Abstract
Corals thrive in a variety of environments, from low wave and tidal energy lagoons, to high energy tidal reef flats, but remain dependent upon suitable substrate. Herein we reviewed the phenomenon of free-living corals (coralliths), examined whether they have the capacity to create their own stable habitat in otherwise uninhabitable, poor substrate environments through ‘free-living stabilization’, and explore their potential ecological role on coral reefs. This stabilization could be achieved by coral settlement and survival on mobile substrate, with subsequent growth into free-living coralliths until a critical mass is reached that prevents further movement. This allows for secondary reef colonization by other coral species. To preliminarily test this hypothesis we provide evidence that the potential to support secondary coral colonisation increases with corallith size. Due to the limited diversity of corallith species observed here and in the literature, and the lack of physiological differences exhibited by coralliths here to static controls, it seems likely that only a small selection of coral species have the ability to form coralliths, and the potential to create their own stable habitat.
Similar content being viewed by others
Introduction
Individual corals and patch reefs can be found in a variety of environments, including those dominated by unsuitable substrate such as sand or mobile rubble1,2. Although prograding into such habitats is possible across millennial time scales (where corals grow, break, and re-grow from the reef edge into muddy or soft sediments)3, herein we explore whether coral survival and reef formation in otherwise unsuitable environments can be achieved via the creation of stable habitat by free-living corals. These ‘coralliths’4 are documented in reefs in all major reef systems worldwide4,5,6,7,8,9,10,11,12,13,14, and have occurred since at least the middle Pleistocene7. They are characterized by an unusual ability to survive mechanical stress from movement and abrasion4, and they settle on unstable substrate such as coral rubble10. Porites is one of the most common corallith genera, and this genus is known to be relatively resilient to environmental pressures15,16.
The idea of free-living organisms stabilizing their own habitat and supporting high biodiversity is well-established in the algal field, and the individual thalli of certain algal species may interlock creating a three-dimensional stable ‘reef’ on an otherwise homogenous, sandy substrate17,18. However, commonly known free-living coral species such as Fungia spp. and Heliofungia spp. do not interlock, and even at their maximum size they are still considered mobile. Thus, they can only provide a stable substrate for coral settlement once dead and ‘cemented’ onto stable substrate by crustose coralline algae (CCA). Conversely, free-living massive coral species (coralliths) have no upper size limit, and can become stable while still alive.
Given the variety of species and sizes of documented coralliths, we refer to coralliths here as living coral colonies that are not attached to the substrate (and hence can be moved by waves, currents and grazing), and constitute genera which are not typically considered to be ‘free-living’ (as opposed to Fungia spp). Once coralliths reach a stable size and support secondary colonization, they are no longer ‘free-living.’ However, large storm events could still cause movement and relocation of these coralliths. For the purposes of this study, coralliths that have ceased to move in response to wave and current action are termed ‘stable coralliths.’
Herein, we examined whether coralliths can (1) grow to ‘stable’ dimensions in a reef environment dominated with rubble, and (2) act as stable substrate for further coral colonization. We hypothesized that such coralliths may differ physiologically from corals embedded in the reef framework to reflect that they will be subject to rapid changes in light availability from sudden movement, and as such may employ different protective mechanisms to prevent (photo) damage, including different pigments and photosynthetic mechanisms. We further hypothesized that the ensuing dataset could be used to test the ‘free-living stabilization hypothesis’ (Fig. 1), in which free-living coral species establish stable substrate for coral colonization by settling and surviving on a mobile substrate as coralliths. They subsequently grow until a point is reached at which movement ceases and stable habitat is formed; other corals can then colonise the corallith.
The free-living stabilization hypothesis. Only certain massive species can survive settlement on mobile rubble. These species form free-living ‘coralliths’ (a), which increase in size until a critical threshold is reached and no further movement occurs, creating a stable substrate for the settlement of other species (b,c). X indicates free-living P. lutea coralliths covered completely by tissue. Y provides examples of how a free-living P. lutea corallith of near-critical mass has been turned over, revealing bare substrate suitable for settlement. Z is an example of a large, static coral with suspected corallith origins (given lack of stable substrate in area) that has reached critical mass and is immobile. Scale bars are 10 cm. Illustrations by L. McWhinnie.
Materials and Methods
Location and ecology
The seaward (west) side of Vavvaru Island, Lhaviyani Atoll (5.4184°N, 73.3548°E), Maldives was used as the study location during March 2015; it is separated from the nearest Maldivian atoll by ~31 km, and is a typically seaward reef system of the area. Triplicate 25 m × 2 m belt transects were conducted parallel to the shore on the reef flat at near- (70 m from the shore), mid- (140 m), and far-shore (210 m) locations; all three locations were at identical depths (1 m). Beyond the reef flat and at the reef wall, depth dropped to ~ 30 m. Reef flat diel temperatures ranged from 27.1–31.4 °C, with an average of 29.0 °C (Table 1). Maximum photosynthetically active radiation (PAR, using an Odyssey PAR logger [Dataflow Systems Ltd]), calibrated against a LICOR) averaged 1250 µmol m2 s−1 at 1 m depth. Water chemistry was measured from nine random water samples taken from across the reef flat from where the sites were located (Table 1). Total alkalinity and dissolved inorganic carbon were analyzed as described below. Seabed substrates (not including live coral) varied between sites; the near-shore site featured predominantly branching rubble and sand while the mid-shore site was similar, though with numerous, dead massive corals. The far-shore site’s benthos was dominated by branching coral rubble, sand and larger, dead massive corals. Along each transect, the number and size (maximum dimension) of all coralliths, and total number of non-free living coral individuals were non-invasively recorded. To determine whether large, static coral colonies were mobile and had originated as coralliths, the base substrate was examined to determine whether it was part of a larger bedrock feature. Where possible, this was tested (in instances where this would not cause damage to the corals or associated organisms) by gently pushing the corallith from one side to see if there was any movement. Following ecological surveys, 10 free-living Porites lutea coralliths of ~10 cm diameter from an area between near- and mid-shore transect sites abundant in suitable coralliths and static controls, were sampled on all sides for physiological measurements to provide life history ‘context’ of coralliths compared to static corals (for specific details see below). As static controls, 5 small P. lutea colonies (similar in size to the coralliths and assumed to be approximately the same age), were carefully removed from static substrate (CCA cemented reef framework) in the same localized area, between near- and mid-shore transect sites. Following physiological experimentation in situ and in the laboratory, all specimens were returned alive to their site of collection.
Physiology
To assess whether coralliths are physiologically specialized for a mobile life in comparison to static conspecifics, standard physiological parameters including photosynthetic efficiency, calcification, oxygen production, and reflectance profiles (which are a function of pigmentation, structure and morphology) were examined.
PAM fluorometry
Rapid light curves (RLC) were conducted on the upper and lower (orientation as found) surfaces of all P. lutea coralliths (n = 10) and control static colonies (n = 5) in mid-afternoon (15:00–16:00) using a Diving - Pulse Amplitude Modulated fluorometer (Walz, GMBH), following Hennige et al.19.
Incubations
Calcification rates and oxygen production (net photosynthesis) were assessed through controlled incubations in sealed 650 ml chambers in situ during the afternoon (15:00–16:00) over two sequential days, for coralliths (n = 10), and controls (n = 5) respectively. Water samples were taken at the beginning and end of the 30 min incubation, analysed on-site for dissolved oxygen concentration with a YSI Pro2020 O2 meter, and poisoned on-site with HgCl2 for total alkalinity and dissolved inorganic carbon analysis20. Total alkalinity was determined via semi-automated titration (Metrohm 848 Titrino plus)20 combined with spectrometric analysis using bromocresol indicator (Smart pH cuvettes, Ocean Optic Ltd and Hach DR 5000™ UV–Vis spectrophotometer; analytical precision: ±11 μmol kg−1)21, and dissolved inorganic carbon using an Automated Infra Red Inorganic Carbon Analyzer (AIRICA, Marianda instruments analytical precision: ±2 μmol kg−1). Certified seawater reference materials were used for oceanic CO2 (Batch 141, Scripps Institution of Oceanography, University of California, San Diego) as standards to quantify analytical precision20.
Calcification rates were calculated using the alkalinity anomaly technique22. Surface areas of the coralliths using the tinfoil method, and images were analysed with Image Tool as outlined in Hennige et al.23. The volume of each coral was calculated by displacement in seawater.
Reflectance
Immediately following in situ experimentation, samples were returned to the laboratory for reflectance measurements. Single measurements were taken from each sample on the upper and lower surfaces according to the in situ orientation at time of sampling (measurements on upper surface only for static control corals), following methodology described in Burdett et al.24 with an Ocean Optics USB 2000+ spectrometer.
Sectioning
Coralliths (n = 3) were covered in EpoHeat epoxy resin (Buehler) and placed in a vacuum oven at 60 °C for 4 hours to harden epoxy before single cross- and longitudinal-sectioning. Flat sections were polished and imaged using a flatbed scanner.
Statistics
For data that met normal distribution assumptions and equal variances, analyses of variance were used (one-way ANOVA). Where normality assumptions were not met, Kruskal–Wallis tests were used with Dunn’s Multiple comparison. To assess potential correlations between photosynthetic parameters and coral abundances, a Pearson’s two-tailed correlation was used once normality had been tested and met. To compare linear regressions of fluorescene data, analysis of covariance was used. To compare observational percentage occurrence data between surveys, a X2 test was used. All statistical analysis were conducted in Prism v. 5.0c. for Mac, GraphPad Software, San Diego, CA, USA. An alpha level of 0.05 was used for all statistical tests.
Results
Ecological surveys
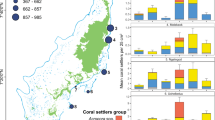
Coralliths were found at all transect sites, with varying sizes illustrating the progression from small coralliths to large, stable coralliths (Fig. 1). At all sites, the most common corallith size was 6–10 cm (Fig. 2); significantly more coralliths were observed within this size range at the far-shore site than either the mid- or near-shore sites (F (2,6) = 5.29, p = 0.047). P. lutea coralliths accounted for ~73% of the total number of coralliths present (Fig. 2).
Size frequency distribution of coralliths using maximum length and 5 cm bins from triplicate 25 × 2 m belt transects 70 m (near), 140 m (mid), and 210 m (far) parallel from the seaward facing side of Vavvaru Island, Maldives. The contribution of P. lutea to the frequency of total coralliths is denoted with hatching. Dashed line indicates the minimum diameter of living coralliths observed to have secondary colonization by other corals (15 cm). The inset graph shows the total number of other (non-corallith) coral species along the transects on live coralliths, dead coralliths and large secondary rubble, with different letters indicating significantly different groups (Dunn’s Multiple Comparison, p = 0.03). The percentage of those that were found on living coralliths is also represented. Error bars represent standard error.
Both total corallith abundance and the frequency of larger coralliths (maximum lengths of >1 m) increased with distance from the shore (Fig. 2). P. lutea coralliths larger than 1 m were not observed in the sampling transects, but they were observed nearby; maximum dimensions of some were in excess of 3 m. The abundance of non-corallith forming species significantly increased from the near to far-shore site (H = 7.20, p < 0.05), and significantly correlated with the increasing abundance of coralliths (Pearson r = 0.93, p < 0.01, r 2 = 0.87). There was a significant positive correlation between the number of secondary species and corallith size (r = 0.73, p = 0.03, r 2 = 0.53). The percentage of corals found living on live coralliths significantly increased from 0% at the near-shore site to ~3.5%, and ~12% at the mid- and far-shore sites respectively (χ 2 2 = 11.25, p = 0.004 between mid- and far-shore sites) (Fig. 2). Other corals observed were settled on either dead coralliths or on large rubble fragments. The minimum size of a corallith on which other corals settled was 15 cm (max. length), with multiple species being often found on coralliths over 30–45 cm (when bare skeleton was available for settlement; Fig. 2).
The coralliths examined were mostly spherical or ellipsoidal in shape, reflecting the substrate they originally settled on (a small fragment or branch of coral), or the history the corallith had had in terms of periods of stability5. On sectioning the coralliths, CCA were observed at the interface between the corallith species and the base rubble (Fig. 3)4, which likely provided the crucial cues to promote coral larval settlement25.
Cross (a) and longitudinal (b) section of massive Porites lutea corallith, grown around a branching coral fragment core. Brown line denoted by r is resin uptake by the coral skeleton during sample preparation. Inset (c) is a close up of the interface between the core coral fragment and the subsequent P. lutea colonization. The arrow in (c) indicates the presence of crustose coralline algae. Scale bar is 0.5 cm in (a) and (b), and 0.2 cm in (c).
Physiological assessment
To determine whether coralliths had unique physiology (compared to static controls) that may facilitate their dynamic life history, their photophysiology, O2 production, calcification rates, and reflectance were examined. Photophysiological parameters were measured on the upper and lower surfaces of coralliths (as found in the field), and compared to nearby static P. lutea colonies (which were attached to more typical coral substrate of exposed bedrock surface) to identify any characteristics that may be associated with corallith survival. The coralliths examined were visually and physiologically similar on both upper and lower surfaces (Fig. 4). Corallith reflectance profiles were similar on the upper and lower corallith surfaces (Fig. 4), although the static P. lutea corals had higher reflectance between 410 and 690 nm (Fig. 4).
(a) Average reflectance profiles of the upper and lower surfaces of Porites lutea coralliths with shaded Standard Error (SE) (n = 10) from 400–680 nm compared to the top of static P. lutea controls (dotted SE for clarity, n = 5). (b) Derived maximum photochemical efficiency (Fq’/Fm’ (max)) and light saturation coefficient (EK) of coralliths and static P. lutea controls. Linear regression for combined upper and lower surfaces of coralliths (in figure with 95% confidence intervals), y = −0.0007x + 0.6828, r2 = 0.64, and for control corals, r2 = 0.74, y = −0.0005x + 0.7594.
Initial photosynthetic efficiency (Fq’/Fm’), and the light saturation coefficient, (EK), which describes the transition between light-limited and light-saturated photochemical efficiency, did not differ between static controls or the upper / lower surfaces of the coralliths (H 2 = 0.91, p = 0.64), (H 2 = 0.03, p = 0.98) (Fig. 4b). The derived maximum photosynthetic efficiency, Fq’/Fm’ (max) correlated significantly with EK for coralliths (r = −0.68, p < 0.01), as described previously by Hennige et al.19 in the context of coral photoacclimatory potential across light gradients. The significant difference in linear regression intercepts between static controls and coralliths (ANCOVA; F (3,40) = 5.71, p < 0.01) (Fig. 4b), highlights that at similar light saturation coefficients, Fq’/Fm’ (max) was lower in coralliths. The slopes from both linear regressions were not significantly different (ANCOVA; F (3, 37) = 0.29, p < 0.83)
Oxygen production rates did not differ significantly between coralliths and static controls (0.61 ± 0.19; 1.73 ± 1.12 µmol O2 cm−2 h−1 respectively). Instantaneous calcification rates (alkalinity anomaly techniques) were also highly variable between individuals, and did not significantly differ (coralliths: −0.97 to 1.70 µmol CaCO3 cm−2 h−1; static controls: −0.08 to 1.54 µmol CaCO3 cm−2 h−1).
Discussion
The common occurrence of P. lutea coralliths likely reflects the relatively hardy nature of the Porites genera in terms of environmental resilience16, acclimatory ability23, and its low aspect growth form of small, sunken polyps with robust corallite structure. While massive Porites sp. colonies can slowly colonize muddy or soft sediments by prograding3, this is the first time that their pivotal ecological role in forming stable habitat from free-living colonies (over annual-decadal timescales) has been documented. Crucially, the free-living stabilization hypothesis does not rely on gradual, iterative colonization from a nearby reef, so can be applied to soft sediments, rubble deposits, high-energy environments, and degraded reefs. The free-living stabilization hypothesis can also explain the spatially separated formation of coral reefs in otherwise uninhabitable areas for corals. This complements observations in the fossil record, where Porites sp. corals have been identified as pioneer colonizers of isolated patch reefs on soft sediment established during the Pleistocene2, indicating that coralliths, and in particular Porites sp., have been performing a fundamental role of habitat stabilization for tens of thousands of years. Other common corallith species identified in this study included Psammocora haimeana and Cyphastrea chalcidicum, both of which have also been noted to be relatively resilient to bleaching16, highlighting the resilient nature of coralliths. The shallow sites assessed here may also be exposed to periods of elevated temperature when tidal flushing is low, promoting the occurrences of coralliths able to withstand periods of higher temperature. Parrotfish grazing marks were noted on many coralliths, which will contribute to mobilization and turning.
Since atoll islands are dynamic in nature26 and in general migrate from the windward to leeward platform27, the three belt-transect zones used in this study also differ in terms of their exposure age, with the near-shore transect having the youngest exposure for coral settlement compared to the far-shore site. This is supported by the increasing presence of larger coralliths with increasing distance from the shore: at the far-shore site, the coralliths have had a longer period of time to grow, reach a critical mass, and support subsequent growth of other coral species. This would also explain why there are a greater total number of coralliths (across all size classes) at the far-shore site, as they have been exposed to many more coral recruitment events.
The critical mass for a corallith to become a stable coral colony will depend upon the strength of the local hydrodynamics, the initial substrate upon which settlement occurred, and environmental perturbations. In a low tide or wave energy system, ‘stability’ and lack of subsequent movement would be achieved with a much smaller mass or size than in a high energy system with frequent strong tidal surges or strong wave action. If initial settlement of the coral larvae was on a large irregular shaped rubble branch, then subsequent growth could also be irregular, providing stability compared to completely spherical coralliths. In the system examined here, critical size where movement decreased and the substrate-facing parts of the coral died (Fig. 1b) was a minimum of ~15 cm diameter, but was more commonly ~30 cm diameter. Once a corallith approaches its critical mass, its stationary residence time will increase, and at this point it could start to encrust surrounding substrate, or CCA from touching substrate could start to encrust any dead parts of the corallith. Successful encrusting would further increase the stability of the corallith. An interesting question is whether ocean acidification would reduce the efficiency of this process, as acidification could increase the erosion of suitable substrate28,29, and reduce the growth and development of reef-stabilising CCA30. This could increase the critical mass needed to become stable, as ‘secondary stabilisation’ through CCA or encrusting may be less efficient, and delay colonisation of the corallith by other coral species.
The increasing occurrence of non-corallith forming coral species with increasing distance from the shore is explained by an increase in the amount of settlement substrate over time as the coralliths grow and stabilize. Suitable substrate includes both living coralliths with dead patches (accounting for settlement of ~12% of non-corallith forming species at the far-shore site), and dead coral substrate. Dead coral substrate was noted to consist of dead coralliths which had reached critical mass before death, and degradation of previous generations of secondary settlement coral species, in many cases from growth on coralliths (alive and dead). Thus, once coralliths have reached a critical mass and created a stable substrate, settlement by other coral species enables self-perpetuating engineering of stable substrate for further corals to colonize.
Physiologically, coralliths were similar on their upper and lower surfaces (indicating regular turning), and did not differ significantly from static controls. While previous studies19 have documented consistent correlations between Fq’/Fm’ (max) and Ek across depths and between different species, coralliths from this study have a significantly different linear relationship than controls of the same species from the same light environment (Fig. 4b). The previous light histories of coralliths used in this study are unknown (i.e. how frequently they have been moving). Given that Fq’/Fm’ max is related to the ‘poise’ for non-photochemical quenching19, further investigation may reveal photoacclimatory ‘trade-offs’ between ensuring no photodamage occurs to symbionts on surfaces suddenly exposed to high light, and energetic investment into photoprotection mechanisms.
Different in hospite symbiont communities between coralliths and static controls may have influenced subtle differences in reflectance spectra31. The key to the success of free-living stabilization is for a coral to survive settlement on mobile substrate, to withstand mechanical damage from frequent movement, and be able to acclimate quickly to changes in environmental conditions, allowing growth to be maintained whilst minimizing cellular damage. Further studies are needed to investigate whether Porites sp. coralliths share the general environmental resilience of established Porites sp. colonies32,33, or whether they are less resilient to stressors such as temperature increases due to energy expenditure on tissue repair from abrasion.
Given their key ecological function, coralliths could play a role in conservation management, restoration, and policy practices. The use of artificial reefs and coral transplants in degraded areas are considered two major coral reef conservation strategies34. Whilst some success has been noted, these strategies have a number of documented problems, including failed coral attachment in new environments, high mortality rates, and depletion of corals from ‘donor reefs’34. As demonstrated here, coralliths can potentially naturally provide new, stable habitat over relatively rapid (decadal) timescales, and can support an increasing number of species as the coralliths grow. In places where no management systems are in place, coralliths may thus play an important role in continuous provision of new colonization substrate to promote coral reef growth.
Data Availabilty
The datasets generated during the current study are available in the British Oceanographic Data Centre, https://www.bodc.ac.uk/data/published_data_library/catalogue/10.5285/4f8efa6e-3c92-5f3e-e053-6c86abc0b543/35).
References
Jones, J. A. Morphology and development of southeastern Florida patch reefs. Proceedings of the Third International Coral Reef Symposium Vol. 2: Geology, 231–235 (1977).
Crame, J. A. Ecological stratification in the Pleistocene coral reefs of the Kenya coast. Palaeontology 24, 609–646 (1981).
Tudhope, A. W. & Scoffin, T. P. Growth and structure of fringing reefs in a muddy environment, South Thailand. J. Sediment Res. A 64, 752–764 (1994).
Glynn, P. Rolling stones among the scleractinia: Mobile coralliths in the Gulf of Panama. Proceedings of the Second International Coral Reef Symposium 2, 183–198 (1974).
Scoffin, T. P., Stoddart, D. R., Tudhope, A. W. & Woodroffe, C. Rhodoliths and coralliths of Muri Lagoon, Rarotonga, Cook-Islands. Coral Reefs 4, 71–80 (1985).
Richards, Z. T., Bryce, M. & Bryce, C. New records of atypical coral reef habitat in the Kimberley, Australia. J. Mar. Biol. 363894, 8 (2013).
Sorauf, J. E. & Harries, P. J. Rotatory colonies of the corals Siderastrea Radians and Solenastraea sp (Cnidaria, Scleractinia), from the Pleistocene Bermont Formation, south Florida, USA. Palaeontology 52, 111–126 (2009).
Riegl, B., Piller, W. E. & Rasser, M. Rolling stones: First report of a free living Acropora anthocercis (Brook) from the Red Sea. Coral Reefs 15, 149–150 (1996).
Lewis, J. B. Spherical growth in the Caribbean coral Siderastrea radians (Pallas) and its survival in disturbed habitats. Coral Reefs 7, 161–167 (1989).
Idjadi, J. A. & Edmunds, P. J. Free-living colonies of Porites in Moorea, French Polynesia. Bull. Mar. Sci. 72, 1025–1031 (2003).
Capel, K. C. C., Segal, B., Lindner, A. & Bertuol, P. Corallith beds at the edge of the tropical South Atlantic. Coral Reefs 31, 75–75 (2012).
Roff, G. Corals on the move: morphological and reproductive strategies of reef flat coralliths. Coral Reefs 27, 343–344 (2008).
McGregor, H. V., Fischer, M. J., Gagan, M. K., Fink, D. & Woodroffe, C. D. Environmental control of the oxygen isotope composition of Porites coral microatolls. Geochim. Cosmochim. Acta 75, 3930–3944 (2011).
Rodriguez-Martinez, R. E. & Jordan-Dahlgren, E. Epibiotic and free living Porites astreoides. Coral Reefs 18, 159–161 (1999).
Levas, S. J., Grottoli, A. G., Hughes, A., Osburn, C. L. & Matsui, Y. Physiological and biogeochemical traits of bleaching and recovery in the mounding species of coral Porites lobata: implications for resilience in mounding corals. PLoS One 8, e63267 (2013).
Marshall, P. A. & Baird, A. H. Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19, 155–163 (2000).
Foster, M. S. Rhodoliths: Between rocks and soft places. J. Phycol. 37, 659–667 (2001).
McCoy, S. J. & Kamenos, N. A. Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. J. Phycol. 51 (2015).
Hennige, S. J. et al. Photoacclimation, growth and distribution of massive coral species in clear and turbid waters. Mar. Ecol. Prog. Ser. 369, 77–88 (2008).
Dickson, A. G., Sabine, C. L. & Christian, J. R. Guide to best practices for ocean CO2 measurements. PICES Special Publication 3 (2007).
Yao, W. S. & Byrne, R. H. Simplified seawater alkalinity analysis: Use of linear array spectrometers. Deep-Sea Res. Pt I 45, 1383–1392 (1998).
Hennige, S. J. et al. Hidden impacts of ocean acidification to live and dead coral framework. Proc. Royal Soc. B: Biol. Sci. 282, 20150990 (2015).
Hennige, S. J. et al. Acclimation and adaptation of scleractinian coral communities along environmental gradients within an Indonesian reef system. J. Exp. Mar. Biol. Ecol. 391, 143–152 (2010).
Burdett, H. L. et al. Dynamic photoinhibition exhibited by red coralline algae in the red sea. BMC Plant Biol. 14, 139 (2014).
Tebben, J. et al. Chemical mediation of coral larval settlement by crustose coralline algae. Sci. Rep. 5, 10803 (2015).
Kench, P. S. & Brander, R. W. Response of reef island shorelines to seasonal climate oscillations: South Maalhosmadulu atoll, Maldives. J. Geophys. Res. 111 (2006).
Webb, A. P. & Kench, P. S. The dynamic response of reef islands to sea-level rise: Evidence from multi-decadal analysis of island change in the Central Pacific. Glob. Planet. Change 72, 234–246 (2010).
Secretariat of the Convention on Biological Diversity. An Updated Synthesis of the Impacts of Ocean Acidification on Marine Biodiversity (Eds: S. Hennige, J.M. Roberts & P. Williamson). Montreal, Technical Series No. 75, 99 pp (2014).
Muehllehner, N., Langdon, C., Venti, A. & Kadko, D. Dynamics of carbonate chemistry, production, and calcification of the Florida Reef Tract (2009-2010): Evidence for seasonal dissolution. Global Biogeochem. Cy. 30, 661–688 (2016).
Jokiel, P. L. et al. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27, 473–483 (2008).
Hennige, S. J., Suggett, D. J., Warner, M. E., McDougall, K. E. & Smith, D. J. Photobiology of Symbiodinium revisited: bio-physical and bio-optical signatures. Coral Reefs 28, 179–195 (2009).
Madin, J. S. et al. A trait-based approach to advance coral reef science. Trends Ecol. Evol. 31, 419–428 (2016).
Roff, G. et al. Porites and the Phoenix effect: unprecedented recovery after a mass coral bleaching event at Rangiroa Atoll, French Polynesia. Mar. Biol. 161, 1385–1393 (2014).
Abelson, A. Artificial reefs vs coral transplantation as restoration tools for mitigating coral reef deterioration: Benefits, concerns, and proposed guidelines. Bull. Mar. Sci. 78, 151–159 (2006).
Hennige S. J., Burdett H., Perna G., Kamenos N. Ecology and physiology of coralliths at Vavvaru Island, Maldives. British Oceanographic Data Centre - Natural Environment Research Council, UK. doi:10/b7mb. (2017).
Acknowledgements
This work was supported by various grants. An Independent Research Fellowship from the Natural Environment Research Council (NERC) to SJH (NE/K009028/1 and NE/K009028/2). An Independent Research Fellowship from the Marine Alliance for Science & Technology for Scotland to HB. An Independent Research Fellowship from the Royal Society of Edinburgh/Scottish Government (RSE 48701/1) and NERC (NE/H010025) to NAK. A Gilchrist Educational Trust with the Gilchrist Fieldwork Award administered by the Royal Geographical Society (with IBG), and a Research Incentive Grant from the Carnegie Trust for the Universities of Scotland to HB, SH and NK (grant #70013). Field sampling was under permission from the Maldives Ministry of Fisheries and Agriculture ((OTHR) 30-D/lNDIV/2015).
Author information
Authors and Affiliations
Contributions
S.J.H. and H.B. jointly conceived the hypothesis, S.J.H., H.B., G.P., and N.K. collected ecological and physiological data. S.J.H., H.B., G.P., A.T. and N.K. discussed results, and all authors substantially contributed to the drafting of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hennige, S.J., Burdett, H.L., Perna, G. et al. The potential for coral reef establishment through free-living stabilization. Sci Rep 7, 13322 (2017). https://doi.org/10.1038/s41598-017-13668-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13668-7
This article is cited by
-
Scleractinian diversity in the upper mesophotic zone of Ludao (Taiwan): a museum collection with new records from Taiwanese waters
Marine Biodiversity (2021)
-
The potential for coral reef establishment through free-living stabilization
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.