Abstract
Little is known about the impact of age at menarche on preterm birth. The aim of this study was to examine the association between age at menarche and preterm birth. A total of 11,016 Chinese women who gave birth to live singleton infants were recruited from the Healthy Baby Cohort between 2012 and 2014 in the province of Hubei, China. Age at menarche was reported via face-to-face interviews and was categorized into five groups (≤11, 12, 13, 14 and ≥15 years). Gestational age was estimated using maternal last menstrual period. Preterm birth was defined as delivering a live singleton infant at <37 weeks’ gestational age. Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Earlier menarche (≤11 years) was associated with an increased prevalence of preterm birth (OR: 1.67, 95% CI: 1.18, 2.36) compared with menarche age at 13 years after controlling for the potential confounders. The findings of our study suggested that a history of earlier menarche might be useful for identifying women at higher risk of preterm birth.
Similar content being viewed by others
Introduction
Preterm birth (PTB, <37 completed weeks of gestation), an important adverse pregnancy outcome, affects approximately 14.9 million infants worldwide in 20101. Preterm infants are at increased risk of developing neonatal and long-term complications2. Thus, identifying women at risk of PTB at an early life stage may allow early health monitoring and intervention.
Menarche, the age at onset of first menstruation, is an indicator of puberty3. Secular trends in earlier menarche have been observed worldwide4,5,6. Potential health effects of age at menarche have received a great deal of attention. Earlier menarche has been linked to several adverse health consequences in later life, such as breast cancer7, type 2 diabetes8, and cardiovascular diseases9. In addition, earlier menarche has been associated with adverse pregnancy outcomes, such as ectopic pregnancies10, miscarriage11, and low birth weight12. However, little is known about the influence of age at menarche on PTB. Previous studies have suggested that earlier menarche was associated with higher estradiol levels13,14, elevated C-reactive protein levels15, and increased plasma glucose levels in the adulthood16,17. These changes were reported to be related to an increased risk of PTB18,19,20,21,22,23. In addition, earlier menarche was associated with PTB risk factors, such as obesity24,25, infection26,27, and psychological stress28,29. To our knowledge, except for one study reported no significant association between age at menarche and PTB among 2115 women from the 1958 British birth cohort study30, we are not aware of any epidemiological studies that have investigated the relationship between them.
The aim of this study was to examine the association between age at menarche and prevalence of PTB among Chinese women, using data from the Healthy Baby Cohort (HBC) study. We hypothesized that earlier menarche was associated with an increased prevalence of PTB.
Results
Characteristics of the participants
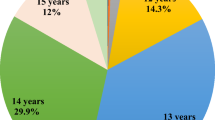
The present study included 11,016 live singleton infants, 653 (5.9%) were born preterm. The mean gestational age was 39.1 ± 1.4 weeks. The mean maternal age was 28.2 ± 3.7 years and the mean age at menarche was 13.2 ± 1.2 years. The proportion of women who reported their menarche at ages ≤11, 12, 13, 14 and ≥15 years was 5.1%, 23.2%, 35.7%, 23.8% and 12.2%, respectively. Characteristics of the participants are showed in Table 1. Women with earlier menarche were more likely to have higher pre-pregnancy body mass index (BMI), shorter gestational age, and higher prevalence of diabetes and hypertension during pregnancy (all P for trend <0.05).
Association between age at menarche and PTB
Table 2 shows the association between age at menarche and prevalence of PTB. Compared with menarche at age 13 years, earlier menarche (≤11 years) was associated with an increased prevalence of PTB (OR: 1.67, 95% CI: 1.18, 2.36) after adjustment for age, educational level, occupational status, pre-pregnancy BMI, lifestyle factors, the use of assisted reproductive technologies, maternal reproductive history, and maternal history of diseases.
Subgroup analyses
The results of subgroup analyses stratified by maternal age, pre-pregnancy BMI, and parity are presented in Table 3, Table 4 and Table 5, respectively. No significant interactions were found between age at menarche and maternal age, pre-pregnancy BMI, and parity on prevalence of PTB (all P for interaction >0.05).
Discussion
In the present study, we investigated the association between age at menarche and PTB among Chinese women. We found that earlier menarche was significantly associated with an increased prevalence of PTB after adjustment for potential confounders.
A study of 2115 women from the 1958 British birth cohort study reported no significant relationship between age at menarche and PTB30, a finding that was inconsistent with our results. They used retrospectively collected self-reported information on PTB, which may lead to potential recall bias. In addition, the differences in race, sample size, and categories of age at menarche might be potential explanations for the inconsistent findings. More studies are needed to verify the effect of age at menarche on PTB.
Although the potential mechanisms underlying the association between earlier menarche and prevalence of PTB are unclear, our findings are biologically plausible. Women who experienced earlier menarche have higher levels of estradiol in adulthood13,14. It has been reported that higher levels of estradiol increased the risk of PTB18,19. In addition, earlier menarche was associated with elevated C-reactive protein levels, which is a marker of inflammation15. Studies have shown that maternal C-reactive protein levels during pregnancy were positively associated with risk of PTB20,21. Furthermore, earlier menarche was associated with metabolic changes including insulin resistance and increased plasma glucose levels16,17,31. Previous studies have demonstrated increasing risk of PTB with increasing maternal plasma glucose levels among women without diabetes mellitus22,23. We did not found lower prevalence of PTB among women with later menarche (≥15 years). The possible reason is that later menarche may reflect unhealthy medical conditions such as polycystic ovary syndrome (PCOS)32. Women with PCOS also have these metabolic problems, which may increase the risk of PTB33.
Another possible explanation for the relationship between earlier menarche and PTB might be obesity. Earlier menarche increased the risk of obesity in adulthood24,25, which is an important risk factor for PTB34,35. In our study, adjustment for pre-pregnancy BMI (as a continuous variable) in the regression models did not alter the significant association between earlier menarche and prevalence of PTB. In addition, we performed a subgroup analysis according to pre-pregnancy BMI; the association between earlier menarche and prevalence of PTB was consistent across subgroup stratified by pre-pregnancy BMI. Therefore, our results suggested that the association between earlier menarche and prevalence of PTB is independent of pre-pregnancy BMI.
This study has many strengths, including the large sample size, and the inclusion of a wide range of potential confounding variables. Moreover, the use of standard questionnaires and medical records enhanced the reliability of the data.
However, there are several limitations of the present study that should be acknowledged. First, age at menarche was assessed retrospectively, which may result in recall bias. However, previous studies indicated that recalled age at menarche reported during adulthood is highly correlated with original childhood data36,37. Second, Because of the lack of detailed information on types of PTB (i.e., spontaneous or iatrogenic PTB), we could not estimate the association between age at menarche and specific types of PTB. Third, the gestational age was calculated from maternal last menstrual period (LMP) in our study, which might result in an inaccurate calculation of gestational age and misclassification of PTB events. However, it has been reported that gestational age estimates by LMP and ultrasound were well correlated, and the estimates of gestational age by LMP was generally reliable and valid38,39. Fourth, our study was conducted in Chinese population. Due to the ethnic/racial differences in menarche, our findings may not be generalizable to other populations. Fifth, although a variety of potential confounding variables were adjusted, we cannot rule out the residual confounding by other unmeasured factors such as stress, maternal nutritional status and history of PTB. However, given that 85.3% of the participants were primiparous, no adjustment for history of PTB should not pose a problem for this study.
In conclusion, our study suggested that earlier menarche is a risk factor for PTB. A history of earlier menarche may be useful for identifying women at an elevated prevalence of developing PTB. Future studies are needed to confirm this finding and to clarify the underlying mechanisms.
Methods
Study participants
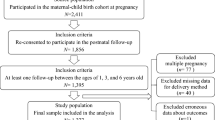
The HBC study is an ongoing prospective birth cohort, which was conducted to investigate the environmental and genetic factors that affect child health and development. Between September 2012 and October 2014, a total of 11,311 women who gave birth to live singleton infants were recruited from the Women and Children Medical and Healthcare Center of Wuhan, Hubei province, China. Each participant was required to provide blood and urine samples, and complete a standard questionnaire by face-to-face interviews. In the present study, we excluded women if they gave birth to an infant with a birth defect or if they had missing information on age at menarche. In total, 295 women were excluded, and 11,016 participants were included for the final analysis.
This study was approved by the Medical Ethics committee of the School of Public Health, Tongji Medical College, Huazhong University of Science and Technology. All participants provided written informed consent. All the methods in the present study were carried out in accordance with the approved guidelines.
Assessment of age at menarche
Information on age at menarche was obtained from questionnaires based on a question: ‘How old were you when you had the first menstrual period?’ In our study, age at menarche was recorded in years. For the present analysis, we categorized age at menarche into five groups (≤11, 12, 13, 14 and ≥15 years).
Ascertainment of PTB
Gestational age was estimated as the difference between the first day of the LMP and the delivery date. The first day of the LMP was obtained from the prenatal care record during the first antennal care visit (before 12 weeks), and the delivery day were obtained from medical records. PTB was defined as delivering a live singleton infant at <37 weeks’ gestational age.
Assessment of covariates
Participants was interviewed during the period of institutional delivery by trained nurses in the hospital. Information on socioeconomic characteristics (maternal age at delivery, educational level, and occupational status), and lifestyle factors (alcohol consumption before pregnancy, smoking before pregnancy, passive smoking during pregnancy, and physical activity frequency during pregnancy) was collected by questionnaires. Passive smoking was defined as exposure second-hand smoking during pregnancy (the father or other persons smoking in the household or workplace)40. Data on maternal reproductive history (history of spontaneous abortion, history of induced abortion, and parity), maternal history of diseases, and the use of assisted reproductive technologies were obtained from medical records. Parity was defined as self-reporting of the number of live births. Pre-pregnancy weight was self-reported at the first prenatal care visit (usually at the first trimester), and height was measured using a stadiometer at the first prenatal care visit. Pre-pregnancy BMI was calculated as pre-pregnancy weight in kilograms divided by height in meters squared.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD), and categorical variables were expressed as number and percentage. To test for linear trend across categories of age at menarche, we assigned the median values of each group of age at menarche and fitted this as a continuous variable in a separate regression model.
Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between age at menarche and prevalence of PTB. We used menarche age of 13 years as the reference group, because 13 years old is the median age of menarche of the study participants. Covariates for the adjusted models were selected based on their established or potential association with PTB35,41,42,43, including maternal age (continuous), educational level (high school or below, college or above), occupational status (employed or unemployed), pre-pregnancy BMI (continuous), alcohol consumption before pregnancy (yes or no), smoking before pregnancy (yes or no), passive smoking during pregnancy (yes or no), physical activity frequency during pregnancy (never/rarely, 1–2 days/week, 3–4 days/week, 5–6 days/week or daily), history of spontaneous abortion (yes or no), history of induced abortion (yes or no), parity (1 or ≥2), the use of assisted reproductive technologies (yes or no), hypertension during pregnancy (yes or no), and diabetes during pregnancy (yes or no). We performed a multiple imputation analysis to handle missing data44. Twenty imputations were used, and all the adjustment variables listed in the regression analysis above were included in the imputation model, along with the outcome (PTB).
To assess a potential effect modification, analyses were stratified by maternal age (<28 or ≥28 years, the median value of age at delivery), and pre-pregnancy BMI (<20 or ≥20 kg/m2, the median value of pre-pregnancy BMI) and parity (1 or ≥2). Tests for interaction across subgroup were performed using Wald test.
All statistical analyses were performed with SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). Significance tests were two-tailed and P value < 0.05 were considered significant.
References
Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172 (2012).
Saigal, S. & Doyle, L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269 (2008).
DiVall, S. A. & Radovick, S. Pubertal development and menarche. Ann N Y Acad Sci 1135, 19–28 (2008).
Harris, M. A., Prior, J. C. & Koehoorn, M. Age at menarche in the Canadian population: secular trends and relationship to adulthood BMI. J Adolesc Health 43, 548–554 (2008).
Cheng, G. et al. Beyond overweight: nutrition as an important lifestyle factor influencing timing of puberty. Nutr Rev 70, 133–152 (2012).
Song, Y. et al. Trends of age at menarche and association with body mass index in Chinese school-aged girls, 1985-2010. J Pediatr 165, 1172–1177 (2014).
Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 13, 1141–1151 (2012).
Elks, C. E. et al. Age at menarche and type 2 diabetes risk: the EPIC-InterAct study. Diabetes Care 36, 3526–3534 (2013).
Canoy, D. et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation 131, 237–244 (2015).
Sandler, D. P., Wilcox, A. J. & Horney, L. F. Age at menarche and subsequent reproductive events. Am J Epidemiol 119, 765–774 (1984).
Martin, E. J., Brinton, L. A. & Hoover, R. Menarcheal age and miscarriage. Am J Epidemiol 117, 634–636 (1983).
Coall, D. A. & Chisholm, J. S. Evolutionary perspectives on pregnancy: maternal age at menarche and infant birth weight. Soc Sci Med 57, 1771–1781 (2003).
Emaus, A. et al. 17-beta-estradiol in relation to age at menarche and adult obesity in premenopausal women. Hum Reprod 23, 919–927 (2008).
Apter, D., Reinilä, M. & Vihko, R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer 783–787 (1989).
Mueller, N. T. et al. Earlier age at menarche is associated with higher diabetes risk and cardiometabolic disease risk factors in Brazilian adults: Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Cardiovasc Diabetol 13, 22 (2014).
Dreyfus, J. et al. Age at menarche and cardiometabolic risk in adulthood: the Coronary Artery Risk Development in Young Adults Study. J Pediatr 167, 344–352 (2015).
Heys, M. et al. Age of menarche and the metabolic syndrome in China. Epidemiology 18, 740–746 (2007).
Mazor, M. et al. Maternal plasma and amniotic fluid 17β-estradiol, progesterone and cortisol concentrations in women with successfully and unsuccessfully treated preterm labor. Arch Gynecol Obstet 258, 89–96 (1996).
Mazor, M. et al. Human preterm birth is associated with systemic and local changes in progesterone/17β-estradiol ratios. Am J Obstet Gynecol 171, 231–236 (1994).
Pitiphat, W. et al. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol 162, 1108–1113 (2005).
Catov, J. M., Bodnar, L. M., Ness, R. B., Barron, S. J. & Roberts, J. M. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol 166, 1312–1319 (2007).
HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008, 1991–2002 (2008).
Lowe, L. P. et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care 35, 574–580 (2012).
Yang, L. et al. Adiposity in relation to age at menarche and other reproductive factors among 300 000 Chinese women: findings from China Kadoorie Biobank study. Int J Epidemiol 46, 502–512 (2016).
Pierce, M. & Leon, D. Age at menarche and adult BMI in the Aberdeen children of the 1950s cohort study. Am J Clin Nutr 82, 733–739 (2005).
Ibitoye, M., Choi, C., Tai, H., Lee, G. & Sommer, M. Early menarche: A systematic review of its effect on sexual and reproductive health in low- and middle-income countries. PLoS One 12, e0178884 (2017).
Copeland, W. et al. Outcomes of early pubertal timing in young women: a prospective population-based study. Am J Psychiatry 167, 1218–1225 (2010).
Graber, J. A. Pubertal timing and the development of psychopathology in adolescence and beyond. Horm Behav 64, 262–269 (2013).
Graber, J. A., Seeley, J. R., Brooks-Gunn, J. & Lewinsohn, P. M. Is pubertal timing associated with psychopathology in young adulthood. J Am Acad Child Adolesc Psychiatry 43, 718–726 (2004).
Hennessy, E. & Alberman, E. Intergenerational influences affecting birth outcome. II. Preterm delivery and gestational age in the children of the 1958 British birth cohort. Paediatr Perinat Epidemiol 12, 61–75 (1998).
Wilson, D. A., Derraik, J. G., Rowe, D. L., Hofman, P. L. & Cutfield, W. S. Earlier menarche is associated with lower insulin sensitivity and increased adiposity in young adult women. PLoS One 10, e0128427 (2015).
Sadrzadeh, S. et al. Birth weight and age at menarche in patients with polycystic ovary syndrome or diminished ovarian reserve, in a retrospective cohort. Hum Reprod 18, 2225–2230 (2003).
Kjerulff, L. E., Sanchez-Ramos, L. & Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol 204, 558 (2011).
Cnattingius, S. et al. Maternal obesity and risk of preterm delivery. JAMA 309, 2362–2670 (2013).
Khatibi, A. et al. Prepregnancy maternal body mass index and preterm delivery. Am J Obstet Gynecol 207, e211–217 (2012).
Must, A. et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol 155, 672–679 (2002).
Cooper, R. et al. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health 60, 993–997 (2006).
Deputy, N. P. et al. Validity of gestational age estimates by last menstrual period and neonatal examination compared to ultrasound in Vietnam. BMC Pregnancy Childbirth 17, 25 (2017).
Neufeld, L. M., Haas, J. D., Grajéda, R. & Martorell, R. Last menstrual period provides the best estimate of gestation length for women in rural Guatemala. Paediatr Perinat Epidemiol 20, 290–298 (2006).
Vardavas, C. I. et al. The independent role of prenatal and postnatal exposure to active and passive smoking on the development of early wheeze in children. Eur Respir J 48, 115–124 (2016).
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371, 75–84 (2008).
Morgen, C. S., Bjork, C., Andersen, P. K., Mortensen, L. H. & Nybo Andersen, A. M. Socioeconomic position and the risk of preterm birth–a study within the Danish National Birth Cohort. Int J Epidemiol 37, 1109–1120 (2008).
Juhl, M. et al. Physical exercise during pregnancy and the risk of preterm birth: a study within the Danish National Birth Cohort. Am J Epidemiol 167, 859–866 (2008).
Sterne, J. A. et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338, b2393 (2009).
Acknowledgements
We thank all the study participants and the entire Healthy Baby Cohort team for their generous help. This study was supported by the National Natural Science Foundation of China (81273083, 21437002), the Fundamental Research Funds for the Central Universities (2014TS051) and the Hubei Province Health & Family Planning Scientific Research Project (WJ2015MA026).
Author information
Authors and Affiliations
Contributions
H.L. contributed to analysis and interpretation of data, and drafted the article. L.S., B.Z., and W.X. contributed to conception and design. L.S. Y.L. and X.Z. assisted in the analysis and interpretation of the data. B.L. Z.C., A.Z. and L.Z. contributed to acquisition of data. S.X. and Y.W. contributed to conception and design, interpretation of the results and critical revision of the manuscript for important intellectual content. All authors aided in the design of the study, in the interpretation of the data and critical revision of the manuscript for important intellectual content, and all authors approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Song, L., Shen, L. et al. Age at menarche and prevalence of preterm birth: Results from the Healthy Baby Cohort study. Sci Rep 7, 12594 (2017). https://doi.org/10.1038/s41598-017-12817-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12817-2
This article is cited by
-
Early age at menarche is associated with an increased risk of preeclampsia and adverse neonatal outcomes: a 6‑year retrospective study
Archives of Gynecology and Obstetrics (2023)
-
Earlier maternal menarche is associated with shorter newborn telomere length
European Journal of Pediatrics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.