Abstract
Sphingosine-1 phosphate receptor 1 (S1P1) is critical for the egress of T and B cells out of lymphoid organs. Although S1P1 agonist fingolimod is currently used for the treatment of multiple sclerosis (MS) little is known how S1P1 signaling regulates Th17 and Treg cell homeostasis. To study the impact of S1P1 signaling on Th17 and Treg cell biology, we specifically deleted S1P1 in Th17 and Treg cells using IL-17A Cre and Foxp3 Cre mice, respectively. Deletion of S1P1 in Th17 cells conferred resistance to experimental autoimmune encephalomyelitis (EAE). On the other hand, permanent deletion of S1P1 in Treg cells resulted in autoimmunity and acute deletion rendered mice more susceptible to EAE. Importantly, our study revealed that S1P1 not only regulated the egress of Treg cells out of lymphoid organs and subsequent non-lymphoid tissue distribution but also their phenotypic diversity. Most of the Treg cells found in S1P1-deficient mice as well as MS patients on fingolimod therapy had an activated phenotype and were more prone to apoptosis, thus converted to effector Treg. Our results provide novel insight into the functions of S1P1 and potential impact of long term fingolimod use on Th17 and Treg cell biology and general health in MS patients.
Similar content being viewed by others
Introduction
Sphingosine 1 phosphate receptor 1 (S1P1) is a G-protein coupled receptor expressed by endothelial cells and lymphocytes, including Treg cells. S1P1 activates various signaling cascades, including PI3K-Akt-mTOR upon binding to its natural ligand sphingosine-1 phosphate (S1P)1. S1P1 was previously shown to play a critical role in the egress of both T and B cells out of thymus and lymphoid organs2,3,4. A gradient of S1P which is high in blood and lymph, and low in tissues, is created by tight regulation of its production5,6. This gradient of S1P coupled with ligand binding-triggered receptor internalization forms the basis of the egress mechanism for T and B cells7. Fingolimod (FTY720 or GilenyaTM) is a structural analog of sphingosine-1; upon binding to S1P1, it induces its internalization and desensitization, thereby causing sequestration of lymphocytes in lymphoid tissues8. Although approved for the treatment of multiple sclerosis9, in some patients, cessation or initiation of fingolimod therapy resulted in exacerbation of MS and/or formation of tumefactive lesions in the brain through yet unexplored mechanisms10,11,12,13,14.
Th17 cells are required for the pathogenesis of multiple autoimmune and chronic inflammatory conditions, including EAE, a murine model of MS. Although S1P1 was genetically targeted broadly in all CD4+ T cells previously, T helper lineage specific knockout murine models of S1P1 have not been studied, thus, it is unknown how S1P1 or fingolimod modulates the biology of Th17 lineage independently of its effects on other helper T cell lineages. CD4+Foxp3+ regulatory T cells (Treg), on the other hand, are crucial for preventing autoimmunity and restraining effector T cell responses during protective immunity15,16. Similarly, the role of S1P1 in exclusively committed Treg cell homeostasis has been less clear, as the mice used in previous reports had deleted S1P1 in all CD4+ T cells.
Recent studies revealed that non-lymphoid tissue (NLT) resident Treg cells assume different phenotypic features than those in circulation or lymphoid tissues (LT)16,17. NLT Treg cells resemble conventional effector CD4+ T cells, and express high levels of CD44, low levels of CD62L and CCR7 and are named effector Treg (eTreg) 18. eTreg cells also express CD103, KLRG1 and ICOS. eTreg cells were shown to be dependent on ICOSL stimulation provided by antigen presenting cells (APC) for their homeostasis in tissue microenvironments lacking IL-2 and appear to be more prone to apoptosis19. In contrast, LT or circulatory Treg cells inversely express the above-mentioned molecules. They are named central Treg (cTreg) and, conversely, cTreg cells rely more on IL-2 than ICOS for their homeostasis and are resistant to apoptosis19. This dichotomous phenotypic subdivision of murine Treg and survival mechanisms are also valid for human Treg cells20. Human cTreg cells can be defined as CD4+CD45RA+CD45RO−CD25+CD127−Foxp3low. Conversely, human CD4+Foxp3+ eTreg cells are CD45RA−CD45RO+CD25highCD127−Foxp3high. More recently, C-C chemokine receptor 4 (CCR4) was defined as a marker of human eTreg along with other effector non-Treg T cells, and was targeted for depletion of exclusively eTreg cell populations21. The studies using broad deletion of S1P1 in T cells (using the CD4cre system) showed improved Treg generation and function in the absence of this receptor22. In contrast, S1P1 overexpression in CD4 T+ cells reduced their differentiation into Treg cells and functions through PI3K-Akt-mTOR axis and its effect on Smad3 transcription factor22,23. However, in these studies S1P1 deletion was not unique to Treg cells. More importantly, it remains unknown how S1P1 regulates function and egress of specifically committed Treg cells.
By permanent and/or temporal genetic deletion of S1P1, herein we show that S1P1 regulates proper Th17 and Treg cell distribution across peripheral organs and homing to the central nervous system and their functions as well as EAE development in mice. We also show that S1P1 regulates phenotypic diversity of murine and human Treg cells by controlling central to effector Treg cell switch. Our data provides novel insights into the egress-dependent and independent functions of S1P1 and potential impact of long term fingolimod use on Treg cell homeostasis.
Results
S1P1 regulates generation and peripheral distribution of Th17 cells and susceptibility to EAE
To uncover the role of S1P1 on Th17 cell biology we crossed S1P 1 Flox to IL-17A Cre ROSA RFP mice. Red fluorescent reporter (RFP) allowed us to gate on all the IL-17A expressing T cells when necessary (including true-Th17 and ex-Th17 which are RFP+ but no longer expressing IL-17A). Deletion of S1P1 from Th17 cells rendered these mice completely resistant to the development of EAE induced by MOG35–55 immunization (Fig. 1a). There were significantly fewer infiltrating leukocytes, including Th17 and Th1 cells, in the central nervous system (CNS) of S1P 1 Flox IL-17A Cre mice compared with controls (Fig. 1b). These results are in line with previous findings that S1P1 regulates T cell egress out of lymphoid organs, thus they are unable to home to CNS during EAE (21–24). To ensure that the past observations on CD4+ T cells are also applicable specifically to Th17 lineage, we examined Th17 cell distribution across the peripheral organs in S1P 1 Flox IL-17A Cre ROSA RFP and control mice, before (not shown) and after MOG35–55 immunization (Fig. 1c,d). As expected, we observed a significant reduction in the percentages of Th17 cells in the lung, spleen and colon (Fig. 1c,d). Surprisingly, Th17 cell ratio in the lymph nodes also decreased, even after immunization, in contrast to the proposed role of S1P1 for egress of T cells out of lymph nodes (LN) and the expected entrapment of these cells. These results suggest that Th17 generation and/or survival may be regulated by S1P1. We did not see a conclusive difference in the apoptosis of peripheral LN Th17 cells between control and S1P 1 Flox IL-17A Cre ROSA RFP mice, suggesting a proliferation or differentiation defect (Supplemental Fig. 1a). In fact, when LN lymphocytes were rechallenged with MOG35–55 in the presence or absence of IL-23 following the first immunization with MOG35–55, Th17 cell expansion and IL-17A production were greatly diminished in S1P 1 Flox IL-17A Cre ROSA RFP mice compared with controls (Fig. 1e,f) which may be due to either reduced frequency of Th17 cells or a compromised responsiveness to antigen and IL-23.
S1P1 is required for peripheral organ distribution and in vivo generation of Th17 cells. (a) EAE scores of S1P 1 Flox IL-17A Cre ROSA RFPand control mice (S1P 1 WT IL-17A Cre ROSA RFP) that were immunized with MOG35–55 and Complete Freund’s Adjuvant. N = 5 mice per group, the experiment was repeated three times. (b) IL-17A and IFN-γ expression of CD4+ T cells from central nervous system (CNS) of S1P 1 Flox IL-17A Cre ROSA RFP and control mice 15 days after MOG35–55 immunization (left). The cells were cultured 4 h with PMA/ionomycin and Golgi Plug for intracellular staining. Quantification of flow plots, (right).* indicates p-value <0.05. (c) Th17 and Th1 cell distribution across the peripheral organs in S1P 1 Flox IL-17A Cre ROSA RFP and control mice, 7 days after MOG35–55 immunization, and quantification was shown in (d). (e) Representative flow plot of RFP+ CD4+ cells after rechallenge with media, MOG35–55 or MOG35–55 + IL-23 following the first immunization. (f) IL-17A and IFN-γ production by S1P 1 Flox IL-17A Cre ROSA RFP and control mice in MOG35–55 + IL-23 rechallenge condition.
Treg-specific deletion of S1P1 causes lymphadenopathy and multi-organ inflammation
To gain insight into how S1P1 regulates Treg biology, we crossed S1P 1 Flox to Foxp3 Cre mice4. Foxp3 Cre locus harbors an internal ribosomal entry site (IRES)-driven YFP and allows tracking of Treg cells. Treg-specific deletion of S1P1 has been verified (Supplemental Fig. 1b). S1P 1 Flox Foxp3 Cre mice started to develop systemic autoimmunity at 8 weeks of age and died at around 13 weeks. We observed skin lesions around the eye with low penetrance (~41%) in these mice (Fig. 2a). At necropsy, we observed enlarged lymph nodes and spleen (Fig. 2b). As the disease progressed, lymphoid organs were observed at necropsy to be smaller than normal. The colon and cecum of 6-week-old S1P 1 Flox Foxp3 Cre mice was thickened with ill-formed feces suggestive of typhlocolitis. Histologically, the colon showed mild multifocal lymphocytic and neutrophilic colitis with protozoal bloom, mesenteric edema and mild lymphocytic steatitis (data not shown). Lung and liver had mild lymphocytic accumulations in the perivascular and periportal areas, respectively (Fig. 2c,d). Consistent with poor weight gain (Fig. 2e) and multi-organ inflammation, TNF-α and IFN-γ levels were highly elevated in the serum of 8-week-old S1P 1 Flox Foxp3 Cre mice, which suggests a systemic inflammation (Fig. 2f). Of note, S1P 1 Flox Foxp3 Cre mice were also anemic (Fig. 2g).
Treg-specific deletion of S1P1 causes autoimmunity. (a) Representative image of skin lesions that develop around the eyes of 8-week-old S1P 1 Flox Foxp3 Cre compared to S1P 1 WT Foxp3 Cre mice. (b) Representative splenomegaly and lymphadenopathy in 8 weeks-old S1P 1 Flox Foxp3 Cre mice compared to S1P 1 WT Foxp3 Cre mice. (c) Representative section of liver and lung stained with hematoxylin & eosin (H&E) depicting higher lymphocytic infiltrates in 6 weeks-old S1P 1 Flox Foxp3 Cre mice. Arrows point the lymphocytic infiltrates. PV, portal vein in liver; PV, pulmonary vein in lung; B, bronchiole. (d) Colon inflammation and discoloration in 8-week-old S1P 1 Flox Foxp3 Cre mice compared to S1P 1 WT Foxp3 Cre mice. (e) Weight chart indicating lack of weight gain over time in S1P 1 Flox Foxp3 Cre mice. (*) indicates significance, p < 0.05. (f) Elevated systemic TNF-α and IFN-γ levels in the serum of 8 weeks-old S1P 1 Flox Foxp3 Cre mice. (*) indicates significance, p < 0.05. (g) Colorimetric assay measuring hemoglobin in adult S1P 1 Flox Foxp3 Cre mice (*) indicates significance, p < 0.05. A total of 7 to 15 mice per group used for experiments (e–g). (h) Activation status of CD4+ or CD8+ T cells in the blood of 8-week-old S1P 1 Flox Foxp3 Cre compared to S1P 1 WT Foxp3 Cre mice by CD44 and CD62L staining (left) and quantified (right). 3 mice per group used.
Both CD4+ and CD8+ T cells in circulation, as well as lymphoid and non-lymphoid organs (Fig. 2h) presented with an activated phenotype. T cells in S1P 1 Flox Foxp3 Cre mice expressed high levels of CD44 and low levels of CD62L. Additionally, activated CD4+ T cells from S1P 1 Flox Foxp3 Cre mice produced higher quantities of IL-4 and IFN-γ in various organs, including liver, LN and colons compared to their WT counterparts (Supplemental Fig. 1c and data not shown). In the gut lamina propria of S1P 1 Flox Foxp3 Cre mice, CD4+ T cells also produced more IL-17A compared to control S1P 1 WT Foxp3 Cre mice. Collectively, these results indicated that Treg cells in S1P 1 Flox Foxp3 Cre mice are unable to suppress adaptive immunity, including Th1, Th2, and Th17 cells, possibly due to defects in mobility and/or functions of Treg cells.
S1P1 regulates Treg cell egress out of lymphoid organs and thus non-lymphoid tissue distribution
S1P1 was shown to be critical for the egress of both CD4+ and CD8+ T cells as well as B cells out of thymus and lymphoid tissues3,4. This requirement was demonstrated genetically by conditionally and globally deleting the receptor; and biochemically, using agonists of S1P1 receptor. Although the impact of FTY720 treatment on murine Treg cell egress has been assessed24,25, the impact of S1P 1 on Treg cells, independently of the effects on other cells, and how S1P 1 regulates specifically Treg cell egress out of thymus and lymphoid organs and homing to various tissues has not been studied. We therefore examined the tissue distribution of Treg cells in adult and neonatal S1P 1 Flox Foxp3 Cre mice. In adult (8-week-old) mice, we detected increased Treg cell frequency in the thymus (Fig. 3a,b) consistent with previous reports10,11,22. This could be due to defective egress out of the thymus or an increased differentiation of Treg cells from CD4+CD25+ precursors as suggested by others3,4,22. We did not however observe a difference in the in vitro differentiation efficacy of naïve CD4+ T cells into Treg cells in the presence of TGF-β between WT and S1P 1 Flox Foxp3 Cre (Supplemental Fig. 2b). Similar to the thymus, examination of lymph nodes (brachial, axillary and inguinal) revealed elevated numbers of Treg cells in these organs, which corroborates past studies that suggested a critical role for S1P1 in the egress of Treg cells out of lymphoid tissues. In line with this, blood Treg cell levels were greatly diminished in S1P 1 Flox Foxp3 Cre mice. The fact that the majority of peripheral Treg cells in S1P 1 Flox Foxp3 Cre mice are Helios+ suggests that S1P1 is dispensable for the egress of Treg cells out of the thymus, and that compensatory egress receptors may be in place (Supplemental Fig. 2a).
S1P1-deficient Treg cells exit thymus but show impaired secondary lymphoid tissue egress and reduced non-lymphoid tissue localization. a) Lymphocytes from various organs of 8-week-old control S1P 1 WT Foxp3 Cre or S1P 1 Flox Foxp3 Cre mice were stained for CD4. Treg (CD4+Foxp3+) distribution was analyzed by flow (representative zebra plots in (a) and quantified in (b), n = 4–7 mice), (*) indicates p < 0.05 quantified. (c) Thymus, spleen and LN from 6–8-week-old mice of indicated genotype were stained for CD45, CD3, CD4, CD25, and CD25+CD4+CD3+ Treg cells gated and among them RFP+ or YFP+ Treg cells visualized. A representative plot is provided.
To establish the role of S1P1 in the trafficking behavior of Treg cells under both homeostatic and inflammatory conditions, we performed cell fate mapping experiments. Thus, we crossed S1P 1 Flox Foxp3 Cre mice with ROSARFP mice. In these mice Treg cells are labeled with the red fluorescent protein (RFP) once Cre recombinase, which is under the endogenous Foxp3 promoter, has been activated. Thus, in female Foxp3 Cre het/S1P 1 Flox homo /ROSA RFP het mice 50% of the Treg cells in lymph nodes is expected to be labeled by both RFP and YFP. The females of this particular mouse strain do not develop inflammation since half of Treg cells behave like WT, whereas males of Foxp3 Cre /S1P 1 Flox homo/ROSA RFP develop very severe systemic inflammation. Thus, by studying both males and females of this particular strain we can study the role of S1P1 in the trafficking behavior of Treg under both homeostatic and inflammatory conditions. Indeed, we found that almost half of the Treg cells were labeled by both RFP and YFP in LNs but almost-none of these cells were detected in spleens of these animals (Fig. 3c). This result strongly suggests that egress of Treg cells from the thymus is S1P1-independent, however S1P1-mediated signaling is needed for Treg cells to migrate to the spleen.
We also assessed the non-lymphoid tissue resident Treg cell numbers. Adult S1P 1 Flox Foxp3 Cre mice had greatly reduced frequency of Treg cells in the colon and lung (Fig. 3a,b). All of the above changes were also readily detectable in 7 to 10-day-old mice, shortly after the initiation of Treg cell development (Supplemental Fig. 2c,d). Additionally, the reduction in Treg cell frequency in liver and spleen was more discernible in young mice than adults. Collectively, these data reveal that S1P1 may be dispensable for thymic egress but is required for egress from lymph nodes, and thus possibly indirectly impacts non-lymphoid tissue distribution of Treg cells.
Treg specific acute deletion of S1P1 renders mice more prone to EAE
To study Treg-specific S1P1 deletion temporally, we crossed S1P 1 Flox mice to Foxp3 Cre-ERT2 mice, which express enhanced green fluorescent protein (eGFP) fused to a Cre recombinase–estrogen-receptor-ligand-binding-domain protein from the 3′ untranslated region of Foxp3. This allows deletion of the receptor only after tamoxifen administration. Such acute deletion also provides a time window to study the impact of exclusively S1P1 deficiency before the systemic inflammation occurs. Acute deletion of S1P1 after five consecutive daily tamoxifen injections resulted in greatly diminished Treg cell percentages and absolute number in circulation, lung and liver. In contrast, lymph node and thymus- resident Treg cells were significantly increased. This was observed only after tamoxifen injection and only in S1P 1 Flox Foxp3 Cre-ERT2 but not control S1P 1 WT Foxp3 Cre-ERT2 mice (Supplemental Fig. 3).
To explore how S1P1 deletion would impact disease onset and progression, we induced experimental autoimmune encephalomyelitis (EAE) by immunization with myelin oligodendrocyte (MOG35–55) peptide emulsified in CFA (Complete Freund’s Adjuvant) after acute deletion of S1P1 with four consecutive tamoxifen injections. S1P 1 Flox Foxp3 Cre-ERT2 mice developed more severe EAE with earlier disease onset compared to control S1P 1 WT Foxp3 Cre-ERT2 mice, which also received tamoxifen (Fig. 4a). The severity of the disease correlated with lymphocyte infiltration to the brain and spinal cord, thus, higher number of lymphocytic infiltrates and IFN-γ+ and IL-17A+ single and double producer CD4+ T cells were observed in S1P 1 Flox Foxp3 Cre-ERT2 mice at the peak of the disease (Fig. 4b). Similar to the reduction of S1P1-deficient Treg cells observed in non-lymphoid tissues at steady state, the ratio of Treg cells among CD4+ T cells in the central nervous system (CNS) of S1P 1 Flox Foxp3 Cre-ERT2 mice during EAE was reduced by 4–5-fold (Fig. 4c). While the proportion of S1P1-deficient Treg is dramatically reduced in the CNS, their absolute number is slightly higher in comparison to control mice (Supplemental Fig. 3b). These data are in line with our previous finding that the ratio between Treg and the effector T cells is the determining factor that dictate the development of EAE. Treg are unable to suppress EAE if they are outnumbered by effector T cells26. Of note, injection site draining lymph nodes of S1P 1 Flox Foxp3 Cre mice contained 2–3-fold more Treg cells compared to their WT counterparts, which is in accord with the steady state data (Fig. 4c). Interestingly, despite this increase in nodal Treg cellularity, we observed significantly more IL-17A+ CD4+ T cells in the draining lymph nodes of S1P 1 Flox Foxp3 Cre-ERT2 mice (Fig. 4d). This suggests that S1P1-deficient Treg cells may be functionally defective in vivo. This defect appears to be more prominent in the containment of Th17, but not Th1 cells in the LN.
Acute deletion of S1P1 in Treg cells renders mice more susceptible to EAE. (a) Treatment schematic (Top), and EAE scores after acute deletion of S1P1 in S1P 1 Flox Foxp3 Cre-ERT2 and control S1P 1 WT Foxp3 Cre-ERT2 mice. (b) Absolute number of CNS-infiltrating cells (left), and intracellular staining of CNS-infiltrating CD4+ T cells at the peak of disease in test and control groups as treated in (a). (c) Representative plot for percentage of Treg cell in pLN and CNS at the peak of disease (left) and quantification of Treg percentage (right) in CNS and pLN. (d) Th17 cells in the pLN at the peak of the disease, intracellular staining of draining pLN lymphocytes 4 hours after stimulation with PMA/Ionomycin.
S1P1 regulates Treg cell transcriptional program and inhibits central to effector Treg phenotypic switch
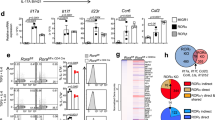
To examine the functional and phenotypic impact of S1P1 on Treg cell biology we sorted CD4+YFP+ Treg cells from 8-week-old WT and S1P 1 Flox Foxp3 Cre mice, and analyzed the global transcriptional changes via RNA-sequencing. A select list of genes is presented in the heat map in Fig. 5a. The selected genes were validated via real-time qPCR (Supplemental Fig. 4a). Along with previously defined upregulation of CD69, CD103, CTLA-4, ICOS, CD25, we realized that transcriptional changes suggested enrichment of the effector Treg cell subset in S1P 1 Flox Foxp3 Cre mice: the levels of CCR7 and CD62L were strongly downregulated, CD44, KLRG1, CD69, CD103, CTLA-4 and ICOS mRNA levels were upregulated. Consistent with the mRNA data, CD44highCD62Llow eTreg cells were more predominant than CD44lowCD62Lhigh cTreg cells and the surface expression of KLRG1, CD69, CD103, CTLA-4, ICOS molecules were elevated in Treg cells obtained from S1P 1 Flox Foxp3 Cre mice (Supplemental Fig. 4b–d). In addition, both mRNA and protein levels of Foxp3 were increased in S1P1-deficient Treg cells. (Not shown) As previously demonstrated to be a characteristic of eTreg cells, we observed higher rates of apoptosis in Treg cells purified from S1P 1 Flox Foxp3 Cre mice (Supplemental Fig. 4e). Since S1P 1 Flox Foxp3 Cre mice have autoimmunity, it is possible that the increased number of effector Treg cells that we observed in the S1P 1 Flox Foxp3 Cre mice could be the result of systemic inflammation and does not necessary imply that S1P1 regulates the transition from cTreg to eTreg. Thus, we examined the S1P1KO Treg phenotype in two non-inflammatory settings. To this end, we used S1P 1 Flox Foxp3 Cre/WT healthy female mice which have 50% WT 50% S1P1 KO Treg cells due to random X-inactivation, owing to Foxp3 being an X-linked gene. YFP+ Treg cells sorted from the LN of these non-inflamed mice were phenotypically similar to those obtained from S1P 1 Flox Foxp3 Cre mice with inflammation with regard to mRNA and protein expression (Fig. 5b–f). More specifically, we detected higher cell surface expression of KLRG1, CD69, CD103, CTLA-4, ICOS and elevated frequency of CD44highCD62Llow eTreg cells and reduced frequency of CD44lowCD62Lhigh cTreg cells. These changes were also reflected in the MFI surface expression of CD44 and CD62L (Fig. 5b–f). Similarly, Foxp3 mRNA and protein levels were upregulated in S1P1KO Treg cells compared with WT Treg (Fig. 5c). We obtained identical results with mixed bone marrow chimeric mice (not shown). These data suggest that S1P1 inhibits central to effector Treg cell conversion.
S1P1 deletion intrinsically alters Treg transcriptional program and promotes central to effector Treg switch. (a) Heat Map of differentially regulated transcripts in Treg cells purified from 8 week-old control S1P 1 WT Foxp3 Cre and S1P 1 Flox Foxp3 Cre mice and (b). Surface expression of indicated Treg associated markers on YFP+ WT (dashed line) or S1P1 KO (solid filled line) Treg cells isolated from the LN of healthy female S1P 1 WT Foxp3 Cre/WT mice as control, or S1P 1 Flox Foxp3 Cre/WT mice as KO source (harboring 50% WT 50% S1P1KO Treg due to random X inactivation), and (c) quantification of YFP-Foxp3 protein expression by MFI. d) YFP+ WT or YFP+S1P1KO Treg cells among LN lymphocytes of healthy female S1P 1 WT Foxp3 Cre/WT or S1P 1 Flox Foxp3Cre/WT mice gated. Higher percentages of eTreg cells (CD44+CD62Llow) are observed (bar graphs in (d) bottom panel) and MFI values of CD44 and CD62L are charted for quantification purposes in (e). (f) YFP/Foxp3+ Treg cells were sorted from control S1P 1 WT Foxp3 Cre/WT or S1P 1 Flox Foxp3 Cre/WT female mice and RNA was extracted. Relative gene expression of indicated eTreg associated genes was quantified by real time qPCR. Higher levels of indicated genes detected in S1P1KO Treg cells. (g) In vitro suppression assay performed with Treg cells sorted from 8-week-old WT or S1P 1 Flox Foxp3 Cre mice. (*) indicates <0.05. n = 3–5 mice per group.
Moreover, to further dissociate the impact of inflammation from S1P1 signaling itself we examined Treg cells in our acute S1P1 deletion model. Post-tamoxifen treatment, S1P1-deficient splenic Treg cells had an effector memory phenotype as shown by the expression of higher levels of CD44, CD103 and CD69 and low CD62L (Supplemental Fig. 5a). In accord with the protein data, sorted Treg cells from lymph nodes and spleen of S1P 1 Flox Foxp3 Cre-ERT2 mice post-tamoxifen treatment had elevated expression of various effector memory Treg-associated genes, including cd103, blimp-1, Ccr4 and ifr4 (Supplemental Fig. 5b). We also detected higher Foxp3 mRNA in these mice. Consistent with the previous reports suggesting an apoptosis prone nature for effector Treg cells16,18, splenic Treg cells from S1P 1 Flox Foxp3 Cre-ERT2 mice post-tamoxifen treatment were significantly more annexin V+ (Supplemental Fig. 5c). Altogether, these results are in line with our observations in the chronic deletion and chimeric mice, and thus, support the notion that S1P1 regulates the egress of Treg cells as well as their phenotypic diversity.
Lastly, to test the functionality of S1P1KO Treg cells, we performed an in vitro suppression assay. S1P1-deficient and WT Treg cells were both capable of inhibiting the proliferation of effector T cells (Fig. 5g) Of note, we also did not detect a difference in IL-2 responsiveness of S1P1KO and WT Treg cells, as measured by STAT5 phosphorylation and dose-dependent proliferation in response to IL-2 (Supplemental Fig. 5d,e). Collectively, these results suggest that S1P1 expression on Treg cells is crucial for their in vivo suppression function by controlling their trafficking behavior and their transition from cTreg to eTreg.
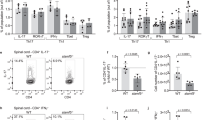
Effector Treg cells are enriched in fingolimod-receiving MS patients
To assess if the effects of S1P1 signaling observed in mice also occur in human Treg cells, we compared blood samples obtained from relapsing remitting MS (RRMS) patients treated with the S1P1 inhibitor, fingolimod, to subjects with MS treated with dimethyl fumarate treatment, or no therapy, with age and gender matched healthy controls (Fig. 6). The patients treated with fingolimod showed significant lymphopenia in their blood, as expected (not shown). More importantly, Treg frequency with respect to total CD4+ T cells was no different between the patient cohorts, (Supplemental Fig. 6a) but the fingolimod-treated subjects had a significantly elevated percentage of CD4+CD45RO+CD25highCD127low memory Treg cells in the blood compared to patients under dimethyl fumarate, no treatment or healthy controls (Fig. 6a and Supplemental Fig. 6a). A small fraction of human memory Treg cells in peripheral blood, similar to murine eTreg cells, also express CD103. The frequency of CD103+ memory T cells was significantly higher in fingolimod-treated MS patients in both Treg cells (Fig. 6b) and their T helper (TH) cell counterpart (Fig. 6c). Comparison of PBMCs from the same patients before and after fingolimod treatment demonstrates that fingolimod promotes the conversion of naïve/central Treg cells to a memory/effector phenotype over time (Fig. 6d–f and Supplemental Fig. 6b). More specifically we analyzed CD103+ Treg cells before and after treatment, which followed a similar pattern (Fig. 6g and h). Additionally, when we evaluated Treg cells by expression of Foxp3 and CCR4, we found that the subset of Foxp3+CCR4+ Treg cells is greatly expanded after fingolimod treatment, with Foxp3+CCR4− cells remaining the same (Fig. 6i and j). It is noteworthy that CD4+CD25−CD127high Th cells also exhibit enhanced CD103 expression, suggesting that S1P1 signaling may maintain a central memory/naïve program in conventional T cells as well (Fig. 6c and h). Thus, these observations are in line with the mouse data presented above and support our observations that S1P1 signaling inhibits the switch from a central to an effector memory Treg cell phenotype. Therefore, in the absence of S1P1 signaling, either through genetic deletion or down-modulation of cell surface expression by biochemical intervention through an antagonist (fingolimod), phenotypic conversion of cTreg to eTreg occurs.
Fingolimod promotes the conversion of cTreg cells to eTreg cells in MS patients. (a) Percentage of CD4+CD45RO+CD25+CD127− memory Treg cells in healthy controls, untreated MS patients (no meds), MS patients treated with fingolimod or dimethyl fumarate. Percentage of CD103+ Treg cells (CD4+CD45RO+CD25+CD127−) (b) and CD103+ T helper cells (CD4+CD45RO+CD25−CD127+) (c) in the same cohort of MS patients and healthy controls as in (a). Data are mean ± SEM of five or six donors in each group. *p < 0.05; **p < 0.01; ***p < 0.001 (ANOVA). Comparison of the number of naïve (CD45RO−) (d) or memory (e) Treg cells (CD45RO+) in MS patients before (gray bars) and after (black bars) fingolimod treatment. (f) Increase in Memory Treg cells in individual MS patients after fingolimod treatment. CD103 expression on Treg (g) and T helper (h) cells from MS patients before (grey bars) and after (black bars) fingolimod treatment. Percentage of Foxp3+CCR4+ (i) and Foxp3+CCR4− (j) T cell subsets in MS patients before (filled circle) and after (filled square) fingolimod treatment. Data are mean ± SEM of five to seven donors. *p < 0.05; **p < 0.01; ***p < 0.001 (two-tailed paired t test). All data are presented as frequency among total CD4+ T cells.
Discussion
The role of S1P1 in regulating Th17 biology has not been investigated previously. Genetic deletion of S1P1 from Th17 cells in this study revealed novel information in this regard. We show that this receptor is necessary for Th17 cell homing to peripheral organs, CNS and for EAE pathogenesis. Additionally, our data suggest that Th17 generation may be regulated by S1P1 as indicated by reduced Th17 levels in S1P 1 Flox IL-17A Cre mice following immunization with MOG35–55. Involvement of S1P1 in Th17 development has been suggested by Garriss et al. recently and is supported by our observations.
Previous work by Matloubian et al. and Allende et al. demonstrated a requirement for S1P1 receptor for the egress of T cells out of thymus and lymph nodes. Although these studies suggested that Treg cells would also rely on S1P1, direct and Treg-specific assessment of its functions was lacking. More recently, Liu et al. investigated this elegantly by using CD4cre deletion of S1P1 which alters receptor levels in conventional CD4+ T cells and thus Treg precursors as well. They utilized CD2 promoter driven S1P1 transgenic mice, which strengthened their findings. Their observations revealed an unprecedented inhibitory role for S1P1 during differentiation of Treg cells from CD4+CD25+Foxp3− precursors. In other words, CD4-specific deletion of S1P1 resulted in more and functionally better Treg cells whereas CD2-driven overexpression of S1P1 yielded fewer Treg cells with poor suppressive functions. However, several points remained elusive in these previous works. Does S1P1 regulate the functions of committed Treg cells, that is, after Foxp3 is turned on? Does altering S1P1 level exclusively in Treg cells result in the same phenotype as mutants that target the receptor broadly, including conventional T cells? Is the lymphoid and non-lymphoid tissue distribution of Treg cells altered by specific S1P1 ablation? S1P 1 Flox Foxp3 Cremice proved useful for addressing these questions. Despite the reports suggesting that S1P1-deficient Treg cells are better suppressors, S1P 1 Flox Foxp3 Cre mice had an autoimmune phenotype resembling that of scurfy mice with some delay in development of fulminant autoimmunity. Although both CD4+ and CD8+ T cells, as well as B cells, were activated, CD8+ T cells showed massive expansion and were the major source of elevated systemic TNF-α and IFN-γ in these mice. This phenotype was suggestive of some in vivo functional impairment unique to Treg cells.
Because lymphocyte egress is the primarily studied cellular response regulated by S1P1, close examination of Treg cell tissue distribution across lymphoid and non-lymphoid organs in S1P 1 Flox Foxp3 Cre mice revealed major defects. Consistent with the previous work investigating conventional T cell egress3,4, and Treg cell egress using CD4cre system22, Foxp3Cre-specific S1P1 deletion resulted in higher frequency of Treg cells in thymus and LNs. Liu et al.’s work using CD4Cre system in vitro showed that Treg cell differentiation from S1P1-deficient CD4+ naïve T cells is more efficient. However, in this system, S1P1 was deleted in all T cells prior their commitment to either conventional or regulatory T cells. The observation in our study that in vitro differentiation of naïve CD4+ T cells obtained from S1P 1 Flox Foxp3 Cre mice is equivalent to that of WT indicates that S1P1 deletion after commitment to the Treg cell lineage does not impact their differentiation efficacy at least in vitro in the presence of TGF-β. However, in vivo, the evidence Liu et al. presents regarding refractory role of S1P1 in Treg development is also supported by our data. Treg cells are found in increased numbers in LNs and comparable numbers in the spleens of S1P 1 Flox Foxp3 Cre adult mice while there is a buildup of Treg cells in thymus in those mice. Moreover, Foxp3 Cre het/S1P 1 Flox homo/ROSA RFP het mice also show S1P1 KO Treg homing to LNs, therefore S1P1 may not be an absolute requirement for Treg cell egress out of the thymus, as it is conceivable that Treg cells may be utilizing receptor/s other than S1P1 to egress thymus for blood. Treg cells also express the remaining four S1P receptors with S1P4 levels being comparable to S1P1. One or more of the receptors may play a role in this process and explain the leaky egress in the absence of S1P1 25. Further study is needed to verify this. Our data, supporting previous studies, also show that egress of Treg cells out of lymph nodes into lymph requires S1P1, as evidenced by increased Treg cells in LNs. Due to this defective lymphoid tissue egress, we hypothesize that non-lymphoid tissue homing of Treg cells are adversely affected. Indeed, our examination of various non-lymphoid organs such as lung, liver and colon showed reduced Treg cellularity in both S1P 1 Flox Foxp3 Cre mice and after acute deletion of S1P1 in the tamoxifen inducible system. Given the critical role of tissue resident Treg cells in preventing autoreactive T cells at these sites, multi-organ inflammation in S1P 1 Flox Foxp3 Cre mice could partly be explained by reduced Treg cellularity in the peripheral organs. These observations also underline the requirement for the presence of Treg cells in the tissues to suppress autoimmunity rather than lymph nodes. This finding provides some insight into repercussions of long term fingolimod use in MS patients. Our results suggest that patients under fingolimod treatment are expected to have a reduction in tissue resident Treg cells, since fingolimod also causes downregulation of S1P1. Although the number of Foxp3− conventional T cells would follow a similar decrease in response to fingolimod, a more precise kinetic analysis of Treg vs non-Treg T cell disappearance from tissues is needed to evaluate the impact of Treg loss on activation of autoreactive tissue-resident T cells. Nevertheless, such reduction in tissue-resident Treg numbers may result in loss of tolerogenic properties of antigen presenting cells which may become crucial in driving autoreactive T cells in some microenvironments and individuals once lymphopenia is resolved due to cessation of treatment.
Another major finding of this report is that S1P1 signaling plays a critical role in maintaining central memory phenotype of Treg cells. This was demonstrated by comparison of the gene expression profile, as well as cell surface marker expression of Treg cells obtained from S1P 1 Flox Foxp3 Cre mice to that of controls. Similarly, S1P1-deficient Treg cells obtained from female S1P 1 Flox Foxp3 Cre/WT or bone marrow chimeras or, S1P 1 Flox Foxp3 Cre-ERT2 mice after acute receptor deletion assumed effector phenotype indicating that the enrichment of effector Treg cells is due to the absence of S1P1 signaling, but not due to inflammatory signals. More importantly, this phenomenon could be recapitulated in MS patients treated with S1P1 antagonist fingolimod, but not other drugs such as Tecfidera, nor in healthy controls. How S1P1 regulates the cTreg to eTreg conversion is not clear. We could not implicate blimp-1 in this process because it was upregulated in the absence of S1P1 in the inflammatory setting but not in non-inflammatory setting. It was previously shown that irf4 is required for conversion of cTreg into eTreg. We also observed an increase in irf4 transcript levels in Treg cells obtained from S1P 1 Flox Foxp3 Cre-ERT2 mice after acute receptor deletion. However, it is unknown if overexpression of irf4 is sufficient for this conversion, as is how S1P1 signaling restrains irf4 expression.
Compelling evidence from past in vitro and in vivo studies indicates that FTY720 (fingolimod)-conditioned Treg cells become more suppressive with regard to suppression of proliferation of target CD4+ T cells, or inhibition of IL-4 and IFN-γ cytokine production by target cells24,25. Additionally, they suppress airway inflammation in a mouse model more efficiently25. More recent reports showed enhanced in vitro suppressive function of S1P1KO Treg cells. Conversely, reduced suppressive capacity of S1P1 overexpressing transgenic Treg cells has been reported22,23. However, Treg cell suppression of target cells can be achieved by various means, and not all aspects of suppression have been tested in those studies. Despite the fact that eTreg cells appear to express components of the molecular machinery utilized for target cell suppression at high levels (ICOS, CTLA4, CD25, Foxp3) and appear to be potent suppressors in vitro, the question still remains as to what would happen if the balance between eTreg vs cTreg were to shift in favor of the former in an organism in vivo. The acute deletion model was proved to be more informative in this regard. Our data from the EAE model showed higher IL-17A producing CD4+T cells in the draining lymph nodes of MOG35–55 peptide immunized S1P 1 Flox Foxp3 Cre-ERT2 mice after acute receptor deletion, despite the presence of 3-fold higher numbers of eTreg cells compared with WT. We hypothesize that this may likely be as a result of improper access of eTreg cells to T cell zones due to their reduced expression of CCR7, which is required for this process19. Thus, in S1P 1 Flox Foxp3Cre mice, in addition to the reduced non-lymphoid organ access of Treg cells, Treg cells that are trapped in the secondary lymphoid organs will have problems accessing the T cell zones, and will therefore be unable to control autoreactive T cells that were primed with migrant DCs carrying autoantigens from tissues that are devoid of Treg cells. Alternatively, S1P1KO Treg cells may also be functionally incapable in certain aspects of their suppression machinery. This hypothesis has merit, given that Garris et al. have recently established that STAT3 is activated by S1P1 signaling27. A more recent study showed FTY720 can downmodulate STAT3 phosphorylation in a murine model which has overactive STAT3 due to an internalization-defective S1P1 28. Prior to this finding, it was shown that STAT3-deficient Treg cells were unable to perform suppression of exclusively Th17 cells in vivo, despite their intact in vitro suppression ability29,30. Our observation of higher IL-17 responses in the draining lymph nodes of S1P 1 Flox Foxp3 Cre-ERT2 mice after acute receptor deletion and immunization corroborates this notion of a customized Treg cell subset for the suppression of Th17 cells. However, in our hands, addition of S1P1 agonists did not impact IL-6 or IL-23 driven STAT3 phosphorylation. We utilized phospho-flow technique in our studies. Additionally, the previous papers tested this phenomenon on cells which have already higher pSTAT3 levels. These differences might account for the variation between our and previous results.
Treg cells are critical for immunologic tolerance, and containment of protective immune responses. As such, they are of critical importance for the suppression of myelin specific autoreactivity during multiple sclerosis. A growing number of reports revealed unexpected exacerbation in MS features of patients who are switching to fingolimod or of those who stopped its use, suggesting an unknown immunomodulation. In this study, we attempted to explore fingolimod’s potential effects on Treg cells through S1P1 using Treg-specific genetic deletion of the receptor. In summation, our results show that S1P1 is critical for the lymphoid tissue exit of Treg cells, and subsequent localization to non-lymphoid tissues. Thus, disruption of S1P1 signaling permanently in Treg cells causes autoimmunity in mice, whereas a temporal disruption renders mice more susceptible to the development of EAE, the animal model of multiple sclerosis. More importantly, our results reveal that, apart from egress, S1P1 regulates various aspects of cTreg to eTreg phenotypic conversion; as such, loss of S1P1 signaling promotes conversion to effector phenotype in both mice and humans, which might have repercussions for the functions of Treg cells. These results provide novel insight in to the possible effects of long-term fingolimod use in MS patients on Treg cells.
Methods
Human Samples
Frozen PBMCs were obtained from participants in the Benaroya Research Institute (BRI) Immune Mediated Disease (IMD) Registry; informed consent was obtained after the nature and possible consequences of the studies were explained. Three patient cohorts (based on disease modifying therapy treatment: Gilenya (fingolimod), Tecfidera (dimethyl fumarate) and no treatment) and healthy controls were selected for these studies. In addition, frozen PBMCs from the same MS patients prior and under Gilenya treatment were analyzed. The diagnosis of RRMS was based on Revised McDonald Diagnostic Criteria for MS (C. H. Polmanetal., Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”)31. All subjects in the MS patient/no treatment cohort were off immunomodulating and immunosuppressive therapies at the time of study and for at least 3 months before the blood draw. Control subjects were recruited from the BRI IMD Registry and were selected because of a lack of autoimmune disease or any family history of autoimmunity. The research protocols were approved by the Institutional Review Board at BRI. All methods for human studies involving human samples were performed in accordance with the relevant guidelines and regulations.
Mice
S1P 1 Flox mouse was a gift from Dr. Richard L. Proia and bred in house to Foxp3 CreYFP, which was a gift from Dr. A. Rudensky, at specific pathogen free conditions. Foxp3Cre-ERT2-YFP mice were purchased from Jackson Laboratories (STOCK Foxp3tm9(EGFP/cre/ERT2) Ayr/J) and bred to S1P 1 Flox and then for some experiments to ROSA RFP on C57BL/6 background. All the mice were bred and maintained under specific pathogen-free conditions. The experiments were approved by the Institutional Animal Care and Use Committee of Seattle Children’s Research Institute. All methods for mice studies involving mouse samples were performed in accordance with the relevant guidelines and regulations.
EAE induction
EAE was induced by s.c. immunization of mice at the flanks with an emulsion of MOG35–55 peptide (100 μg) emulsified in CFA supplemented with 4 mg/ml Mycobacterium tuberculosis extract H37Ra (Difco). Additionally, the animals received 200 ng pertussis toxin (List Biological Laboratories) i.p. on days 0 and 2. Clinical signs of EAE were assessed daily and scored according to the following criteria: 0, no signs of disease; 1, limp tail; 2, hind limb weakness; 3, hind limb paralysis; 4, hind limb and forelimb paralysis.
Tamoxifen treatments
Tamoxifen (Sigma, T5648) was dissolved in corn oil (Sigma, C8267) to a stock solution of 15 mg/ml by overnight shaking at 37 °C and kept at 4 °C until use. Mice were injected intraperitoneally with 5ul/g of stock tamoxifen solution (final 0.75 mg tamoxifen/g mice). Injections were repeated for 5 consecutive days and mice were euthanized on day 6 or 7. For EAE experiments, mice received 4 injections of tamoxifen, and were then immunized, followed by another injection day 7 after EAE induction. For some experiments, 2 consecutive injections were performed and mice were euthanized on day 2 or 3.
Autoantibody measurement
Anti-dsDNA ELISA on blood serum was performed as described previously32. Total serum levels of IgM, IgG, IgG1, IgG2b, and IgA were determined with the ELISA kit from Southern Biotech.
In vitro suppression assay
The assay was performed as described by Chaudhry et al.30.
In vitro differentiation into Treg cells
CD62Lhigh CD44low naïve CD4+ T cells were sorted from WT or S1P 1 Flox Foxp3 Cre mice (LN and spleen combined) after initial positive enrichment step with CD4+ microbeads (Miltenyi Biotech). 1 × 105 T cells were cultured in 24-well plates with 5 × 105 irradiated antigen presenting cells (CD4− fraction) in the presence of 5ng/ml TGF-β and 2 µg/ml anti-CD3 in RPMI supplemented with 10% FBS. 5 days later Foxp3+ cells were quantified by YFP or Foxp3 staining.
Recall responses
Splenocytes or inguinal lymphocytes were prepared from indicated mice at the 7th day of immunization (with Complete Freund’s Adjuvant and MOG35–55 emulsion) and restimulated with 100 µg MOG35–55 plus recombinant IL-23 or without IL-23 (1 × 105 cells/well) in 96-well plate. Five days later RFP+ cells were visualized by Flow cytometry. Cells were stimulated 4 h with PMA/Ionomycin/Golgi Plug to perform intracellular staining for IL-17A and IFN-γ.
Treg cell proliferation and pSTAT5 detection
Treg cells were sorted from 6 week-old WT or S1P 1 Flox Foxp3 Cre mice (LN and spleen combined) after initial positive enrichment step via CD4+ microbeads (Miltenyi Biotech). Treg cells (2.5 × 104) were co-cultured with 1.25 × 105 irradiated antigen presenting cells (CD4− fraction) and 1 µg/ml anti-CD3 plus increasing concentrations of IL-2 (0, 1, 100, 1000 Units) in 96-well round bottom plates in RPMI supplemented with 10% FBS. 16 h prior to harvesting, cells were pulsed with 1µCi3H-Thymidine. At 72 h, cells were harvested and thymidine incorporation was measured with a scintillation counter.
To examine IL-2 signaling, 1 × 106 lymphocytes from LN of WT or S1P 1 Flox Foxp3 Cre mice or female S1P 1 Flox Foxp3 Cre/WT mice were stimulated with varying concentrations of IL-2 for 20 minutes at 37 °C in 96-well plate (100 µl volume). Cells were fixed with the addition of equal volume of 4% Formalin for 10 minutes. Cells were permeabilized with Perm Buffer III (BD Biosciences) and staining of pSTAT5 was performed per manufacturer’s protocol.
Generation of bone marrow chimeras
The recipient Rag −/− mice were irradiated 4–6 h before transfer at 550 rad and treated with antibiotics (Baytril) for 2 weeks. Irradiated mice received retro-orbitally 50/50 mixture of CD45.1+WT and CD45.2 S1P 1 Flox Foxp3 CreYFP mice bone marrow cells (5 × 106) which are depleted of T cells by CD90.2 microbeads (Miltenyi Biotech). The mice were analyzed 5–8 weeks post-transfer.
RNA sequencing
Total RNA was purified from Treg cells sorted via FACSAria gating on Foxp3YFP+ CD4+ cells. Total RNA was purified using the RNeasy mini kit (Qiagen). RNA sequencing was performed at the Genomic Core Facility Southwestern Medical Center, University of Texas as per the facility protocols.
Flow cytometry
For flow cytometry, cells from spleen and LNs were isolated and surface stained with the appropriate antibodies. For intracellular cytokine staining, single-cell suspensions were cultured directly in RPMI containing 10% fetal bovine serum (FBS) and Golgi plug for 4–5 h or re-stimulated in RPMI containing 10% FBS with 50ng/ml of phorbol 12-myristate 13-acetate and 1 μg/ml of ionomycin in the presence of Golgi plug. Cells were then fixed and stained according to the instructions from the manufacturer, using an intracellular cytokine staining kit (BD Biosciences). Human PBMC’s were cultured in RPMI 1640 medium supplemented with 2 mM glutamine, 1% (v/v) nonessential amino acids, 1% (v/v) sodium pyruvate, penicillin (50 U/ml), streptomycin (50 μg/ml) (all from Invitrogen), and 5% heat-inactivated human serum. Cells were stained on the surface with the appropriate antibodies. When indicated, cells were then fixed with Fix and Perm buffer (eBioscience), according to the manufacturer’s instructions and stained for Foxp3.
Following antibodies were used for staining: Mice:CD4 (GK1.5, eBioscience), CD44 (IM7, BioLegend), CD62L (MEL-14, eBioscience), CD69 (H1.2F3, eBioscience), CD25 (PC61.5, eBioscience), CD103 (2-E-7, eBioscience), KLRG1 (2F1/KLGR1, BioLegend), ICOS (C398.4 A, BioLegend), CTLA4 (UC10–4B9, eBioscience), CD8 (53–6.7, eBioscience), GranzymeB (NGZB, eBioscience), TNF-α (MP6-XT22, eBioscience), IFN-γ (XMG1.2, BioLegend), IL-17A (eBio17B7, eBioscience), IL-4 (11B11, eBioscience), Foxp3 (MF-14, BioLegend), CD86 (GL1, eBioscience), CD38 (Clone 90, eBioscience), MHCII (M5/114.15.2, eBioscience), CD42d (1C2, eBioscience), Ter119 (TER-119, BioLegend), CD45.1 (A20, eBioscience), CD45.2 (104, eBioscience), Helios (22F6, BioLegend), pSTAT5 (SRBCZX, eBioscience), AnnexinV (88–8103–72, eBioscience), Fixable Viability Dye (FVD) (65-0865-14, eBioscience). Human: CD127 (HIL-7R-M21 BDBiosciences and A019D5, Biolegend), CD4 (OKT4, Biolegend), CD25 (BC96, Biolegend), CD103 (Ber-ACT8, Biolegend), CD45RA (HI100, eBioscience), CD45RO (UCHL1, Biolegend), Foxp3 (259D, Biolegend), CCR4 (TG6/CCR4, Biolegend).
Quantitative reverse transcription–polymerase chain reaction
Treg cells were sorted by gating on CD4+ Foxp3YFP+ or CD4+CD25+ T cells. Tissues were collected in Trizol or RLT buffer (Qiagen RNEasy Kit) and homogenized with the Pro200 Homogenizer (Pro Scientific) and total RNA was extracted. TaqMan one-step RT-PCR (Applied Biosystems, Foster City, CA) or SYBR green q-PCR was performed with a 7500 Real Time PCR System per the instructions of the manufacturer (Applied Biosystems). Expression of the tested genes was normalized to the housekeeping ribosomal protein L19 (rPL19) mRNA. Arbitrary relative expression units were calculated by division of expression of the gene of interest by rPL19 mRNA expression. Primer and probe sequences for each target are available upon request.
Enzyme-linked immunosorbent assay
Supernatants were taken and used for ELISA. Assay was performed according to manufacturer’s guidelines. TNF-alpha Ready-Set-Go! ELISA kit was purchased from eBioscience. IL-17A and IFN-γ ELISA Max™ Set Standard were purchased from BioLegend.
Histology
Organs were fixed in 10% formalin for overnight and transferred in to 70% ethanol. Paraffin embedded blocks were sectioned (4–5 μm) and hematoxylin and eosin stained. Slides were scored blindly on a scale of 0–12 if needed, as previously described.
Statistical analysis
The p values for all Figures were calculated with a paired 2-tailed Student’s t test. In addition, some Fig. 6 statistics were calculated by one-way ANOVA where indicated. A p value <0.05 was considered significant. Error bars denote ±SEM as indicated.
References
Chi, H. Sphingosine-1-phosphate and immune regulation: Trafficking and beyond. Trends in Pharmacological Sciences 32, 16–24 (2011).
Cinamon, G. et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 5, 713–720 (2004).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Allende, M. L., Dreier, J. L., Mandala, S. & Proia, R. L. Expression of the Sphingosine 1-Phosphate Receptor, S1P1, on T-cells Controls Thymic Emigration. J. Biol. Chem. 279, 15396–15401 (2004).
Pappu, R. et al. Promotion of Lymphocyte Egress into Blood and Lymph by Distinct Sources of Sphingosine-1-Phosphate. Science (80-.). 316, 295–298 (2007).
Schwab, S. R. et al. Lymphocyte Sequestration Through S1P Lyase Inhibition and Disruption of S1P Gradients. Science (80-.). 309, 1735–1739 (2005).
Cyster, J. G. & Schwab, S. R. Sphingosine-1-Phosphate and Lymphocyte Egress from Lymphoid Organs. Annu. Rev. Immunol. 30, 69–94 (2012).
Mandala, S. et al. Alteration of Lymphocyte Trafficking by Sphingosine-1-Phosphate Receptor Agonists. Science (80-.). 296, 346–349 (2002).
Cohen, J. A. et al. Oral Fingolimod or Intramuscular Interferon for Relapsing Multiple Sclerosis. N. Engl. J. Med. 362, 402–415 (2010).
Bourdette, D. & Gilden, D. Fingolimod and multiple sclerosis: Four cautionary tales. Neurology 79, 1942–1943 (2012).
Jander, S., Turowski, B., Kieseier, B. C. & Hartung, H.-P. Emerging tumefactive multiple sclerosis after switching therapy from natalizumab to fingolimod. Mult. Scler. J. 18, 1650–1652 (2012).
Gross, C. M., Baumgartner, A., Rauer, S. & Stich, O. Multiple sclerosis rebound following herpes zoster infection and suspension of fingolimod. Neurology 79, 2006–2007 (2012).
Centonze, D., Rossi, S., Rinaldi, F. & Gallo, P. Severe relapses under fingolimod treatment prescribed after natalizumab. Neurology 79, 2004–2005 (2012).
Visser, F., Wattjes, M. P., Pouwels, P. J. W., Linssen, W. H. J. P. & van Oosten, B. W. Tumefactive multiple sclerosis lesions under fingolimod treatment. Neurology 79, 2000–2003 (2012).
Josefowicz, S. Z., Lu, L.-F. & Rudensky, A. Y. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 30, 531–564 (2012).
Liston, A. & Gray, D. H. D. Homeostatic control of regulatory T cell diversity. Nat. Rev. Immunol. 14, 154–65 (2014).
Gratz, I. K. & Campbell, D. J. Organ-specific and memory treg cells: specificity, development, function, and maintenance. Front. Immunol. 5, 333 (2014).
Cretney, E. et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12, 304–11 (2011).
Smigiel, K. S. et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 211, 121–36 (2014).
Sakaguchi, S., Miyara, M., Costantino, C. M. & Hafler, D. A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10, 490–500 (2010).
Sugiyama, D. et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. USA. 110, 17945–50 (2013).
Liu, G. et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 10, 769–77 (2009).
Liu, G., Yang, K., Burns, S., Shrestha, S. & Chi, H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat. Immunol. 11, 1047–56 (2010).
Sehrawat, S. & Rouse, B. T. Anti-inflammatory effects of FTY720 against viral-induced immunopathology: role of drug-induced conversion of T cells to become Foxp3+ regulators. J. Immunol. 180, 7636–47 (2008).
Sawicka, E. et al. The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+T-regulatory cells and enhances their functional activity. J. Immunol. 175, 7973–80 (2005).
Korn, T. et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 13, 423–31 (2007).
Garris, C. S. et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol. 14, 1166–72 (2013).
Tsai, H.-C. et al. Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY720 during neuroinflammation. JCI Insight 1, e86462 (2016).
Kluger, M. A. et al. Stat3 programs Th17-specific regulatory T cells to control GN. J. Am. Soc. Nephrol. 25, 1291–302 (2014).
Chaudhry, A. et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326, 986–91 (2009).
Polman, C. H. et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria’. Ann. Neurol. 58, 840–846 (2005).
Becker-Herman, S. et al. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J. Exp. Med. 208, 2033–42 (2011).
Acknowledgements
We would like to thank Piper M. Treuting and Brian Johnson from Histology and Imaging Core Facility at UW for his help with histology, and Devin Margolies for assistance with animal husbandry. This work is supported by the following NIH grants: R56 AI113906 to M.O and R01 NS081687 to EB.
Author information
Authors and Affiliations
Contributions
A.E. performed all the experiments, except Figure 6, wrote the manuscript and helped experimental design. R.D. generated Figure 6, revised and wrote the associated sections of the manuscript. A.K.S. and M.F. helped Figure 5 and genotyping, and revised the manuscript. M.O., E.B. conceived the experimental design, provided funding and supervised the study. J.H.B. and M.K. provided the human patient samples and revised the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eken, A., Duhen, R., Singh, A.K. et al. S1P1 deletion differentially affects TH17 and Regulatory T cells. Sci Rep 7, 12905 (2017). https://doi.org/10.1038/s41598-017-13376-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13376-2
This article is cited by
-
Co-modulation of T cells and B cells enhances the inhibition of inflammation in experimental hypersensitivity pneumonitis
Respiratory Research (2022)
-
Characterisation of peri-implantation endometrial Treg and identification of an altered phenotype in recurrent pregnancy loss
Mucosal Immunology (2022)
-
Sphingosine 1-phosphate receptor-targeted therapeutics in rheumatic diseases
Nature Reviews Rheumatology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.