Abstract
Rock outcrop is an important habitat supporting plant communities in karst landscape. However, information on the restoration of higher biotic populations on outcrops is limited. Here, we investigated the diversity, biomass changes of higher vascular plants (VP) and humus soil (HS) on karst outcrops during a restoration process. We surveyed VP on rock outcrops and measured HS reserved by various rock microhabitats in a rock desertification ecosystem (RDE), an anthropogenic forest ecosystem (AFE), and a secondary forest ecosystem (SFE) in Shilin County, southwest China. HS metrics (e.g. quantity and nutrients content) and VP metrics (e.g. richness, diversity and biomass) were higher at AFE than at RDE, but lower than at SFE, suggesting that the restoration of soil subsystem vegetation increased HS properties and favored the succession of VP on rock outcrops. There was significantly positive correlation between VP metrics and HS amount, indicating that the succession of VP was strongly affected by availability and heterogeneity of HS in various rock microhabitats. Thus, floral succession of rock subsystem was slow owing to the limited resources on outcrops, although the vegetation was restored in soil subsystem.
Similar content being viewed by others
Introduction
Karst landscapes constitute approximately 12–15% of the global terrestrial surface1. Depend on the local rainfall and the purity of bedrock, the land surface may consist of various morphologies of rock outcrops and rock-soil patterns2,3,4 (Fig. 1). Human activities (e.g. firewood harvesting and grazing) cause trees growing on soil subsystems to be destroyed leaving rock outcrops exposed. This process is called rocky desertification when the exposed rocks ratio reaches ≥ 30% of the land surface in China1, and it is considered a serious environmental problem globally5. For example, a recent report revealed that the rocky desertification area is about 1.2 × 107 ha and constitutes 25.6% of total karst terrain in China1. Large efforts have been made on researching vegetation degradation/recovery mechanisms of soil subsystems5,6, but not on rock outcrops despite their ecological importance.
Carbonate outcrops are formed from different corrosion rates of carbonate rock due to varied chemical compositions, and climatic and geological conditions at different localities2,4. Thereby, outcrops gradually form diverse microhabitats (e.g., rocky pit, rocky crevice, rocky surface, rocky gully, etc.) comprising high heterogeneity7,8. These microhabitats can intercept and gather soil particles, animal residues, vegetation litter and other exotic substances transported by rainfall and air flow from great distances9,10. We refer to these mixed substances as the humus soil on rock outcrops (HS) in this study (Fig. 1).
Plants and their associated soil communities are interlinked and influence each other in ecosystem development11,12. The interrelationship between them has been well studied13, and remains important for explaining vegetation dynamics14. Plant-soil feedbacks can contribute to the coexistence of plant species15, alter plant community structure, and act as drivers of vegetation succession12,16. Plants can influence soil properties through changing inputs of chemical compounds and organic matter, shaping the hydrological process, changing surface soil temperatures, and providing habitats and resources for rupicolous organisms17. Changes to soil chemicals and physical attributes that are caused by plants in turn influence the growth, productivity and reproductive success of individual plants, and the assemblage and floristic composition of these plant communities14,18. Flora and scarce soil are common in various rock microhabitats on karst outcrops9,19. However, few studies focused on the relationship between the flora and soil in those microhabitats.
Plants on rock outcrops have been proven to substantially contribute to regional biodiversity20,21. On rock outcrops, the HS constitutes a suitable growth medium for plants by supplying nutrients, energy, and water sources. Moreover, living organisms (or rock-dwellers) on rock outcrops also fix atmospheric carbon (C) and nitrogen (N) to maintain HS fertility and rock outcrop subsystem productivity1,22. Yet there has been, and remains, some confusion concerning the relationship between such microhabitats’ conditions and the rock-dwellers. Particularly, little quantitative research addresses the HS and its ecological function on rock outcrops. In addition, the physical environment of rock subsystems is stressful for plant growth because of low water and nutrients availability, intense solar radiation, great temperature fluctuations, and the shallow soil layer19,23. Especially during the dry season in semihumid region (e.g. Shilin), plant growth suffers from severe drought stress24. Floral succession in rock subsystems can be considered as an important aspect when evaluating karst degradation and restoration.
The succession of life in (and on) the rock outcrops plays an important role in natural rocky desertification restoration25,26. At an early stage of vegetation succession, various types of algae27,28,29, fungi30, and cyanobacteria and heterotrophic bacteria colonize where conditions are most favorable20. Successively mosses, lichens21,31,32, and other higher plants become established in different successional periods33. Biological changes of soil subsystems also greatly affect the successional process of rock vegetation. Angelini and Silliman34 demonstrated that primary foundation species facilitate secondary foundation species by increasing habitat complexity and quality to enhance biodiversity. The restoration of degraded karst areas may take so long that human interventions, including reforestation, water and soil conservation are required to accelerate the restoration process5,26. Previous studies have concentrated on the ecological significance of vegetation restoration in soil subsystems but data concerning floral changes in rock subsystem are limited, and no study addresses the effects of soil subsystem vegetation on plants of rock outcrops.
In this study, we described the vascular plants (VP) and HS on rock outcrops across three karst landscapes, assuming that they were the three typical stages in restoration of rocky desertification. We aim to answer the following questions: (1) How much is the amount of HS on rock outcrops and how does it impact on VP? (2) Do the diversity, density, lifeforms, species composition, and distribution of VP on rock outcrops differ in rocky desertification restoration? (3) Did the rock outcrop subsystem restore following revegetation in soil subsystem? (4) What factors drive rock vegetation succession?
Results
HS on rock outcrops
The average amounts of HS per unit area was about 10.3 times higher in the SFE (405.54 ± 174.26 g/m2) than in the AFE (39.36 ± 16.27 g/m2), while for the RDE it was only 3.1 times higher than in the AFE (Fig. 2). On average, the moisture content of the HS showed the following pattern: SFE > AFE > RDE, and it was significantly different among ecosystems. N and P concentration were slight higher at AFE than at RDE, but significant lower than SFE. K concentration was highest at AFE, and lowest at SFE.
Amount of humus soil and its nutrients content for three study sites in Shilin County, southwest China. Different letters above the x-axis represent significant differences (P < 0.05) among different sites. RDE: rocky desertification ecosystem, AFE: anthropogenic forest ecosystem, SFE: secondary forest ecosystem.
VP on rock outcrops
Sixty-one species, from 58 genera of 37 families were identified in the 180 subplots (Appendix), of which 88.5% were spermatophytes, and 11.5% were ferns. SFE had the highest species richness and RDE had the lowest (Table 1). Seven species (11.4%) occurred in all three ecosystems, while 41 (67.2%) were only identified in one or two ecosystems. Thirteen species were found at both RDE and AFE. Of species unique to an ecosystem, 27 were identified only at SFE, while it was 9 and 5 at AFE and RDE respectively.
On average, the number of individual per subplot was 34.5 times higher at SFE than that at AFE, and 12.1 times higher than that at RDE (Appendix). Most species (approximately 77.5~96.0%) had less than 50 individuals. Paraboea neurophylla and Selaginella tamariscina were the dominant species for RDE, Bidens pilosa for AFE, and Pilea pumila var. hamaoi and Pyrrosia lingua for SFE.
Average species per subplot ranged from 0.92 to 3.53 and decreased in the order: SFE > RDE > AFE (Table 1), and showed the same trend as γ-diversity. Similarly, the Shannon-Wiener index and Simpson index were both significantly lower for RDE than the other ecosystems. No significant difference was found between AFE and SFE.
The VP communities showed significant differences among different ecosystems in the NMDS ordination scatterplot (Fig. 3). Each of the ecosystems showed distinct VP assemblages. Plots in the SFE were more bunched, while plots in RDE were more scattered.
Similarity of VP assemblages in 85 subplots of three karst outcrops in Shilin County, southwest China. Two-dimensional scatterplot of NMDS based on Bray-Curtis index (stress = 0.15; r² = 0.97 for non-metric fit and r² = 0.86 for linear fit of ordination distances with observed dissimilarities). RDE: rocky desertification ecosystem, AFE: anthropogenic forest ecosystem, SFE: secondary forest ecosystem.
Herbs were the dominant species in each ecosystem, followed by shrubs, lianas and trees (Fig. 4). More than half (58.1%) of VP were herbs at RDE, and the proportion was significantly lower than at the other two ecosystems. Yet, the proportion of shrubs at RDE was statistically significantly higher than at AFE and SFE. The percentages of lianas were surprisingly low for the three ecosystems. A few trees occurred at AFE and SFE but not at RDE. Generally, deciduous species were richer than evergreen. The proportional representation of deciduous and heliophyte species among ecosystems showed a decreasing order: RDE > AFE > SFE. SFE had the greatest evergreen and sciophytes, followed by AFE, and RDE had the lowest. In addition, the heliophytes were proportionally 5.6 times greater than sciophytes at RDE, while the opposite was true at SFE.
Species richness (a) and species composition by life forms (b) and (c), and life habits (d) of VP in subplots for three study sites in Shilin County, southwest China (mean ± S.E. (error bars)). Different letters above the bars represent significant differences (P < 0.05) among different sites. RDE: rocky desertification ecosystem, AFE: anthropogenic forest ecosystem, SFE: secondary forest ecosystem.
The relationship between plant and physical factors
Correlation analysis showed that the numbers of individuals, species richness, and biomass of VP were positively related to the amount of HS, canopy coverage, rock outcrops ration, and relative humidity (Table 2). HS on rock outcrops was the most important variable to explain the number of individuals, species richness, and biomass.
A large proportion of vascular individuals were biased in favor of the RW, RS and RP microhabitats at SFE (Fig. 5a). Yet most individuals were recorded in RC and RP at RDE and AFE. The VP individuals in any of the microhabitat per square meter at SFE were higher than at AFE and RDE. A vast majority of HS was accumulated in RP at SFE, while HS was mainly stored in RT and RP at RDE. Only a small subset could be found at RW (Fig. 5b). In particular, the HS at RP for SFE was evidently higher than in the other two ecosystems.
VP individuals (a) and HS (b) in microhabitats per unit area for three study sites in Shilin County, southwest China. RC: rocky crevice, RG: rocky gully, RP: rocky pit, RS: rocky surface, RT: rocky terrace, RW: rocky wall. RDE: rocky desertification ecosystem, AFE: anthropogenic forest ecosystem, SFE: secondary forest ecosystem.
Discussion
Over the large karst area, such practice as reforestation in the soil subsystem was usually adopted to control rocky desertification and accelerate ecosystem restoration5,35,36. Much research has focused on soil-vegetation restoration processes5,6,23. However, there is no information concerning the rock-vegetation conversion and its relationship with the soil retained on rock outcrops. Furthermore, there is no research addressing the changing pattern of VP and HS on the highly fragile rock subsystem during the restoration of vegetation in soil subsystem. Our data indicated that a divers VP can be found on rock outcrops with declining density at the various microhabitats holding different amounts of HSs in the SFE. With the removal of forest from the soil sub-ecosystem, VP and HS will be highly diminished. On the other hand, the loss of VP and HS will be reversed with the restoration of tree plantations in the soil subsystem.
Vascular flora on rock outcrops have a broad distribution in a range of habitats from savannas to humid tropical forests19. However, little research considers the changing pattern along the vegetative gradient on the nearby soil subsystem. Our results suggested that VP metrics obviously changed with the degradation and restoration of soil subsystem vegetation (Fig. 4, Table 1). When the canopy coverage in soil subsystem at SFE was reduced to that of RDE (Table 3), the species richness of VP lost 48.8% and diversity indices dropped by approximately 50% (Table 1). Moreover, NMDS ordination analysis showed a strong separation between forest (e.g. AFE and SFE) and non-forest (e.g. RDE) sites (Fig. 3). The VP were characterized by calcification and xerophilization at RDE, such as dense growth (Paraboea neurophylla), desiccation-tolerant (Selaginella tamariscina), or deep rooted (Ficus tikoua)37. Similarly, the VP also changed with the restoration of soil subsystem vegetation. These changes in community structure and composition followed a trend towards SFE but remained different from it. Certain life forms (i.e., shrubs, herbs, deciduous and evergreen plants) and space distribution of VP at AFE were in an intermediate stage of developmental sequence between RDE and SFE.
HS also changed following the soil subsystem vegetation degradation, as did the restoration process, in both quantity and quality. Average amount and nutrient content of HS at RDE were significantly lower than at SFE (Fig. 2). Almost 30% quantity, 35% moisture, 33% N, and 40% P in HS of SFE accounted for that at RDE. Nevertheless, the moisture and nutrients content (except K) at AFE were slightly higher than those of RDE. In addition, the HS amount and its nutrient concentration at AFE sharply decreased compared with SFE, like the variation pattern of VP individuals per square meter and microhabitats. These results indicated that the VP and HS were highly sensitive to the vegetation changes on nearby soil subsystems, and neither of them have improved substantially despite the successful vegetation restoration in soil subsystems.
The availability and heterogeneity of HS are important factors for the slow succession of VP since a strong correlation was found between HS and VP metrics (Table 2). Our results showed that the individuals and species richness of VP were significantly positive correlated with the amount of HS on rock outcrops. Moreover, the variation of species richness was similar to that of HS among three sites (Figs 2, 4). Thus HS, to some extent, may induce the successful establishment of VP by supplying sufficient water and preventing nutrients leaching into runoff water or seeping through the fissures, and also acts as a medium for roots proliferation. The amount and quality differences in HS patches would increase the heterogeneity of substrate fertility, and significantly influence karst biodiversity. However, because of the limited retention capacity of HS on rock outcrops10, much water, redundant organic carbon and nutrients were transported to nearby soil subsystems in local karst landscape1,38.
The low HS quantity can be attributed to the extremely slow rate of soil formation because little residue results from carbonate rock weathering39. On average, it takes 300–73800 years to form 1 cm thick soil based on different estimation methods involving the dissolution test of carbonate rock39. The magnitude of quantity and quality in HS, and associated ecological function at AFE cannot reach the levels within the regional natural forests (e.g. SFE). Therefore, the floral succession on rock outcrops remained slow in plantation forest. Additionally, HS tended to amass at the “funnel” shaped microhabitats (RC and RP), whereas few residua were detained at the rain-washed places (Fig. 5b). The finite and shallow substrate layer, combined with low availability of resources made it difficult for flora to develop in rock subsystem. Perhaps this could help explain the species richness of our carbonate outcrops (61) being lower than other outcrops, such as 86 for granite outcrops40, and 142 for crystalline outcrops19. Research showed that soil communities changed over time to favor late successional plant species41, although the soil depth had litter influence on community structure during the initial years of restoration42. Plant-soil feedbacks, the successful drivers of succession12, play a vital role in explaining slow outcrops flora succession. In return, our study provided an obvious evidence that availability and heterogeneity of resources (e.g. nutrients and soil heterogeneity) have a strong influence on plant community structure during the restoration of rock desertification.
In addition, the flora on rock outcrops exhibits interesting patterns of distribution and affinity to the substratum40. Porembski43 reported many species on inselbergs distributed in ephemeral flush plot and a shallow depression with more nutrients and soil. Our study showed that distinctive species were usually found in diverse microhabitats with a shallow soil layer, forming a patchy size distribution ranging from a few centimeters across to hundreds of square centimeters. This agrees with other studies where numerous individual plants were biased in favor of rocky habitats where considerable amounts of HS was easily accumulated10,44. Outcrops which appear bare at a distance, however, can bear many vascular plant species, some of which have the striking ability to grow on a surface with almost no soil40. SFE reflected this; there was very little HS in RW but many VP. More than 160 g/m² of HS occurred at RP with only relatively few individuals (Fig. 5). This uncommon phenomenon could suggest that the species diversity in rock vegetation may be influenced by other factors (e.g., microclimate, or physiological characteristics of plants).
Many factors are likely to influence the succession of plants in rocky habitats. Angelini and Silliman34 observed that vegetation species initially settled in the community (e.g., soil subsystem vegetation) which may facilitate the establishment of subsequent species by improving the habitat. Clements et al.45 claimed the presence of karst microhabitats supported high floral diversity. Both primary communities and diverse topographies result in a difference of microclimate, and further affect the structure and distribution of VP. Additionally, seed and establishment limitations restrict plant recruitment at early stages during ecological restoration36,46. Compared with soil subsystem vegetation, more stressful establishment conditions (e.g., low coverage, scanty soil, and intensive human intervention) resulted in slow flora succession on outcrops.
Ecological restoration is not just a matter of planting trees, it involves assisting the restoration of a damaged and destroyed ecosystem47. Rock outcrop is a critical component of karst landscapes and typically supports the vast majority of its entire floristic diversity45, even in the severe environment of RDE. Certain attempts have been made to alleviate the rate of environmental degradation in the vulnerable karst area6,19, and have made good progress5. Most of the eco-efficiency indexes that evaluate rocky desertification control, mainly come from soil subsystem vegetation, such as coverage, biomass, and diversity indexes48. Due to the different VP variation pattern during rocky desertification restoration, its succession should be taken into consideration when constructing an evaluation index system of rocky desertification restoration, especially in areas with a high rock-to-soil area ratio.
Methods
Study area
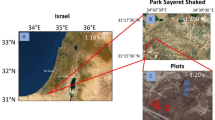
This study was performed in Shilin County (24°30′–25°03′N, 103°10′–103°40′E), Yunnan Province, southwest China, where karst landforms are composed of Permian, Carboniferous and Devonian carbonate rocks49. The karst landscapes consist of rock gaps, rock ditches, small rock caves and rock slots. Altitudes range from 1600 to 2200 m above sea level. A subtropical plateau monsoon climate prevails with a mean annual precipitation of 967.9 mm, 80–88% of which falls between May and October. Mean annual temperature is 16.2 °C, mean maximum temperature of the warmest month (July) is 20.7 °C, and mean minimum temperature of the coldest month (January) is 8.2 °C 36. Red earth and calcareous soil are the primary soil types.
Three typical local ecosystems were selected for this study:
-
(1)
A rocky desertification ecosystem (RDE), where most of the trees of the primary forest were removed or cut because of human activities (e.g., firewood harvesting, animal grazing), followed by the establishment of shrubs and herbs, such as Sophora viciifolia, Spiraea salicifolia, Diospyros duetorum, Bidens pilosa, Heteropogon contortus, Themeda triandra. Rock outcrops are exposed. The rock surface is covered by cyanobacteria film, and a few VP occur in microhabitats formed on rock outcrops.
-
(2)
An anthropogenic forest ecosystem (AFE), with trees including Pinus yunnensis, Koelreuteria paniculata, Photinia × fraseri, and Pyracantha fortuneana which were planted after a land preparation on a RDE site in 2005. Currently, these trees are higher than most of rock outcrops. The site is 4 km away from the RDE. Tian et al.29 investigated the species and communities of epilithic cyanobacteria films in this area. There are more VP on rock outcrops compared with RDE. No restoration measures were applied to the vegetation on rock subsystem and they are the result of succession.
-
(3)
A secondary forest ecosystem (SFE), is an evergreen broadleaved forest dominated by Neolitsea homilantha, Olea yunnanensis, Cyclobalanopsis glaucoides and Pistacia weinmannifolia, with mixed deciduous species such as Albizia mollis, Carpinus mobeigiana and Pistacia chinensis. It was a god forest of a local village, the forest is dense, has a clear vertical stratification, and outcrops are covered by the tree canopy. Cyanobacteria, lichen, bryophytes, and many VP live on the rocks50. The site is 26.5 km away from RDE, within the natural reserve of Shilin Geopark.
The specific degradation process of RDE is difficult to ascertain for the complicated reasons51. For AFE, the soil subsystem vegetation has almost been restored after a period of more than 20 years. The time was confirmed by the record of Stone Forest Scenic Area Administration. There were no trees in soil subsystem before restoration according to the description of local people, although there was no detailed record about it. The previous situation of AFE could be regarded as same as RDE. Vegetation is well preserved in the SFE with slight human disturbance. All three ecosystems are located on the same karst rock base, and have similar geographical features. We conceptualized the RDE as the degradation stages of SFE, and the AFE and SFE as the ecological restoration stages of RDE by employing a space-for-time substitution method52. The coordinates and environmental conditions of the study sites are shown in Table 3.
Plot design
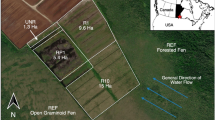
Comprehensive fieldwork was undertaken between October and November 2014. Six transects of 10 m wide were established along the hill slope within each of the three ecosystems and then each transect was divided into 10 plots with plastic line (Fig. 6). At the center of each plot (10 m × 10 m), a 2 m × 2 m sample collection subplot was established. There were 180 subplots in total for the three ecosystems.
Vascular plant identification and classification
Within each 2 m × 2 m subplot, all vascular species (VP) growing on rock outcrops were identified. Some plants were too small to identify. We only recorded the recognizable individuals more than one centimeter high. Next, all the recorded VP were collected, sealed within bags and brought back to laboratory, dried for 72 h at 70 °C to attain constant weight to calculate biomass. Such biomass obtained for the rock outcrops must be regarded as conservative. This is because a few shrubs and lianas had roots extending into soil, but only the part that was growing on rock outcrops was collected and counted as biomass.
Based on our investigation and analysis, the VP were grouped into life forms of trees, shrubs, herbs and lianas53, and further grouped into deciduous and evergreen, and into heliophyte and sciophyte54 for comparison among the three ecosystems.
Humus soil collection and chemical determination
The microhabitats on rock outcrops were divided into six categories: rocky crevice (RC), rocky gully (RG), rocky pit (RP), rocky surface (RS), rocky terrace (RT), rocky wall (RW)8. Their specific features are shown in Table 4. The numbers of microhabitat type within each subplot were recorded. A maximum of three for each microhabitat type were selected to collect HS depending on the total individuals counted. As much HS as possible was collected, stones and other impurities were removed, and it was then air-dried and weighed. The total amount of HS for each microhabitat in each subplot was multiplied by the number of each microhabitat type to calculate the mean weight of replicates.
The HS samples from each transect were combined into one sample (six samples for each ecosystem), passed through a 0.25 mm sieve, and subjected to chemical analysis. Total N was determined using a Vario MAX CN elemental analyzer. The P and K content were determined with an ICP-AES (iCAP6300, Thermo Fisher Scientific U.S.A) after mixing with HNO3-HClO4.
Statistical analyses
VP diversity was estimated using (1) α-diversity, evaluated as species richness per subplot, (2) γ-diversity, the total species numbers in each karst outcrops, (3) Shannon-Wiener index, (4) Simpson index55.
We calculated VP similarities using Sorensen’s similarity index55. Moreover, to estimate the differences in VP community across ecosystem types, a similarity matrix of 85 plots (eliminated without plants) × 61 species was subjected to non-metric multidimensional scaling (NMDS) with function metaMDS 56 in the package vegan 57 in R 3.3.2 58. NMDS is a good ordination method because it can use rank information and map ranks non-linearly onto ordination space to measure community dissimilarities based on species data.
One-way ANOVA, followed by a Tukey test for multiple pair-wise comparison, were used to assess the differences in diversity of VP among the three study sites. All data were checked for homogeneity of variances using Bartlett’s test and normality using the Shapiro-Wilk test and when the assumptions could not be satisfied after transformation, comparisons of non-parametric data were made using the Kruskal-Wallis test and Wilcoxon rank sum test. We performed all calculations and statistical analyses in R 3.3.2 58.
Kruskal-Wallis test and Wilcoxon rank sum test were used to assess the difference in the quantity and nutrients concentration of HS among ecosystems. Spearman’s correlation coefficients were used to express the relationship between physical factors and number of individuals, species richness and biomass.
References
Wang, D. J., Shen, Y. X., Li, Y. H. & Huang, J. Rock outcrops redistribute organic carbon and nutrients to nearby soil patches in three karst ecosystems in SW China. Plos One. doi:10.1371/journal.pone.0160773 (2016a).
Gams, I. Origin of the term “karst”, and the transformation of the classical karst (kras). Environ. Geol. 21, 110–114 (1993).
Šebela, S., Slabe, T., Liu, H. & Pruner, P. Speleogenesis of selected caves beneath the Lunan Shilin and caves of fenglin karst in Qiubei, Yunnan. Acta Geol. Sin-Engl. 78, 1289–1298 (2004).
Ford, D. C. & Williams, P. W. Karst hydrogeology and Geomorphology. (John Wiley & Sons, 2013).
Jiang, Z. C., Lian, Y. Q. & Qin, X. Q. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 132, 1–12 (2014).
Kranjc, A., Pang, X. & Conacher, A. History of deforestation and reforestation in the Dinaric Karst. Geogr. Res. 47, 15–23 (2009).
Waltham, T. F. fenglin, cone karst and tower karst. Cave Karst Sci. 35, 77–88 (2008).
Xiong, H. & Yu, F. Characteristics of microclimate change in different habitats of karst moderate rocky desertification area. Guizhou Agr sci. 41, 103–105 (2013).
Viles, H. A. & Gorbushina, A. A. Soiling and microbial colonisation on urban roadside limestone: a three year study in Oxford, England. Build. Environ. 38, 1217–1224 (2003).
Zhu, X. A., Shen, Y. X., He, B. B. & Huang, J. Humus on karst rock outcrops and their adsorption of ammonia nitrogen: a case from three ecosystems of Shilin, Yunnan. Carsol. Sin. 34, 616–623 (2015).
Odum, E. P. The strategy of Ecosystem development. Science. 164, 262–270 (1969).
Bauer, J. T., Mack, K. M. L. & Bever, J. D. Plant-soil feedbacks as drivers of succession: Evidence from remnant and restored tallgrass prairies. Ecosphere. 6, 1–12 (2015).
West, D. C., Shugart, H. H. & Botkin, D. B. Forest succession concepts and applications. (Springer, 1981).
Putten, W. H. et al. Plant–soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276 (2013).
Petermann, J. S., Fergus, A. J., Turnbull, L. A. & Schmid, B. Janzen-Connell effects are widespread and strong enough to maintain diversity in grasslands. Ecology. 89, 2399–2406 (2008).
Comita, L. S., Mullerlandau, H. C., Aguilar, S. & Hubbell, S. P. Asymmetric density dependence shapes species abundance in a tropical tree community. Science. 329, 330–332 (2010).
Dam, N. M. V., Qiu, B. L., Hordijk, C. A., Vet, L. E. M. & Jansen, J. J. Identification of biologically relevant compounds in aboveground and belowground induced volatile blends. J. Chem. Ecol. 36, 1006–1016 (2010).
Nunes, J. A. et al. Soil-vegetation relationships on a banded ironstone ‘island’, Carajás Plateau, Brazilian Eastern Amazonia. An. Acad. Cienc. 87, 2097–2110 (2015).
Gomes, P. & Sobral, L. M. Crystalline rock outcrops in the Atlantic Forest of northeastern Brazil: Vascular flora, biological spectrum, and invasive species. Braz. J. Bot. 36, 111–123 (2013).
Gorbushina, A. A. Life on the rocks. Environ. Microbiol. 9, 1613–1631 (2007).
Lisci, M., Monte, M. & Pacini, E. Lichens and higher plants on stone: a review. Int. Biodeter. Biodegr. 51, 1–17 (2003).
Elbert, W., Weber, B., Büdel, B., Andreae, M. O. & Pöschl, U. Microbiotic crusts on soil, rock and plants: neglected major players in the global cycles of carbon and nitrogen? Biogeosciences Discussions. 6, 6983–7015 (2009).
Wang, S. J., Liu, Q. M. & Zhang, D. F. Karst rocky desertification in southwestern China: geomorphology, landuse, impact and rehabilitation. Land Degrad. Dev. 15, 115–121 (2004).
Shen, Y. X., Yang, G. R. & Huang, J. Comparison of tree sprouting in three regeneration stages of an evergreen broadleaved forest in a karst landscape, SW China. Acta Ecol. Sin. 31, 126–132 (2011).
Antony, C. P., Cockell, C. S. & Shouche, Y. S. Life in (and on) the rocks. J. Biosciences. 37, 3–11 (2012).
Holl, K. D. & Aide, T. M. When and where to actively restore ecosystems? Forest Ecol. Manag. 261, 1558–1563 (2011).
Karsten, U., Schumann, R. & Mostaert, A. Aeroterrestrial algae growing on man-made surfaces in Algae and cyanobacteria in extreme environments (ed. Seckbach, J.) 583–597 (Springer, 2007).
Seckbach, J. Algae and cyanobacteria in extreme environments. (Springer, 2007).
Tian, Y. P., Zhang, J., Song, L. H. & Bao, H. S. A study on aerial cyanophyta (cyanophyta) on the surface of carbonate rock in Yunnan stone forest, Yunnan province, China. Acta Ecol. Sin. 22, 1793–2020 (2002).
Etienne, S. & Dupont, J. Fungal weathering of basaltic rocks in a cold oceanic environment (Iceland): comparison between experimental and field observations. Earth Surf. Proc. Land. 27, 737–748 (2002).
Matthes, S. U., Gerrath, J. A. & Larson, D. W. Abundance, biomass, and productivity of endolithic and epilithic lower plants on the temperate-zone cliffs of the Niagara Escarpment, Canada. Int. J. Plant Sci. 158, 451–460 (1997).
Spitale, D. & Nascimbene, J. Spatial structure, rock type, and local environmental conditions drive moss and lichen distribution on calcareous boulders. Ecol. Res.
Warscheid, T. & Braams, J. Biodeterioration of stone: a review. Int. Biodeter. Biodegr. 46, 343–368 (2000).
Angelini, C. & Silliman, B. R. Secondary foundation species as drivers of trophic and functional diversity: evidence from a tree–epiphyte system. Ecology. 95, 185–196 (2014).
Menz, M. H. M., Dixon, K. W. & Hobbs, R. J. Ecology: Hurdles and opportunities for landscape-scale restoration. Science. 339, 526–527 (2013).
Shen, Y. X., Gao, L., Xia, X., Li, Y. H. & Guan, H. L. Successional distance between the source and recipient influence seed germination and seedling survival during surface soil replacement in SW China. Plos One. 8, 70–77 (2013).
Pérez-García, E. A. & Meave, J. A. Heterogeneity of xerophytic vegetation of limestone outcrops in a tropical deciduous forest region in southern México. Plant Ecol. 175, 147–163 (2005).
Wang, D. J., Shen, Y. X., Huang, J. & Li, Y. H. Rock outcrops redistribute water to nearby soil patches in karst landscapes. Environ. Sci. Pollut. Res. 23, 1–7 (2016b).
Wang, Z. G., Wan, M. C., Sun, J. J. & Zhang, P. C. Review of studies on soil formation rate in karst rocky desertification region of southwestChina. J. Yangtze River Sci. Res. Inst. 32, 64–72 (2015).
Meirelles, S. T., Pivello, V. R. & Joly, C. A. The vegetation of granite rock outcrops in Rio de Janeiro, Brazil, and the need for its protection. Environ. Conserv. 26, 10–20 (1999).
Middleton, E. L. & Bever, J. D. Inoculation with a native soil community advances succession in a grassland restoration. Restor. Ecol. 20, 218–226 (2010).
Baer, S. G., Blair, J. M., Collins, S. L. & Knapp, A. K. Plant community responses to resource availability and heterogeneity during restoration. Oecologia. 139, 617–629 (2004).
Porembski, S. Tropical inselbergs: habitat types, adaptive strategies and diversity patterns. Braz. J. Bot. 30, 579–586 (2007).
Liao, H. K., Li, J., Long, J., Zhang, W. J. & Liu, L. F. Soil characteristics of different microhabitats of Chinese prickly ash in karst mountain areas of Guizhou Province. J. Agro-Environ. Sci. 32, 2429–2435 (2013).
Clements, R., Sodhi, N. S., Schilthuizen, M. & Ng, P. K. L. Limestone karsts of southeast Asia: Imperiled arks of biodiversity. Bioscience. 56, 733–742 (2006).
Sheley, R. L., Mangold, J. M. & Anderson, J. L. Potential for successional theory to guide restoration of invasive-plant-dominated rangeland. Ecol Monogr. 76, 365–379 (2006).
Tollefson, J. President prunes forest reforms. Nature. 486, 13 (2012).
Qi, X. K., Wang, K. L. & Zhang, C. H. Effectiveness of ecological restoration projects in a karst region of southwest China assessed using vegetation succession mapping. Ecol. Eng. 54, 245–253 (2013).
Šebela, S. & Liu, H. Structural geological characteristics of karst caves and major stone forest, Yunnan, China. Acta Carsologica. 43, 115–127 (2014).
Xu, H. Q., Liu, W. Y., Shen, Y. X., Liu, L. H. & Li, Y. H. A preliminary study of epiphytes in semi-humid evergreen broad-leaved forest in Stone-forest Karst Region, Yunnan Province. Guihaia. 26, 43–48 (2006).
Li, Y. H., Feng, Z. Q., Yu, X. Y. & Ma, Z. P. Eventful changes of the vegetation in Shilin National Park and its significance. Carsol. Sin. 24, 46–53 (2005).
Miao, R. H., Qiu, X. L., Guo, M. X., Alamusa & Jiang, D. Accuracy of space-for-time substitution for vegetation state prediction following shrub restoration. J. Plant Ecol. https://doi.org/10.1093/jpe/rtw133 (2016)
Wu, Z. Y. Chinese vegetation. (Science Press, 1980).
Benzing, D. H. Vascular epiphytes: general biology and related biota. (Cambridge University Press, 2008).
Li, S., Liu, W. Y. & Li, D. W. Bole epiphytic lichens as potential indicators of environmental change in subtropical forest ecosystems in southwest China. Ecol. Indic. 29, 93–104 (2013).
McCune, B., Grace, J. B. & Urban, D. L. Analysis of ecological communities. Available at: https://www.researchgate.net/publication/216769990. (MjM Software Design, 2002)
Oksanen J. et al. Vegan: an R package for community ecologists. R package version 2.4-2. Available at: http://github.com/vegandevs/vegan (2016)
R Development Core Team. The R Project for Statistical Computing. R version 3.3.2. Available at: http://www.r-project.org (2016)
Acknowledgements
We are deeply appreciating the two anonymous reviewers for providing constructive comments towards the manuscript. We are grateful to the Biogeochemistry Laboratory, and Xishuangbanna Tropical Botanical Garden for their assistance in chemical tests. We also wish to thank the Stone Forest Scenic Area Administration for assistance in all field investigations. This study was supported by the National Natural Science Foundation of China (41671031) and National Key Research and Development Program (2016YFC0502504).
Author information
Authors and Affiliations
Contributions
X.-A.Z. conducted the experiments, analyzed the data, wrote and revised the manuscript; Y.-X.S. assisted with writing and revising the manuscript; B.-B.H. and Z.-M.Z. helped design the experiment and data analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, X., Shen, Y., He, B. et al. Humus soil as a critical driver of flora conversion on karst rock outcrops. Sci Rep 7, 12611 (2017). https://doi.org/10.1038/s41598-017-13060-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13060-5
This article is cited by
-
Morphological characteristics of rock fissure networks and the main factors affecting their soil nutrients and enzyme activities in Guizhou Province, China
Journal of Mountain Science (2022)
-
The role of soil water retention functions of near-surface fissures with different vegetation types in a rocky desertification area
Plant and Soil (2019)
-
Variation in plant functional groups indicates land degradation on the Tibetan Plateau
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.