Abstract
Bradysia odoriphaga and Bradysia difformis are devastating pests of vegetable, ornamental crops and edible mushrooms causing significant losses. Temperature may be an important factor restricting their population abundance in the summer. To determine the effects of short-term heat shock on adults, their survival, longevity and fecundity data were collected, and antioxidant responses and heat shock protein expression levels were examined. Our results indicated that the survival rates of Bradysia adults decreased rapidly after heat shock ≥36 °C, and the longevity and reproductive capacities were significantly inhibited, indicating that short-term heat shock had lethal and sub-lethal effects. Moreover, the lipid peroxidation levels of B. difformis and B. odoriphaga increased dramatically at 36 °C and 38 °C, respectively. Four antioxidant enzymes activities of B. odoriphaga were greater than those of B. difformis at 38 °C. Additionally, hsp70 and hsp90 expression levels significantly increased after heat stress, and higher expression levels of B. difformis and B. odoriphaga were discovered at 36 and 38 °C respectively, indicating their different heat tolerance levels. Overall, short-term heat shock (≥36 °C) caused significantly adverse effects on Bradysia adults, indicating that it could be applied in pest control, and antioxidant system and hsp genes played important roles in their heat tolerance levels.
Similar content being viewed by others
Introduction
Insects, typical small-bodied poikilotherms, are easily affected by environmental factors that limit their abundance and distribution. Temperature is an important abiotic environmental factor that causes the body temperatures of insects to quickly fluctuate to lethal levels, resulting in rapid metabolic variation, which can lead to disorder, affecting survival and fecundity1,2. Because of ongoing global warming, the average temperature has increased over the past 30 years, and the frequency and extent of heat events have increased during the summer3,4. Thus, interest in the impact of heat stress on insects has grown5,6. Heat stress can have rapid lethal effect on insects, which has been widely reported7,8. However, understanding the sub-lethal impacts of thermal stress on the development and reproduction of surviving insects could provide important basic information for insect ecology study9,10. When the temperature is between the lethal high and physiological limits, insects can be affected by thermal injuries, which result in the loss of life or decline in fecundity9. Moreover, because insects can be killed by short exposure to an extremely high temperature, heat treatments can be applied to control horticultural and stored-product pests, with fewer insecticidal applications, decreasing the environmental threat11,12. During evolution, insects evolved many behavioral and physiological strategies, such as elevating antioxidant defenses and synthesizing heat shock proteins (Hsps), which are critical indicators in thermal tolerance research, to avoid thermal and other stress impairments12,13,14,15. An understanding of the impacts of environmental changes on insects and their adaptive mechanisms is vital in studying insect-climate interactions, and will aid in predicting and explaining the regular occurrences of insects in different seasons and regions16,17.
In general, there is a balance between reactive oxygen species (ROS) generation and scavenging. However, the balance is disturbed under environmental stress. Thermal stress is responsible for increasing the generation of ROS, which causes oxidative damage18. The surplus ROS causes lipid peroxidation (LPO) and disrupts cell membrane fluidity, resulting in cell lesions18. The degree of membrane LPO can be determined indirectly by measuring the malondialdehyde (MDA) concentration19. To maintain homeostasis and prevent ROS damage, organisms have evolved complex adaptation-related mechanisms for eliminating ROS, including molecular antioxidants and anti-oxidative enzymes20. Antioxidative enzymes, including superoxide dismutase (SOD), catalase (CAT), peroxidase (POD) and glutathione-S-transferases (GSTs), are the most important components for protecting cells and maintaining homeostasis involved in various stress conditions by scavenging ROS21,22. Many studies have measured antioxidant responses under thermal stress conditions as indicators of the important physiological adaptation processes of insects, including Corythucha ciliata 23, Bactrocera dorsalis 24 and Plutella xylostella 25.
Inducing Hsp (heat shock protein) gene expression levels is an important physiological adaptation to biotic and abiotic stresses. HSPs act as molecular chaperones that participate in maintaining regular cellular functions and in regulating metabolic activity, thereby protecting cells from oxidative damage14. Among the different heat shock proteins, Hsp70 and Hsp90 belong to two major conserved families and are commonly expressed under thermal and other stress conditions. In addition to preventing oxidative damage, they may also interfere with the signaling events that trigger the apoptotic process26. In previous studies, hsp90 and hsp70, as stress markers, have played important roles in resisting high-temperature stress and in protecting insects from thermal injury and death14,27. The different expression levels of hsp70 and hsp90 correlate positively with the thermotolerance of insect species and populations27,28.
Bradysia odoriphaga Yang et Zhang and Bradysia difformis Frey, two main root maggot flies, are devastating pests of liliaceous vegetables, flowers and edible fungi, and they can coexist on the same host plant in protected cultivation or in open fields29,30,31,32. Their larvae tend to aggregate to attack and damage roots and corm tissues, resulting in moisture loss and even death31,33,34. In Chinese chive fields, the two Bradysia species occur with similar regularities, with outbreaks in the spring, autumn and winter in greenhouses, and population decreases in the summer35,36. Temperature was thought to be an important factor affecting their population dynamics during different seasons. The optimum temperature ranges of the two Bradysia flies were 13–28 °C for B. odoriphaga and 10–25 °C for B. difformis, and a temperature over 30 °C had adverse effects on both species. The development threshold temperature (T 0 ) of B. odoriphaga was 6.29–8.7 °C, while 4.0–8.4 °C for B. difformis 32,34, and we hypothesized B. difformis had a lower optimum temperature range and threshold temperature, indicating greater cold tolerance than B. odoriphaga. Because extreme daytime temperatures can exceed 35 °C for several hours during the summer season in northern China, high temperature was regarded as a critical abiotic factor restricting their occurrence in the summer. However, there are no reports regarding the thermal tolerance levels of the two Bradysia flies against heat stress. Our previous work indicated that the two Bradysia species were sensitive to heat stress, additionally, adults stage being the most sensitive stage to heat shock (unpublished). Other researchers also confirmed that heat shock negatively influences B. odoriphaga 37, but no research about heat tolerance of B. difformis was reported. To manage root maggot flies efficiently in Chinese chive fields, it is important to clarify the impact of high temperature on the survival and fecundity of these pests, which will aid in predicting their occurrences.
In this study, we demonstrated the lethal and sub-lethal effects of heat shock on two Bradysia adults. The physiological responses to heat stress in the two root maggot flies were then determined, including those of the antioxidant systems and hsp gene expression levels. Our findings provide an important theoretical basis for predicting population dynamics and understanding the potential physiological adaptations to heat stress for two important Bradysia flies.
Results
Lethal effects of heat shock
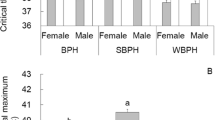
When the temperature excessed 36 °C, heat shock exerted lethal effects on both Bradysia adults (Table 1 and Fig. 1). As an example, the heat shock at 36 °C for 1 h resulted in B. difformis survival rates of 80% (female) and 84% (male), while B. odoriphaga was not affected. When the temperature increased to 38 °C, the B. odoriphaga survival rates were 53% (female) and 62% (male), while those of B. difformis were 28% (female) and 34% (male), and at 40 °C, no B. difformis survived, while the B. odoriphaga survival rates were 11% (female) and 19% (male).
Survival of B. odoriphaga and B. difformis adults after short-term heat shock. The letters, (A and a, B and b, C and c, D and d), mean the survival rates after heat shock at 0.5, 1, 2 and 4 h treatment, respectively. (A,B,C and D) mean the survival rates of female adults, while (a,b,c and d) mean the survival rates male adults. The logistic regression equations indicate the correlation between the treated temperatures (x) and survival rates (y) of two Bradyisa adults, and the inflection points of equations indicate the Ltemp50 (the median lethal temperatures) (95%CL) at every treated time.
We calculated the Ltemp50 according to the logistic regression (Eq. 1; Fig. 1). As the treatment time was prolonged, the Ltemp50 declined. After a 0.5-h exposure, the Ltemp50 values of B. odoriphaga were 39.26 °C (female) and 39.61 °C (male), while those of B. difformis were 38.02 °C (female) and 38.15 °C (male), indicating heat shock (≥36 °C) possessing lethal effects. The Ltemp50 values of B. odoriphaga were 1.24 and 1.46 °C higher than those of B. difformis, which indicated that B. odoriphaga was more heat tolerant than B. difformis.
Sub-lethal effects of heat shock
Longevity
The mean adult longevity values of the two Bradysia species decreased significantly as the exposure time increased at the tested temperature (34, 36 and 38 °C) (Table 2). The female adults longevity of B. difformis ranged from 2.27 (34 °C for 0.5 h) to 0.92 (38 °C for 1 h) d, while that of B. odoriphaga females ranged from 2.47 (34 °C for 0.5 h) to 0.70 (38 °C for 2 h) d. However, the male adults had greater longevity values than females under the same heat shock condition. No B. difformis adults survived 12 h after a heat shock of 38 °C for 2 h, while no B. odoriphaga adults survived 12 h after a heat shock of 38 °C for 4 h. After a 36 °C heat shock for 1 h, the longevity values of B. odoriphaga adults were 2.16 (female) and 2.71 (male) d, shortened by 0.52 and 0.32 d, respectively, compared with at 25 °C, while the values of B. difformis adults were 1.73 (female) and 2.13 (male) d, shortened by 0.95 and 0.83 d, respectively.
Reproductive capacity
When the treatment temperature exceeded 34 °C, heat shocks significantly suppressed the reproductive capacities of two Bradysia adults (Table 2). The female fecundity of B. odoriphaga ranged from 108.96 (36 °C for 0.5 h) to 29.94 eggs (38 °C for 2 h), while that of B. difformis ranged from 46.84 (36 °C for 0.5 h) to 25.00 eggs (38 °C for 1.0 h). The 1-h treatment at 36 °C resulted in B. odoriphaga fecundity of 96.75 eggs, declining by 21.23% compared with at 25 °C, while that of B. difformis was 25.00 eggs, declining by 40.27%. Heat shock at 34 °C exerted slight effects.
Additionally, after heat shock partial female adults could not lay eggs (Table 2). After 1-h exposures at 36 °C and 38 °C, the spawning rates of B. odoriphaga were 86.67% and 76.67%, respectively, while those of B. difformis were 76.67% and 48.33%, respectively.
Although eggs were oviposited successfully, the hatching rates were significantly inhibited (Table 2). At 36 °C with exposure lengths from 0.5 to 4 h, the hatching rate of B. odoriphaga ranged from 91.53% to 68.83%, while that of B. difformis ranged from 82.66% to 56.69%.
Antioxidant responses
Lipid peroxidation (LPO)
The MDA concentrations in B. odoriphaga (female, F2,6 = 61.818, P < 0.001; male, F2,6 = 48.793, P < 0.001) and B. difformis (female, F2,6 = 85.546, P < 0.001; male, F2,6 = 243.311, P < 0.001) increased significantly after heat stress (Fig. 2). The MDA concentration in B. difformis began to increase significantly at 36 °C, while that of B. odoriphaga began to increase significantly at 38 °C. Additionally, at 36 °C and 38 °C, the MDA concentration in B. difformis was greater than in B. odoriphaga adults (F3,8 = 27.352, P < 0.001 at 36 °C; F3,8 = 34.797, P < 0.001 at 38 °C).
Malondialdehyde (MDA) concentrations in B. odoriphaga and B. difformis adults after heat shock. Each value represents the average (±s.e.) of three replicates. Different capital letters over the bars indicate significant differences in MDA concentrations of B. odoriphaga or B. difformis (same single-sex) among different heat stress conditions (P < 0.05), while and the different small letters indicate significant differences of two Bradysia adults at the same heat stress (P < 0.05). Data was analyzed with ANOVA (Tukey’s HSD).
Antioxidant enzyme activities
CAT activities of B. odoriphaga (female, F2,6 = 11.090, P = 0.010; male, F2,6 = 33.927, P = 0.001) and B. difformis (female, F2,6 = 15.524, P = 0.004; male, F2,6 = 27.598, P = 0.001) adults exhibited significant changes in response to heat stress (. 3 A). Compared with at 25 °C, the CAT activities of B. odoriphaga increased by 64% (female) and 90% (male) at 36 °C for 1 h, and those of B. difformis increased by 55% (female) and 73% (male) indicating the higher CAT activities of B. odoriphaga than B. difformis (F3,8 = 10.511, P = 0.004). At a greater heat stress (38 °C), the CAT activities of B. difformis declined compared with at 25 °C, while those of B. odoriphaga declined compared with at 36 °C but still higher than at 25 °C.
POD activities of B. odoriphaga (female, F2,6 = 14.352, P = 0.005; male, F2,6 = 10.744, P = 0.010) and B. difformis (female, F2,6 = 12.022, P = 0.008; male, F2,6 = 12.826, P = 0.007) adults were affected by heat shock (Fig. 3B). The maximum POD activity levels in B. difformis and B. odoriphaga occurred at 36 °C and 38 °C, respectively. At 36 °C, the POD activities of B. difformis were higher than those of B. odoriphaga (F3,8 = 38.317, P < 0.001), unlike at 38 °C (F3,8 = 19.731, P < 0.001). Noticeably, compared with at 25 °C, POD activities of B. difformis were inhibited at 38 °C.
CAT (A), POD (B), SOD (C), GST (D) activities of B. odoriphaga and B. difformis adults after heat shock. Each value represents the average (±s.e.) of three replicates. Different capital letters over the bars indicate significant differences in enzyme activities of B. odoriphaga or B. difformis (same single-sex) among different heat stress conditions (P < 0.05), while and the different small letters indicate significant differences of two Bradysia adults at the same heat stress (P < 0.05). Data was analyzed with ANOVA (Tukey’s HSD).
SOD activities were significantly influenced by heat stress (B. odoriphaga female, F2,6 = 16.711, P = 0.004, male, F2,6 = 31.770, P = 0.001; B. difformis female, F2,6 = 16.364, P = 0.004, male, F2,6 = 14.974, P = 0.005) (Fig. 3C). At 36 °C, the SOD activities of B. odoriphaga increased by 35% (female) and 57% (male) compared with at 25 °C, while those of B. difformis increased by 31% (female) and 40% (male). At 38 °C, the SOD activities of the two species were significantly inhibited compared with at 25 °C, while the activities of B. odoriphaga were still greater than those of B. difformis.
After exposure to 36 and 38 °C, the GST activities of the two species were significantly enhanced (B. odoriphaga female, F2,6 = 16.711, P = 0.004, male, F2,6 = 31.770, P = 0.001; B. difformis female, F2,6 = 16.364, P = 0.004, male, F2,6 = 14.974, P = 0.005) (Fig. 3D). The highest GST activities in B. difformis and B. odoriphaga occurred at 36 °C and 38 °C, respectively. Compared with at 25 °C, the GST activities of B. difformis increased by 75% (female) and 80% (male) at 36 °C, which were greater than those of B. odoriphaga (F3,8 = 14.954, P = 0.001). Conversely, the GST activities of B. odoriphaga were higher than those of B. difformis at 38 °C.
Hsp gene expression
hsp70
The hsp70 expression levels in B. odoriphaga (female, F2,6 = 65.280, P < 0.001; male, F2,6 = 186.099, P < 0.001) and B. difformis (female, F2,6 = 49.447, P < 0.001; male, F2,6 = 52.535, P < 0.001) adults were significantly induced by heat stress (Fig. 4A). At 36 °C, the hsp70 expression in B. odoriphaga was up-regulated by 4.66- (female) and 7.84- (male) fold, while in B. difformis it was up-regulated by 6.72- (female) and 11.08- (male) fold, compared with at 25 °C. In addition, the maximal inductions in B. difformis and B. odoriphaga were obtained at 36 °C and 38 °C, respectively.
Changes in hsp70 (A) and hsp90 (B) expression of B. odoriphaga and B. difformis adults after heat shock. Each value represents the average (±s.e.) of three replicates. Different capital letters over the bars indicate significant differences in gene expression levels of B. odoriphaga or B. difformis (same single-sex) among different heat stress conditions (P < 0.05), while and the different small letters indicate significant differences of two Bradysia adults at the same heat stress (P < 0.05). Data was analyzed with ANOVA (Tukey’s HSD).
hsp90
hsp90 expression levels in B. odoriphaga (female, F2,6 = 217.970, P < 0.001; male, F2,6 = 40.782, P < 0.001) and B. difformis (female, F2,6 = 15.524, P = 0.004; male, F2,6 = 12.012, P = 0.008) adults increased dramatically at the treatment temperatures (Fig. 4B). Compared with at 25 °C, hsp90 expression in B. odoriphaga was up-regulated by 1.13- (female) and 0.81- (male) fold at 36 °C, while in B. difformis it was up-regulated by 1.87- (female) and 3.25- (male) fold. Consistent with the hsp70 results, the maximal inductions of hsp90 in B. difformis and B. odoriphaga were recorded at 36 and 38 °C, respectively.
Discussion
Poikilotherms are usually exposed to various challenges to survival and reproduction in their environments, and temperature is a critical abiotic factor that causes physiological changes in arthropods24. When the temperature exceeds the optimum for insects, short-term heat shock can cause rapid death. In this study, heat shock temperatures over 36 °C exerted significant lethal effects. For all treatments (0.5–4 h), the Ltemp50 of B. odoriphaga was in the range of 36.48–39.61 °C, and that of B. difformis was 35.92–38.15 °C. In northern China, the temperature is normally above 35 °C during the summer months, and the maximum daytime temperature even exceeds 40 °C (Climate Databases, Chinese Academy of Forestry)37. Thus, this environmental condition is extremely adverse to Bradysia species. Because it absorbs the solar radiation, the ground’s surface temperature is greater than the atmospheric temperature, which could aggravate the heat stress. Previous studies confirmed that Bradysia adults, having weak flight capabilities, were mainly active on the ground32,38. Thus, the two Bradysia adults were bound to confront thermal stress in the summer, and the thermal stress resulted in rapid death, restricting their abundance.
In addition to rapid lethal effects, heat stress also exerted various biological stresses on surviving insects, such as suppressing fecundity and longevity1,10. Previous studies confirmed that two Bradysia adults did not oviposit at once after emergence, and the pre-oviposition period ranged from 1.0 to 1.5 d32,33,34, indicating that they were restricted to suffer the heat stress in the daytime before oviposition. Results obtained in this study revealed that heat shocks (36–38 °C for 0–4 h) suppressed the reproductive capacity and longevity of the surviving adults. After exposure to 38 °C for 2 h, few B. difformis survived, and those that did were unable to oviposit and mate. Meanwhile, more B. odoriphaga adults survived, but their fecundity was low. Our findings were partially consistent with the previous reports about other insects. Cheng et al. also found heat shock, except for rapid lethal effects, also resulted in longevity and fecundity suppression of B. odoriphaga adults37. Agasicles hygrophila adults suffered adverse effects after being exposed to 36 and 39 °C for 4.0 h, with the fecundity and offspring hatching rate decreasing39. Similarly, the longevity of Helicovrpa armigera was shortened under heat shock (40–46.5 °C) and the fecundity decreased40. The effects of heat stress on fecundity and longevity in insects may be a result of direct injuries to reproductive systems or metabolic disorders. However, we could not determine whether the reduction in fecundity resulted from damage to the reproductive systems of both sexes or to only one sex.
Heat stress (36–40 °C) exerted significant lethal and sub-lethal effects on two Bradysia adults, but there were significant differences in the heat-tolerance responses between them: B. odoriphaga possessed more heat tolerance than B. difformis indicating that the former maintained a higher survival rate after heat shock exposure and suffered less severe sub-lethal effects. Previous research confirmed that Bemisia tabaci, whose population peaked in summer, did not exhibit significant negative changes after a 1-h heat shock (37–45 °C), while the fecundity of Trialeurodes vaporariorum, whose population peaked under cooler conditions, decreased rapidly10. Thermal adaptability limits the distribution and abundance of Culicoides imicola and Culicoides bolitinos. Compared with C. imicola, C. bolitinos has a wider altitude range and has stronger heat- and cold-stress tolerance levels41. Moreover, B. dorsalis, a widely distributed species, whose heat tolerance is enhanced by heat hardening at 35 °C, 37 °C, 39 °C and 41 °C, has a greater thermal plasticity than Bactrocera correcta, a narrowly distributed species, whose heat tolerance is only enhanced at 39 °C and 41 °C42. Here, the regional distributions of the two Bradysia species in Chinese chive fields were significantly different. B. odoriphaga has a wide distribution in North China, especially in Shandong, Hebei and Beijing, while B. difformis is mainly distributed in the northwest and northeast of China31,32,36. We hypothesized that the different responses to heat shock were related to the population dynamics of the two Bradysia species.
Generally, exposure to high- or low- temperature stress may lead to oxidative damage and generate surplus ROS in insects43,44. To relieve the adverse oxidative stress, insects increase antioxidant defense to maintain a balance in ROS metabolism45. For example, the antioxidant enzyme activities (SOD, POD, CAT and GSTs) of Bactrocera dorsalis 24, Chilo suppressalis 44, Antheraea mylitta 46 and Propylaea japonica 47 were induced by heat stress to protect the insects. In this study, after 36 and 38 °C heat shock treatments, the MDA concentration of B. difformis was greater than that of B. odoriphaga, which indicated that B. difformis suffered more oxidative stress. Moreover, the antioxidant enzyme activities (SOD, POD, CAT and GSTs) varied significantly after heat stress, indicating the protection function of antioxidant enzymes. At 36 °C, the POD, CAT and GST activities of B. difformis were greater than those of B. odoriphaga, while all of the tested antioxidant enzyme activities of B. odoriphaga were greater than those of B. difformis at 38 °C. This phenomenon was consistent with that B. odoriphaga possessed a stronger heat tolerance than B. difformis. Furthermore, the POD and GST activities of B. odoriphaga were induced at a higher temperature (38 °C), suggesting that they were stimulated to protect insects by scavenging ROS at a higher heat stress. Meanwhile, the reduction in the SOD activities in both Bradysia adults at 38 °C, compared with the control, suggested that excessive ROS could decrease SOD activity. Thus, the different heat tolerance levels in the two Bradysia species were related to the different responses of antioxidant enzymes to heat stress.
Hsps of insects are involved in physiological responses to various environmental stresses, especially heat and cold stress15,42. Previous research confirmed that Hsp70 and Hsp90 were two prominent Hsps that play important roles in thermal stress. Our study also confirmed that in both Bradysia adults the expression levels of hsp70 and hsp90 were induced by heat stress, suggesting that these two Hsps were involved in protecting Bradysia adults from thermal stress. At 36 °C, the relative expression of hsp70 and hsp90 in B. difformis increased more significantly than in B. odoriphaga, while the opposite was true at 38 °C. Previous studies indicated that the temperature for the onset of the induction of hsp gene expression (T on ) and the temperature for the maximal induction of gene expression (T max ) of hsp may be useful indicators to evaluate the thermal tolerance of insects48,49. The higher of Ton and Tmax of hsps are, the stronger heat tolerance of insects is, while the lower of Ton and Tmax of hsps are, the stronger cold tolerance of insects is. The T on and T max of five hsps (hsp20, hsp40, hsp60, hsp70 and hsp90) in Liriomyza huidobrensis, which possesses a great heat tolerance, were higher than in Liriomyza sativae, which has a greater cold tolerance49. Similarly, Drosophila virilis, the low-latitude species, possesses a greater heat tolerance than Drosophila lummei, the high-latitude species, and the T max of the expression levels of the hsp genes in the former were greater than in the latter28. Thus, in the current study, we hypothesized that the Ton and Tmax of hsp70 and hsp90 in B. odoriphaga were close to 38 °C, higher than those in B. difformis, close to 36 °C. Indeed, B. odoriphaga possessed a greater heat-tolerance than B. difformis. Moreover, the synthesis of Hsps and antioxidant enzyme proteins consume biological energy50. Thus, the declines in the fecundity and longevity of the Bradysia species may have resulted from a reduction in energy, which was consumed to synthesize stress proteins or supporting enzymatic reactions.
In conclusion, our results confirmed that two Bradysia adults were sensitive to heat shock and that after short-term heat shocks their longevity and fecundity were suppressed. B. odoriphaga possessed the greater heat tolerance, and the difference in the heat tolerance levels between species was related to protective physiological responses, such as antioxidant capacities and hsp expression levels.
Methods
Insect materials
Bradysia odoriphaga Yang and Zhang and Bradysia difformis Frey colonies were originally obtained from a Chinese chive greenhouse field in Tai’an, Shangdong, China, in April 2015. Insect colonies were maintained in the Shandong Provincial Key Laboratory of Applied Microbiology, and reared on Chinese chives for more than 5 generations according to the breeding method32,34. Eggs, larvae and pupae were reared in culture dishes (Φ = 9 cm) covered with wet filter paper, and newly emerged adults were placed in pairs in oviposition containers (3-cm diameter × 1.5-cm height). Insect colonies were maintained in growth cabinets at 25 ± 1 °C with 75 ± 5% relative humidity, and a 12:12 h light:dark cycle.
Heat shock treatment
The treatment methods refer to the methods described by Huang et al.9. Adults (single-sex) that emerged from pupae within a 12-h period were collected in a 10-mL centrifuge tube, and exposed to a water bath at the target temperature (32, 34, 36, 38, 40, 42 and 44 °C) for 0.5, 1, 2 and 4 h. They were allowed to recover at 25 °C for 1 h. The survival number was recorded. The treatment kept at 25 °C was regarded as the control. Each treatment contained 100 individuals for five replicates, and each replicate contained 20 individuals. The median lethal temperatures, Ltemp50 values, were calculated according to the logistic regression (1).
Longevity and reproductive capacity
Above lethal experiments indicated that 34 °C was the highest temperature exerted no lethal effects on adults within 4 h, while almost no B. difformis adults survived at 40 °C. Therefore, after the heat shock (34, 36 and 38 °C for 0.5, 1, 2 and 4 h), the surviving adults were collected as the tested insects. Males and females were paired and placed on oviposition plastic and reared at 25 °C. Adults were checked every 12 h, and the numbers of eggs were recorded until all of the adults died. The average longevity, fecundity and female fertility rate were calculated. The treatment kept at 25 °C was regarded as the control. Every treatment contained 60 pairs of adults for three replicates, and each replicate contained 20 pairs.
Antioxidant responses and hsp70 and hsp90 expression levels
Heat treatment
Above lethal experiments indicated that there were significant differences in survival rate of two Bradysia adults at 36 and 38 °C for 1 h. The new adults (single-sex) were exposed to a water bath at the target temperature (36 and 38 °C) for 1 h, and then allowed to recover at 25 °C for 1 h. The treatment kept at 25 °C was regarded as the control. All of the surviving adults were flash frozen in liquid nitrogen and stored at −80 °C.
Sample preparation and enzyme activity assay
The treated adults were homogenized in a cold mortar with a pestle in 0.05 M phosphate buffer solution, pH 7.8, containing 0.1 mM ethylenediamine tetraacetic acid and 1% polyvinylpyrrolidone. The crude homogenates were centrifuged at 10,000 g for 15 min at 4 °C. The supernatant was gathered for the determination of antioxidant enzyme activities. Protein concentrations were determined using the Bradford assay. The activities of CAT, POD, SOD and GSTs, and the MDA concentration, were determined by spectrophotometry. All spectrophotometric analyses were conducted in a Shimadzu UV-2450 spectrophotometer (Shimadzu, Arlington, MA, USA). Every treatment contained 240 individuals (single-sex) for three replicates, and each replicate contained 80 individuals.
CAT activity was calculated by measuring the consumption of H2O2 at 240 nm for 2 min. The amount (μmol) of H2O2 decomposition per min per mg protein was defined as one unit of CAT activity. The result was expressed as U mg−1 protein.
POD activity was assayed using the guaiacol oxidation method at 470 nm. One unit of POD activity was defined as the amount that catalyzes 1 μmol substrate reaction per minute per mg protein. The result was expressed as U mg−1 protein.
SOD activity was measured based on the inhibition of the nitro blue tetrazolium photochemical reaction at 550 nm. One unit of SOD activity was defined as the amount of enzyme that caused a 50% inhibition of the nitro blue tetrazolium reduction. The result was expressed as U mg−1 protein.
GSTs activity was determined using 1-chloro-2,4-dinitrobenzene and reduced glutathione as the substrate. The change in absorbance was measured continuously for 4 min at 340 nm. Changes in absorbance per min were converted into mmol 1-chloro-2,4-dinitrobenzene conjugated/min/mg protein using a molar extinction coefficient of 9.6 mM−1 cm−1. The result was expressed as mmol min−1 mg−1 protein.
The LPO was determined indirectly by measuring the amount of MDA formed by reacting with thiobarbituric acid to give a red species having a maximum absorption at 532 nm at 37 °C25. The MDA concentration was expressed as nmol of MDA produced per mg protein.
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
Total RNAs were extracted using an RNApure Tissue Kit (DNase I) (ComWin Biotech, Beijing, China). cDNA was synthesized using the SYBR1 PrimeScript RT-qPCR Kit II (Takara Biotechnology, Dalian, China). hsp70 and hsp90 mRNA levels were measured using RT-qPCR. RT-qPCR reactions were performed using the Bio-rad CFX96 Real-Time PCR System (BioRad Laboratories, Hercules City, CA, USA) with SYBR-Green detection. The average threshold cycle (Ct) was calculated per sample. The relative expression levels were calculated with the 2−ΔΔCT method. The relative level of each hsp was defined as the increase (in folds) compared with the amount of β-actin. RT-qPCR primers and the list of accession numbers are provided (Table 3). The process of how to design these primers was supplied in the Supplementary section (Table 1S, Figs 1S and 2S). Each gene was analyzed in triplicate in each of three biologically independent treatments. Every treatment contained 150 individuals (single-sex) for three replicates, and each replicate contained 50 individuals.
Data analysis
In the logistic regression analysis (Eq. 1), the survival rates of these two Bradyisa adults after heat shock were regarded as the dependent variable (y), while the treated temperatures were regarded as the independent variables, and ×0 indicates the Ltemp50 value. We tested the variables for homogeneity of group variances using Levene’s test and normality using the Kolmogorov-Smirnov test prior to statistical analysis. For analysing the difference among different treatments, the survival rate, longevity, fecundity, egg hatching rate and female spawning rate of B. odoriphaga or B. difformis at each heat treatment were regarded as the dependent variable, while the treatment were regarded as the independent variables in one-way ANOVA followed by Tukey’s HSD multiple comparisons. For analysing the difference between two species, the survival rate, longevity (single-sex), fecundity, egg hatching rate and female spawning rate of B. odoriphaga or B. difformis at same heat condition were regarded as the Test variables, while the species were regarded as the Group variables in Independent-Samples T Test comparison.
Similarly, when analysing the enzyme activities and gene expression levels of two Bradysia adults, the MDA concentration, enzyme activities (CAT, SOD, POD and GSTs) and gene expression levels (hsp70 and hsp90) of B. odoriphaga or B. difformis at each heat treatment were regarded as the dependent variable, while the treatments were regarded as the independent variables in one-way ANOVA followed by Tukey’s HSD multiple comparisons. With regards to the difference analysis at the same heat conditions, the values of two Bradyisa species were regarded as the dependent variable, while the species (single-sex) were regarded as independent variables in the above-mentioned method. All analyses were performed with PASW Statistics 18.0.0 (2009; SPSS Inc. Quarry Bay, HK). Figures were constructed using SigmaPlot 12.0.
Data availability
All data generated or analysed during this study are included in this published article.
References
Rinehart, J. P., Yocum, G. D. & Denlinger, D. L. Thermotolerance and rapid cold hardening ameliorate the negative effects of brief exposures to high or low temperatures on fecundity in the flesh fly, Sarcophaga crassipalpis. Physioll. Entomol. 25, 330–336 (2000).
Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 (2006).
Easterling, D. R. et al. Climate extremes: observations, modeling, and impacts. Science. 289, 2068–2074 (2000).
Diffenbaugh, N. S., Pal, J. S., Trapp, R. J. & Giorgi, F. Fine-scale processes regulate the response of extreme events to global climate change. P. Natl. Acad. Sci. USA 102, 15774–15778 (2005).
Meehl, G. A. & Tebaldi, C. More intense, more frequent, and longer lastingheat waves in the 21st century. Science. 305, 994–997 (2004).
Rahmstorf, S. & Coumou, D. Increase of extreme events in a warming world. P. Natl. Acad. Sci. USA 108, 17905–17909 (2011).
Hallman, G. J., Wang, S. & Tang, J. Reaction orders for thermal mortality of third instars of Mexican fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 98, 1905–1910 (2005).
Schou, M. F., Kristensen, T. N., Kellermann, V., Schlötterer, C. & Loeschcke, V. A. Drosophila laboratory evolution experiment points to low evolutionary potential under increased temperatures likely to be experienced in the future. J. Evolution. Biol. 27, 1859–1868 (2014).
Huang, L. H., Chen, B. & Kang, L. Impact of mild temperature hardening on thermotolerance, fecundity, and hsp gene expression in Liriomyza huidobrensis. J. Insect Physiol. 53, 1199–1205 (2007).
Jerbi-Elayed, M., Lebdi-Grissa, K., Le Goff, G. & Hance, T. Influence of temperature on flight, walking and oviposition capacities of two aphid parasitoid species (Hymenoptera: Aphidiinae). J. Insect Behav. 28, 157–166 (2015).
Hansen, J. D., Johnson, J. A. & Winter, D. A. History and use of heat in pest control: a review. Int. J. Pest Manage. 57, 267–289 (2011).
Cui, X. H., Wan, F. H., Xie, M. & Liu, T. X. Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci Biotype B. J. Insect Sci. 8, 1–10 (2008).
Duman, J. G. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu. Rev. Physiol. 63, 327–357 (2001).
Sørensen, J. G., Kristensen, T. N. & Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025–1037 (2003).
Kang, L., Chen, B., Wei, J. N. & Liu, T. X. Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annu. Rev. Entomol. 54, 127–145 (2009).
Chown, S. L. & Terblanche, J. S. Physiological diversity in insects: ecological and evolutionary contexts. Adv. Insect Physiol. 33, 50–152 (2006).
Tantowijoyo, W. & Hoffmann, A. A. Identifying factors determining the altitudinal distribution of the invasive pest leafminers Liriomyza huidobrensis and Liriomyza sativae. Entomol. Exp. Appl. 135, 141–153 (2010).
Lalouette, L., Williams, C. M., Hervant, F., Sinclair, B. J. & Renault, D. Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp. Biochem. Phys. A. 158, 229–234 (2011).
Meng, J. Y., Zhang, C. Y., Zhu, F., Wang, X. P. & Lei, C. L. Ultraviolet light-induced oxidative stress: effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 55, 588–592 (2009).
Joanisse, D. R. & Storey, K. B. Oxidative stress and antioxidants in stress and recovery of cold-hardy insects. Insect Biochem. Molec. 28, 23–30 (1998).
Felton, G. W. & Summers, C. B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 29, 187–197 (1995).
Dubovskiy, I. M. et al. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella. larvae (Lepidoptera: Pyralidae). Comp. Biochem. Phys. C. 148, 1–5 (2008).
Ju, R. T., Gao, L., Zhou, X. H. & Li, B. Physiological responses of Corythucha ciliata adults to high temperatures under laboratory and field conditions. J. Therm. Biol. 45, 15–21 (2014).
Jia, X. F., Dou, W., Hu, F. & Wang, J. J. Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol. 94, 956–963 (2011).
Zhang, L. J. et al. Trade-off between thermal tolerance and insecticide resistance in Plutella xylostella. Ecol. Evol. 5, 515–530 (2015).
Gehring, W. J. & Wehner, R. Heat shock protein synthesis and thermotolerance in Cataglyphis, an ant from the Sahara desert. P. Natl. Acad. Sci. USA 92, 2994–2998 (1995).
Chen, H., Xu, X. L., Li, Y. P. & Wu, J. X. Characterization of heat shock protein 90, 70 and their transcriptional expression patterns on high temperature in adult of Grapholita molesta (Busck). Insect Sci. 21, 439–448 (2014).
Garbuz, D., Evgenev, M. B., Feder, M. E. & Zatsepina, O. G. Evolution of thermotolerance and the heat-shock response: evidence from inter/intraspecific comparison and interspecific hybridization in the virilis species group of Drosophila. I. Thermal phenotype. J. Exp. Biol. 206, 2399–2408 (2003).
Yang, C. K. & Zhang, X. Notes on the fragrant onion gnats with descriptions of two new species of Bradysia (Sciaridae: Diptera). Acta. Agric. Univ. Pekinensis. 11, 153–156 (1985).
Jagdale, G. B., Casey, M. L., Cañas., L. & Grewal, P. S. Effect of entomopathogenic nematode species, split application and potting medium on the control of the fungus gnat, Bradysia difformis, (Diptera: Sciaridae), in the greenhouse at alternating cold and warm temperatures. Biol. Control. 43, 23–30 (2007).
Xue, M., Pang, Y. H., Wang, C. X. & Li, Q. L. Biological effect of liliaceous host plants on Bradysia odoriphaga Yang et Zhang (Diptera: Sciaridae). Acta. Entomol. Sin. 48, 914–921 (2005).
Liu, Q., Gou, Y. P. & Liu, C. Z. Effects of different temperature on the growth, development and fecundity of Bradysia difformis. Plant. Prot. 41, 85–87 (2015).
Zhang, P., Liu, F., Mu, W., Wang, Q. & Li, H. Comparison of Bradysia odoriphaga Yang and Zhang reared on artificial diet and different host plants based on an age-stage, two-sex life table. Phytoparasitica. 43, 107–120 (2015).
Li, W. X. et al. Effects of temperature on the age-stage, two-sex life table of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 108, 126–134 (2015).
Dang, Z. H., Dong, J., Gao, Z. L., Jia, H. M. & Zhang, K. J. Biology and injury of Bradysia odoriphaga on leek in different types of cultivation. J. Agric. Hebei. Univ. 24, 65–68 (2001).
Hu, X., Bai, Y. C., Xu, W. H., Zou, D. Y. & Xu, J. Y. Population dynamics of Bradysia odoriphaga and its relationship with temperature. Curr. Biotechnol. 6, 113–118 (2016).
Cheng, J. X. et al. Effects of Heat Shock on the Bradysia odoriphaga (Diptera: Sciaridae). J. Econom. Entomol. 2, 1–9 (2017).
Zhou, L. et al. Emergence rhythm and oviposition behavior of Bradysia odoriphaga. J. Henan Agric. Univ. 49, 482–487 (2015).
Zhao, M. T. et al. Effects of periodically repeated heat events on reproduction and ovary development of Agasicles hygrophila (Coleoptera: Chrysomelidae). J. Econ. Entomol. 109, 1586–1594 (2016).
Mironidis, G. K. & Savopoulou-Soultani, M. Effects of heat shock on survival and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) adults. J. Therm. Biol. 35, 59–69 (2010).
Vorhees, A. S., Gray, E. M. & Bradley, T. J. Thermal resistance and performance correlate with climate in populations of a widespread mosquito. Physiol. Biochem. Zool. 86, 73–81 (2013).
Hu, J. T., Chen, B. & Li, Z. H. Thermal plasticity is related to the hardening response of heat shock protein expression in two Bactrocera fruit flies. J. Insect Physiol. 67, 105–113 (2014).
An, M. I. & Choi, C. Y. Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters. Comp. Biochem. Phys. B. 155, 34–42 (2010).
Cui, Y. D., Du, Y. Z., Lu, M. X. & Qiang, C. K. Antioxidant responses of Chilo suppressalis (Lepidoptera: Pyralidae) larvae exposed to thermal stress. J. Therm. Biol. 36, 292–297 (2011).
Krishnan, N., Davis, A. J. & Giebultowicz, J. M. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem. Bioph. Res. Co. 374, 299–303 (2008).
Skorpil, V. & Kolman, J. Effects of temperature on modulation of oxidative stress and antioxidant defenses in testes of tropical tasar silkworm Antheraea mylitta. J. Therm. Biol. 38, 199–204 (2013).
Zhang, S. Z., Fu, W. Y., Li, N., Zhang, F. & Liu, T. X. Antioxidant responses of Propylaea japonica, (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 73, 47–52 (2015).
Tomanek, L. & Somero, G. N. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric Marine snails from different thermal habitats: implications for limits of thermotolerance and biogeography. J. Exp. Biol. 202, 2925–2936 (1999).
Huang, L. H. & Kang, L. Cloning and interspecific altered expression of heat shock protein genes in two leafminer species in response to thermal stress. Insect Mol. Biol. 16, 491–500 (2007).
Hoffmann, A. A. Acclimation: increasing survival at a cost. Trends Ecol. Evol. 10, 1–2 (1995).
Acknowledgements
This research was funded by “The National Key Research and Development Program of China (2017YFD0200900)” and “International Commonweal Scientific Research Special Fund-Research and Demonstration of Crop Root Maggot Control Technology (No. 201303027)”. We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The study was jointly conceived by G.Z., Y.L., and M.X. Experiments were designed by G.Z. and M.X.; G.Z. prepared the manuscript; Y.L., M.X., Guixia Ji, Fang Liu, H.Z. and X.S. edited the manuscript. G.Z., Y.L. and Xia Sun carried out experiments.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, G., Xue, M., Luo, Y. et al. Effects of short-term heat shock and physiological responses to heat stress in two Bradysia adults, Bradysia odoriphaga and Bradysia difformis . Sci Rep 7, 13381 (2017). https://doi.org/10.1038/s41598-017-13560-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13560-4
This article is cited by
-
Transgenerational effects of thermal stress on reproductive physiology of fall armyworm, Spodoptera frugiperda
Journal of Pest Science (2023)
-
Biochemical response of Aedes aegypti and Aedes albopictus mosquitoes after exposure to thermal stress and toxin of Bacillus thuringiensis israelensis
International Journal of Tropical Insect Science (2022)
-
Differential manifestation of RONS and antioxidant enzymes in response to singular versus combinatorial stress in Chironomus ramosus
Stress Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.