also depends on nuclear Ca2+ signaling mediated by activation of IP3R in the nucleosome14. This Ca2+ release plays a key role in the regulation of gene expression. The increased nuclear levels of Ca2+ facilitate a rapid change in gene expression that has been linked to cardiomyocyte hypertrophy42. This rapid response may underlie the phenotypic change observed in Herpud1-knockout mice and NRVM treated with Herpud1 siRNA. Since the latter experiments were performed in the absence of external Ca2+, intracellular reservoirs are likely the source of the increased cytosolic and nuclear Ca2+. Herpud1 is apparently capable of regulating the ER Ca2+ pool, as Chan et al.41 showed that Herpud1 overexpression lowers the amount of Ca2+ released by the ER after thapsigargin treatment. Therefore, our results could be explained by an increased availability of Ca2+ channels and/or a larger intracellular Ca2+ pool.
Some limitations of the present study are: a) The evaluation of heterozygous littermates as well as the time course of myocardial function of Herpud1 KO mice over time were not done and future work should study these points. b) Immunohistochemistry of Herpud1 shows some non-specific immunoreactivity in Herpud1 KO mice (Supplementary Fig. 2), in agreement with the detection of a non-specific band in Western blot.
In summary, our data show that Herpud1 plays a significant role in the regulation of Ca2+ levels, possibly by modulating IP3R degradation, and that reducing Herpud1 protein levels allows for activation of a pro-hypertrophic signaling pathway (Fig. 7). These findings also suggest an alternative model for the development of pathological cardiac hypertrophy and a novel putative pharmacological target, as increasing Herpud1 levels may prevent or revert pathological cardiac hypertrophy and myocardial dysfunction.
Methods
Antibodies and reagents
The following reagents were purchased from Sigma-Aldrich: Hank’s medium, DME medium (DMEM), 199 medium (M199), pancreatin, gelatin, Triton X-100, 5-bromo-2′-deoxiuridine, anti-β-tubulin and anti-Rcan1 antibodies, and Herpud1, Mission #1 (universal negative control) siRNAs and Xestospongin C. The fluorescent probe Fura-2 AM, paraformaldehyde, and Hoechst 33342 were obtained from Thermo Fisher Scientific. The proteasome inhibitor MG-132 was purchased from Merck Millipore. Tunicamycin and the anti-Herpud1 antibody were from Enzo Life Science. Anti-IP3R antibody was purchased from Abcam. Anti-polyubiquitin Lys48 from Cell Signaling Technology and anti-β-MHC from Vector Laboratories. Secondary rabbit and rat anti-IgG antibodies conjugated with peroxidase were from Calbiochem. ECL chemiluminescent reagent for Western blotting was purchased from Amersham BioSciences. All SDS-PAGE materials and PVDF membranes were from Bio-Rad Laboratories. The mounting medium for fluorescence was purchased from DAKO Corporation. All other organic and inorganic reagents, salts, acids, and solvents were purchased from Merck unless otherwise specified.
Experimental models
Primary neonatal rat ventricular myocytes (NRVM) or adult rat cardiomyocytes and cardiac fibroblasts were obtained from 1-to-3-day-old and 2-month-old Sprague-Dawley rats, respectively (Animal Facility, Facultad de Ciencias Químicas y Farmacéuticas, Universidad de Chile, Santiago). Genetically-modified Herpud1-knockout mice were obtained from the National Cerebral and Cardiovascular Center, Osaka, Japan. All animals were handled according to the Guide for the Care and Use of Laboratory Animals (NIH, 2011), and the experimental protocols were approved by the Bioethics Committee of the Faculty of Chemical and Pharmaceutical Sciences, University of Chile, Santiago (#BE2014-01).
Cardiomyocyte culture
NRVM were prepared as described previously43. Cells were maintained in DMEM/M199 (4:1) medium. Cells were plated at a density of 1–8 × 103 mm−2 on gelatin-coated 12-well plates or 35- or 60-mm Petri dishes. For Ca2+ and immunofluorescence studies, NRVM were plated on gelatin-coated 25- and 18-mm glass coverslips in 35-mm or 12-well Petri dishes, respectively.
Cardiomyocyte transfection
Small interfering RNAs (siRNA) for Herpud1 and a negative control (Mission #1, SIC001, Sigma-Aldrich) were used according to manufacturer instructions. Cells were grown and transfected with siRNAs at a concentration of 100-200 nM with Oligofectamine™ (Invitrogen): Herpud1 #1: SASI_RN01_00185592 or Herpud1 #2: SASI_RN01_00057861. After 6 h of incubation in Opti-MEM®, the medium was removed and replaced with DMEM/M199. Downregulation of Herpud1was assessed 48 h post-transfection.
Herpud1-KO mice
These mice were generated in the laboratory of Dr. Koichi Kokame (National Cerebral and Cardiovascular Center, Osaka, Japan). Full Herpud1-KO C57BL/6 mice were generated by crossing C57BL/6 wild-type mice with Herpud1+/− mice for 10 generations. Heterozygous mice were obtained from 129/SvJ-derived Go Germline embryonic stem cells (Incyte Genomics). Herpud1-KO mice showed normal development with no apparent phenotypic abnormalities under normal breeding conditions, as described in Eura et al.23. All animals used were 10-to-12-week-old males. Following ultrasound measurements, mice were sacrificed and weighed, and samples from the heart, liver, skeletal muscle, and brain were obtained for further analysis. One tibia was also extracted from each mouse and subjected to digestion with 2 mg mL−1 proteinase K (Sigma-Aldrich) in lysis buffer (50 mM Tris, 100 mM EDTA, 100 mM NaCl, 1% SDS) at 55 °C overnight to clean the bones of cartilage and muscle. Once clean, tibia length was measured for each mouse.
PCR genotyping
The Herpud1-KO mice were identified using PCR genotyping of genomic DNA samples obtained from an ear. The primers used are described below, and the amplification products (94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s; 30 cycles) were separated by agarose gel electrophoresis and stained with SYBR Green/Nucleic acid gel stain (Lonza). Bands were detected using a LAS-3000 Image Analyzer (Fujifilm) equipment. Primers used were: Herpud1 (WT) F: 5′-CCCCTCCCCCTTTGGTTGACA-3′ R: 5′-TCCAGGGGCTTAGACGCTTAC-3′ 343 bp and Herpud1* (KO) F: 5′-CCCCTCCCCCTTTGGTTGACA-3′ R: 5′-TGGACCTGGGAGTGGACACCT-3′ 252 bp.
Western blot analysis
Equal amounts of cell protein extracts were separated by SDS-PAGE (8–15% polyacrylamide gels) and electrotransferred to PVDF membranes. Membranes were blocked with 5% milk in Tris-buffered (pH 7.6), containing 0.1% (v/v) Tween 20 (TBST). Membranes were incubated with primary antibodies at 4 °C and re-incubated with horseradish peroxidase-linked secondary antibody [1:5,000 in 1% (w/v) milk in TBST]. The bands were detected using ECL with exposure to Kodak film or a LAS-3000 Image Analyzer (Fujifilm) and quantified with scanning densitometry. Protein content was normalized to β-tubulin or total protein content as measured using Ponceau red or Coomassie brilliant blue staining (CBB).
Histological and immunocytochemical studies
Hearts from both WT and Herpud1 KO mice were fixed in 4% paraformaldehyde and transferred to 1x PBS, followed by paraffin embedding. Hematoxylin/Eosin (H&E) staining was performed for morphological analyses. Wheat germ agglutinin (WGA) was used for cross-sectional area measurements, quantified from at least 50 cells per group as described by Pedrozo et al.46. Anti Herpud-1 rabbit polyclonal Thermo Fisher (Pa5-29469, 1:200 pH 8.0) was used for immunohistochemistry and immunocytochemistry assays.
Echocardiography
Mice were initially anesthetized in a chamber with isoflurane (3.5%) and then kept under isoflurane anesthesia (1.7%) in a 1:1 air-oxygen mixture. During the procedure, body temperature and heart rate were monitored, establishing 36 °C and 400 beats per min, respectively, as inferior limits. Vevo2100 equipment from PrimeTech with a MicroScan™ Transducer (MS-550) was used. The corresponding images were captured in M-mode and analyzed using the equipment software. All measurements were performed on the papillary muscles.
RNA purification and real-time reverse transcription PCR (RT-PCR)
Total RNA was isolated using TRIzol according to the manufacturer protocol (Invitrogen). Random hexamers were used for reverse transcription reactions with Superscript II (Invitrogen). RT-PCR was performed using PowerUp SYBR Green Master Mix, and the results were analyzed using the Step OneTM (Thermo Fisher Scientific) system. The primers used were:
Itpr1 (rat) F: 5′-GAGAGAAAGCGCACGCCGAGA-3′ R: 5′-GCAATCCCATGTCCGCGAAGAG-3′; Itpr2 (rat) F: 5′-TCTTGGTGGATATGGCAAGGGTCT-3′ R: 5′-AGTTGAAGAAGCCGCTCACGATGT-3′; Itpr3 (rat) F: 5′-CAGAACGACCGCAGGTTTGTCAT-3′ R: 5′-TTCTGCCCATTGTTGGGAACATCG-3′; Nppa (mouse) F: 5′-CTTCTTCCTCGTCTTGGCCT-3′ R: 5′-CTGCTTCCTCAGTCTGCTCA-3′; Nppb (mouse) F: 5′-CATGGATCTCCTGAAGGTGC-3′ R: 5′-CCTTCAAGAGCTGTCTCTGG-3′; Rcan1 (mouse) F: 5′-CCCGTGAAAAAGCAGAATGC-3′ R: 5′-TCCTTGTCATATGTTCTGAAGAGGG-3′; Col1- α: (mouse) F: 5′CTGACGCATGGCCAAGAAGA-3′ R: 5′-TACCTCGGGTTTCCACGTCT-3′; Pabpn1 (rat) F: 5′-GTTGGCAATGTGGACTATGG-3′ R: 5′-AACAGGGACTCATCTAAGGC-3′; 18S (mouse) F: 5′-CGGACAGGATTGACAGATTG-3′ R: 5′-CAAATCGCTCCACCAACTAA-3′. Additionally, one-step quantitative reverse transcription was then performed on the tissue samples using the One-Step RT-PCR Kit (Qiagen). The catalog numbers for the primers (included in the kit) were as follows: QF00503041 for Herpud1, QF00196504 for ITPR2, and QF00289576 for 18S. Data for each transcript were normalized to Pabpn1 or 18S mRNA as an internal control using the 2−ΔΔCt method to calculate relative transcript abundances.
Assessment of cardiomyocyte hypertrophy
Area, perimeter, percent sarcomerization, and sarcomere fluorescence profiles were assessed using epifluorescence microscopy (Carl Zeiss Axiovert 135, LSM Microsystems) in paraformaldehyde-fixed cells permeabilized with Triton X-100 and treated with Hoechst 33342 (to stain nuclei) and rhodamine-phalloidin (to stain F-actin) at a 1:500 dilution (Thermo Fisher Scientific), as previously described44. At least 50 cells from randomly-selected fields were analyzed using ImageJ software (NIH).
Intracellular Ca2+ measurements
Intracellular Ca2+ signals were determined as described previously45. Images were obtained from cultured cardiomyocytes preloaded with Fura-2AM (Thermo Fisher Scientific) using a Spinning Disk IX81 confocal microscope (Olympus). Coverslips were mounted in a 1 mL capacity chamber and placed in the microscope for fluorescence measurements after excitation with a laser line of 340/380 nm. In some selected experiments, the cells were preincubated with MG-132 (1 μM for 3 h)46 or with XeC (100 μM for 30 min)47. In these experiments, a pulse of histamine (Sigma-Aldrich) was added after 60 s at a final concentration of 100 mM to increase Ca2+ movement from the ER to cytosol via IP3R47. The fluorescent images were collected every 2 s and analyzed frame-by-frame with ImageJ software (NIH).
Statistical analysis
Results are shown as representative images or mean ± SEM of at least three independent experiments. All data had a normal distribution, and differences were analyzed using Student’s t-test or ANOVA with a Bonferroni’s post-test. p < 0.05 was the threshold for statistical significance.
References
Heineke, J. & Molkentin, J. D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nature reviews. Molecular cell biology 7, 589–600, https://doi.org/10.1038/nrm1983 (2006).
Weisman, H. F., Bush, D. E., Mannisi, J. A. & Bulkley, B. H. Global cardiac remodeling after acute myocardial infarction: a study in the rat model. Journal of the American College of Cardiology 5, 1355–1362 (1985).
Devereux, R. B., Savage, D. D., Sachs, I. & Laragh, J. H. Relation of hemodynamic load to left ventricular hypertrophy and performance in hypertension. The American journal of cardiology 51, 171–176 (1983).
Molkentin, J. D. et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93, 215–228 (1998).
Pandya, K. & Smithies, O. beta-MyHC and cardiac hypertrophy: size does matter. Circulation research 109, 609–610, https://doi.org/10.1161/CIRCRESAHA.111.252619 (2011).
van Empel, V. P. & De Windt, L. J. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovascular research 63, 487–499, https://doi.org/10.1016/j.cardiores.2004.02.013 (2004).
Conrad, C. H. et al. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 91, 161–170 (1995).
Oka, T., Akazawa, H., Naito, A. T. & Komuro, I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circulation research 114, 565–571, https://doi.org/10.1161/CIRCRESAHA.114.300507 (2014).
Meerson, F. Z. Compensatory hyperfunction of the heart and cardiac insufficiency. Circulation research 10, 250–258 (1962).
Berridge, M. J., Bootman, M. D. & Roderick, H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nature reviews. Molecular cell biology 4, 517–529, https://doi.org/10.1038/nrm1155 (2003).
Nakayama, H. et al. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circulation research 107, 659–666, https://doi.org/10.1161/CIRCRESAHA.110.220038 (2010).
Sankar, N., deTombe, P. P. & Mignery, G. A. Calcineurin-NFATc regulates type 2 inositol 1,4,5-trisphosphate receptor (InsP3R2) expression during cardiac remodeling. The Journal of biological chemistry 289, 6188–6198, https://doi.org/10.1074/jbc.M113.495242 (2014).
Luo, D. et al. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell calcium 43, 165–174, https://doi.org/10.1016/j.ceca.2007.04.017 (2008).
Ljubojevic, S. et al. Early remodeling of perinuclear Ca2+ stores and nucleoplasmic Ca2+ signaling during the development of hypertrophy and heart failure. Circulation 130, 244–255, https://doi.org/10.1161/CIRCULATIONAHA.114.008927 (2014).
Vervloessem, T., Yule, D. I., Bultynck, G. & Parys, J. B. The type 2 inositol 1,4,5-trisphosphate receptor, emerging functions for an intriguing Ca(2)(+)-release channel. Biochimica et biophysica acta 1853, 1992–2005, https://doi.org/10.1016/j.bbamcr.2014.12.006 (2015).
Wilkins, B. J. & Molkentin, J. D. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochemical and biophysical research communications 322, 1178–1191, https://doi.org/10.1016/j.bbrc.2004.07.121 (2004).
Kokame, K., Agarwala, K. L., Kato, H. & Miyata, T. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. The Journal of biological chemistry 275, 32846–32853, https://doi.org/10.1074/jbc.M002063200 (2000).
Liang, G. et al. Polycystin-2 is regulated by endoplasmic reticulum-associated degradation. Human molecular genetics 17, 1109–1119, https://doi.org/10.1093/hmg/ddm383 (2008).
Marutani, T. et al. ER-stress-inducible Herp, facilitates the degradation of immature nicastrin. Biochimica et biophysica acta 1810, 790–798, https://doi.org/10.1016/j.bbagen.2011.04.017 (2011).
Belal, C. et al. The homocysteine-inducible endoplasmic reticulum (ER) stress protein Herp counteracts mutant alpha-synuclein-induced ER stress via the homeostatic regulation of ER-resident calcium release channel proteins. Human molecular genetics 21, 963–977, https://doi.org/10.1093/hmg/ddr502 (2012).
Paredes, F. et al. HERPUD1 protects against oxidative stress-induced apoptosis through downregulation of the inositol 1,4,5-trisphosphate receptor. Free radical biology & medicine 90, 206–218, https://doi.org/10.1016/j.freeradbiomed.2015.11.024 (2016).
Wojcikiewicz, R. J. H. IP3 receptor processing by the ERAD pathway. Faseb J 26 (2012).
Eura, Y. et al. Derlin-1 deficiency is embryonic lethal, Derlin-3 deficiency appears normal, and Herp deficiency is intolerant to glucose load and ischemia in mice. PloS one 7, e34298, https://doi.org/10.1371/journal.pone.0034298 (2012).
Sergeeva, I. A. & Christoffels, V. M. Regulation of expression of atrial and brain natriuretic peptide, biomarkers for heart development and disease. Biochimica et biophysica acta 1832, 2403–2413, https://doi.org/10.1016/j.bbadis.2013.07.003 (2013).
Rothermel, B. A. et al. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proceedings of the National Academy of Sciences of the United States of America 98, 3328–3333, https://doi.org/10.1073/pnas.041614798 (2001).
Yoshikane, H. et al. Collagen in dilated cardiomyopathy–scanning electron microscopic and immunohistochemical observations. Japanese circulation journal 56, 899–910 (1992).
van Laar, T. et al. The novel MMS-inducible gene Mif1/KIAA0025 is a target of the unfolded protein response pathway. FEBS letters 469, 123–131 (2000).
Liu, C. L. et al. Genome-wide analysis of tunicamycin-induced endoplasmic reticulum stress response and the protective effect of endoplasmic reticulum inhibitors in neonatal rat cardiomyocytes. Molecular and cellular biochemistry 413, 57–67, https://doi.org/10.1007/s11010-015-2639-0 (2016).
Bogeholz, N., Muszynski, A. & Pott, C. The physiology of cardiac calcium handling. Wiener medizinische Wochenschrift 162, 278–282, https://doi.org/10.1007/s10354-012-0102-3 (2012).
Hori, O. et al. Role of Herp in the endoplasmic reticulum stress response. Genes to cells: devoted to molecular & cellular mechanisms 9, 457–469, https://doi.org/10.1111/j.1356-9597.2004.00735.x (2004).
Ljubojevic, S. & Bers, D. M. Nuclear calcium in cardiac myocytes. Journal of cardiovascular pharmacology 65, 211–217, https://doi.org/10.1097/FJC.0000000000000174 (2015).
Lorell, B. H. & Carabello, B. A. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 102, 470–479 (2000).
Ikeda, S. et al. Longitudinal strain of right ventricular free wall by 2-dimensional speckle-tracking echocardiography is useful for detecting pulmonary hypertension. Life sciences 111, 12–17, https://doi.org/10.1016/j.lfs.2014.06.024 (2014).
Heinzel, F. R., Hohendanner, F., Jin, G., Sedej, S. & Edelmann, F. Myocardial hypertrophy and its role in heart failure with preserved ejection fraction. Journal of applied physiology 119, 1233–1242, https://doi.org/10.1152/japplphysiol.00374.2015 (2015).
Barrans, J. D., Allen, P. D., Stamatiou, D., Dzau, V. J. & Liew, C. C. Global gene expression profiling of end-stage dilated cardiomyopathy using a human cardiovascular-based cDNA microarray. The American journal of pathology 160, 2035–2043, https://doi.org/10.1016/S0002-9440(10)61153-4 (2002).
Newton, C. L., Mignery, G. A. & Sudhof, T. C. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. The Journal of biological chemistry 269, 28613–28619 (1994).
Ramos-Franco, J., Fill, M. & Mignery, G. A. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophysical journal 75, 834–839, https://doi.org/10.1016/S0006-3495(98)77572-1 (1998).
Vanlingen, S. et al. Ca2+ and calmodulin differentially modulate myo-inositol 1,4, 5-trisphosphate (IP3)-binding to the recombinant ligand-binding domains of the various IP3 receptor isoforms. The Biochemical journal 346(Pt 2), 275–280 (2000).
Perez, P. J., Ramos-Franco, J., Fill, M. & Mignery, G. A. Identification and functional reconstitution of the type 2 inositol 1,4,5-trisphosphate receptor from ventricular cardiac myocytes. The Journal of biological chemistry 272, 23961–23969 (1997).
Backs, J. & Olson, E. N. Control of cardiac growth by histone acetylation/deacetylation. Circulation research 98, 15–24, https://doi.org/10.1161/01.RES.0000197782.21444.8f (2006).
Chan, S. L. et al. Herp stabilizes neuronal Ca2+ homeostasis and mitochondrial function during endoplasmic reticulum stress. The Journal of biological chemistry 279, 28733–28743, https://doi.org/10.1074/jbc.M404272200 (2004).
Zhong, X. et al. Calcium sensing receptor regulates cardiomyocyte function through nuclear calcium. Cell biology international 36, 937–943, https://doi.org/10.1042/CBI20110594 (2012).
Foncea, R. et al. Insulin-like growth factor-I rapidly activates multiple signal transduction pathways in cultured rat cardiac myocytes. The Journal of biological chemistry 272, 19115–19124 (1997).
Pennanen, C. et al. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+ -calcineurin signaling pathway. Journal of cell science 127, 2659–2671, https://doi.org/10.1242/jcs.139394 (2014).
Ibarra, C. et al. Insulin-like growth factor-1 induces an inositol 1,4,5-trisphosphate-dependent increase in nuclear and cytosolic calcium in cultured rat cardiac myocytes. The Journal of biological chemistry 279, 7554–7565, https://doi.org/10.1074/jbc.M311604200 (2004).
Pedrozo, Z. et al. Polycystin-1 Is a Cardiomyocyte Mechanosensor That Governs L-Type Ca2+ Channel Protein Stability. Circulation 131, 2131–2142, https://doi.org/10.1161/CIRCULATIONAHA.114.013537 (2015).
Gutierrez, T. et al. Alteration in mitochondrial Ca(2+) uptake disrupts insulin signaling in hypertrophic cardiomyocytes. Cell communication and signaling: CCS 12, 68, https://doi.org/10.1186/s12964-014-0068-4 (2014).
Acknowledgements
We thank the staff members at the S. Lavandero’s and K. Kokame’s groups for providing technical support and for valuable discussions. This work was supported by Comision Nacional de Investigación Científica y Tecnologica (CONICYT, Chile): Fondo de Financiamiento de Centros de Investigacion en Areas Prioritarias (FONDAP; grant 15130011 to S.L., J.C.R. & Z.P.), Fondo Nacional de Desarrollo Científico y Tecnologico (FONDECYT grant 1161156 to S.L.), PhD fellowships (to N.T., V.G. and M.N.), and grants from Japan Society for the Promotion of Science (to Y.E. and K.K.).
Author information
Authors and Affiliations
Contributions
N.T. performed most of the in vitro and in vivo studies and drafted the manuscript. Y.E. performed in vivo studies with Herpud1-KO mice. E.V., D.R. & J.C.R. performed all the histochemical studies. M.N.-M. performed Ca2+ assays. V.G. performed the qPCR assays. N.T., Z.P., M.C., K.K. and S.L. conceived the study and participated in the experimental design, data analysis and discussion. All authors contributed to the preparation, and discussion of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torrealba, N., Navarro-Marquez, M., Garrido, V. et al. Herpud1 negatively regulates pathological cardiac hypertrophy by inducing IP3 receptor degradation. Sci Rep 7, 13402 (2017). https://doi.org/10.1038/s41598-017-13797-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13797-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.