Abstract
Ethanol has extensive effects on sleep and daytime alertness, causing premature disability and death. Adenosine, as a potent sleep-promoting substance, is involved in many cellular and behavioral responses to ethanol. However, the mechanisms of hypnotic effects of ethanol remain unclear. In this study, we investigated the role of adenosine in ethanol-induced sleep using C57BL/6Slac mice, adenosine A2A receptor (A2AR) knockout mice, and their wild-type littermates. The results showed that intraperitoneal injection of ethanol (3.0 g/kg) at 21:00 decreased the latency to non-rapid eye movement (NREM) sleep and increased the duration of NREM sleep for 5 h. Ethanol dose-dependently increased NREM sleep, which was consistent with decreases in wakefulness in C57BL/6Slac mice compared with their own control. Caffeine (5, 10, or 15 mg/kg), a nonspecific adenosine receptor antagonist, dose-dependently and at high doses completely blocked ethanol-induced NREM sleep when administered 30 min prior to (but not after) ethanol injection. Moreover, ethanol-induced NREM sleep was completely abolished in A2AR knockout mice compared with wild-type mice. These findings strongly indicate that A2AR is a key receptor for the hypnotic effects of ethanol, and pretreatment of caffeine might be a strategy to counter the hypnotic effects of ethanol.
Similar content being viewed by others
Introduction
Ethanol is one of the most highly abused psychoactive compounds worldwide1,2. It produces a variety of acute and chronic effects3, which have a significant socio-economic impact on the individuals, their families, and society. Ethanol can cause premature disability and death, accounting for an estimated 6–9% of all deaths4,5. Extensive clinical studies have documented that acute ethanol has a profound impact on sleep4,6, and acute discontinuation of alcohol in alcoholics results in severe disturbance of sleep architecture6,7,8. In addition, these sleep impairments are so severe that they are primary predictors of relapse in recovering alcoholics9,10. Thus, it is of paramount importance to identify the mechanism underlying the effects of ethanol on sleep-wake regulation. However, the central mechanisms involved in sleep-wake regulation by ethanol remain elusive.
Adenosine, a potent sleep-promoting substance11, is a key mediator of many behavioral and neuronal responses to ethanol12,13,14,15. Dysregulation of adenosine signaling has been implicated in ethanol-use disorders14,15,16,17,18. Ethanol is known to increase adenosine release19,20 and decrease adenosine uptake by inhibiting the type 1 equilibrative nucleoside transporter, which result in increased extracellular adenosine21,22,23. Accumulated extracellular adenosine induces sleep by activating adenosine A1 receptor (A1R) and A2A receptor (A2AR) in the central nervous system24,25. Among adenosine receptors responsible for sleep induction, the role of A2AR is predominant in sleep regulation. Administration of the selective A2AR agonist CGS21680 to the subarachnoid space adjacent to the basal forebrain and lateral preoptic area reliably induces a dramatic increase in non-rapid eye movement (non-REM, NREM) sleep, whereas infusion of A1R agonists produces weak and variable effects26,27,28,29. Furthermore, homeostasis of sleep-wake regulation is unaltered in animals lacking A1R30. However, the role of A2AR in ethanol-induced hypnotic effects is still in debate.
Caffeine, another widely used psychoactive compound31, binds A1R and A2AR with similar affinity as a receptor antagonist. The antagonistic role of caffeine in the ethanol-induced hypnotic effects is controversial. Some studies have suggested that caffeine offsets ethanol-induced changes in information processing, memory, and psychomotor performance32,33,34,35. However, other studies have been unable to confirm these results36,37. Therefore, it is unclear whether caffeine can block ethanol-induced hypnotic effects.
In the present study, we characterized sleep-wake profiles of C57BL/6Slac mice after an intraperitoneal (i.p.) injection of ethanol and found that ethanol increased NREM sleep in a dose-dependent manner. Furthermore, we demonstrated an antagonistic role of caffeine in hypnotic effects of ethanol. Pretreatment but not post-treatment of caffeine abolished the hypnotic effects of ethanol. Because caffeine reduces sleep by blocking A2AR38,39, we assessed possible involvement of A2AR in the hypnotic effects of ethanol using A2AR knockout (KO) and wild-type (WT) mice. After ethanol administration, A2AR WT mice showed an increase in duration of NREM sleep, but A2AR KO mice showed no change in time spent in NREM sleep. These findings indicate that A2AR plays an important role in the hypnotic effects of ethanol. Understanding the molecular mechanism underlying the hypnotic effects of ethanol may provide new therapeutic approaches for treating alcoholism and blocking acute behavioral impairment due to ethanol.
Results
Ethanol increased NREM sleep and decreased wakefulness in C57BL/6Slac mice
To investigate the hypnotic effects of ethanol, electroencephalograms (EEG) and electromyograms (EMG) were recorded for 2 consecutive days in C57BL/6Slac mice. On day 1, the mice were treated with vehicle i.p. at 21:00 in the early phase of the dark (active) period, and the recordings made on that day served as the own control. The animals were then treated with vehicle or ethanol (2.1, 2.5, 3.0, 3.6 g/kg, i.p.) 24 h later. Because mice spend most of their time sleeping during the light period, it is more difficult to evaluate effects of a drug on duration of sleep in the light period than in the dark period. Therefore, the experiments were performed during the dark period when animals spend most of their time in wakefulness. Typical examples of polygraphic recordings and corresponding hypnograms from one mouse given vehicle or 3.0 g/kg ethanol are shown in Fig. 1A. The mouse treated with ethanol spent more time in NREM sleep compared with their own control.
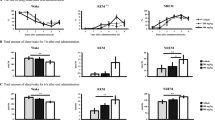
Sleep-wake profiles produced by administration of ethanol in C57BL/6Slac mice. (A) Typical examples of polygraphic recordings and corresponding hypnograms illustrating changes of sleep over 7 h (20:00–03:00) following vehicle (upper panel) or ethanol (lower panel) administration. (B) Changes in NREM sleep, REM sleep, and wakefulness in mice treated with 3.0 g/kg ethanol. (C) Dose-response effects on total time spent in NREM sleep, REM sleep, and wakefulness for 5 h after administration of vehicle and ethanol. Values are mean ± SEM (n = 9–10). (B) *P < 0.05 or **P < 0.01 indicates significant differences compared with their own control as assessed by two-way ANOVA followed by Fisher’s least-significant difference (PLSD) test. (C) **P < 0.01 indicates significant differences compared with the control as assessed by non-paired, two-tailed Student’s t test. The letters “a, b, c, d” indicate different subsets among doses of ethanol as assessed by one-way ANOVA followed by Duncan’s multiple range test.
Time course changes revealed that ethanol at 3.0 g/kg significantly increased NREM sleep (F1,198 = 21.3, P < 0.05) and decreased wakefulness (F1,198 = 18.0, P < 0.05) in C57BL/6Slac mice compared with their own control (Fig. 1B). Ethanol at 3.0 g/kg increased NREM sleep for 5 h following ethanol administration, which was consistent with a reduction in wakefulness during the same period. The effects began within the first hour after ethanol injection and last for 5h. There was no further disruption of sleep architecture during the subsequent period. No time course changes on REM sleep were observed.
Total time spent in NREM sleep, REM sleep, and wakefulness were measured for 5 h after ethanol injection, because 3 g/kg ethanol increased NREM sleep for 5 h. Ethanol dose-dependently increased NREM sleep (F4,47 = 17.9, P < 0.01) and reduced wakefulness (F4,47 = 15.6, P < 0.01) (Fig. 1C). Ethanol at 2.5, 3.0, and 3.6 g/kg increased the total duration of NREM sleep by 1.5-, 1.8-, and 1.6-fold (P < 0.01), respectively, which was consistent with a reduction in wakefulness by 23%, 33%, and 24% (P < 0.01), respectively, compared with their own control in each group. However, ethanol at 2.1 g/kg did not affect the cumulative amount of NREM sleep when measured for 5 h after injection. In contrast, ethanol at 3.6 g/kg reduced REM sleep by 67% when measured for 5 h after ethanol injection (Fig. 1C). However, the other doses of ethanol did not affect the duration of REM sleep. These results clearly indicate that ethanol increases NREM sleep in a dose-dependent manner and reduces REM sleep at a high dose of 3.6 g/kg.
Ethanol shortened sleep latency and altered sleep architecture in C57BL/6Slac mice
To assess initiation of the sleep state after injection, we measured the latencies to NREM and REM sleep, which were defined as the time from vehicle or ethanol injection to the first appearance of a NREM or REM sleep episode that lasted for at least 20 s40. As shown in Fig. 2A (upper panel), injection of ethanol remarkably shortened NREM sleep latency. The latencies to NREM sleep in mice treated with ethanol (2.1, 2.5, 3.0, and 3.6 g/kg) were 11.6, 12.3, 10.6, and 14.2 min, respectively, which were markedly shorter than the latency of 29.4 min after vehicle injection (P < 0.05). The short NREM sleep latency following ethanol injection clearly indicates that ethanol accelerates initiation of NREM sleep. In addition, REM sleep latency was dose-dependently prolonged by ethanol (Fig. 2A, lower panel). The latencies to REM sleep in mice treated with ethanol (2.1, 2.5, 3.0, and 3.6 g/kg) were 122.4, 155.8, 197.2, and 221.4 min, respectively, which were longer than the latency of 55 min after vehicle injection (Fig. 2A, lower panel). Prolonged REM sleep latency in ethanol-injected mice clearly indicates that ethanol inhibits initiation of REM sleep.
Changes in sleep latency and architecture produced by administration of ethanol. Effect of ethanol on NREM and REM sleep latency (A). Numbers of NREM sleep bouts (B), total episode numbers (C), mean durations (D), and stage transitions (E) during the first 5 h after administration 3.0 g/kg ethanol. Values are mean ± SEM (n = 10). (A) *P < 0.05 or **P < 0.01 indicates significant differences assessed by one-way ANOVA followed by Fisher’s PLSD test. (B–E) *P < 0.05 or **P < 0.01 indicates significant differences performed using non-paired, two-tailed Student’s t test.
To better understand the changes in sleep architecture caused by 3 g/kg ethanol, we determined the episode number and mean duration of NREM sleep, REM sleep, and wakefulness, as well as stage transitions between the 3 vigilance stages. Ethanol at 3.0 g/kg increased the number of bouts of NREM sleep with durations of 230–950 s (Fig. 2B). There was no difference in the number of episodes of wakefulness, NREM sleep, and REM sleep (Fig. 2C). In addition, the mean duration of NREM sleep increased by 53% (Fig. 2D, P < 0.01), with a concomitant 50% decrease in wakefulness (Fig. 2D, P < 0.05). The mean transition numbers showed no change during the 5 h immediately following administration of ethanol (Fig. 2E). These results suggest that ethanol increases bouts of longer NREM sleep and mean duration of NREM sleep, which extend the overall duration of NREM sleep.
Pretreatment with caffeine offset ethanol-induced hypnotic effects
It is well known that caffeine induces wakefulness by blocking A2AR39. To determine whether caffeine reduces hypnotic effects of ethanol, caffeine (5, 10, or 15 mg/kg, i.p.) was administered to C57BL/6Slac mice 30 min prior to ethanol injection. As shown in Fig. 3A, pretreatment with caffeine at a dose of 10 mg/kg completely abolished the hypnotic effects induced by ethanol 3 g/kg when compared with their own control (F1,198 = 2.3, P = 0.15).
Sleep-wake profiles produced by ethanol administration with caffeine pretreatment in C57BL/6Slac mice. (A) Changes in NREM sleep, REM sleep, and wakefulness in mice treated with 10 mg/kg caffeine and 3.0 g/kg ethanol. (B) Dose-response effect on total time spent in NREM sleep, REM sleep, and wakefulness for 5 h after administration of vehicle and drugs. Values represent mean ± SEM (n = 10). (A) Comparisons of time course changes in the hourly amounts of each stages assessed by two-way ANOVA followed by Fisher’s PLSD test. (B) *P < 0.05 or **P < 0.01 indicates significant differences compared with their own control as assessed by two-tailed unpaired Student’s t test. The letters “a, b, c, d” indicate different subsets among doses of ethanol as assessed by one-way ANOVA followed by Duncan’s multiple range test.
Total time spent in NREM sleep, REM sleep, and wakefulness was calculated for 5 h after administration of caffeine and ethanol. Ethanol with vehicle pretreatment increased the duration of NREM sleep by 1.5-fold, which was consistent with a 25% decrease in wakefulness (Fig. 3B, P < 0.01). Caffeine alone increased the duration of wakefulness by 1.2-fold, which was consistent with a 32% decrease in NREM sleep (Fig. 3B, P < 0.05). Pretreatment with caffeine at 10 mg/kg or 15 mg/kg 30 min before ethanol did not increase the cumulative amount of NREM sleep when measured for 5 h after injection, compared with their own control. When caffeine was pretreated at 5 mg/kg, ethanol still increased the total duration of NREM sleep (Fig. 3B, P < 0.05). These findings indicate that pretreatment with caffeine dose-dependently reduces hypnotic effects of ethanol.
The latencies to NREM and REM sleep were also determined. As shown in Fig. 4A (upper panel), injection of caffeine with vehicle prolonged the latency to NREM sleep and injection of ethanol with vehicle pretreatment remarkably shortened NREM sleep latency. The latency to NREM sleep in mice treated with vehicle and ethanol was 12.5 min, which was markedly shorter than the latency of 24.8 min in vehicle control (P < 0.05). However, there were no significant differences in NREM latency in response to vehicle administration with vehicle pretreatment versus ethanol administration with caffeine pretreatment at 5, 10, and 15 mg/kg. Pretreatment with caffeine at 15 mg/kg 30 min before injection of ethanol abolished the decrease in NREM sleep latency induced by ethanol with vehicle pretreatment (Fig. 4A, upper panel). These results indicate that caffeine pretreatment counteracts initiation of NREM sleep induced by ethanol.
Changes in sleep latency and architecture produced by administration of ethanol with pre-treatment of caffeine. Effect of ethanol on NREM and REM sleep latency (A). Numbers of NREM sleep bouts (B), total episode numbers (C), mean durations (D), and stage transitions (E) during the first 5 h after administration of 10 mg/kg caffeine and 3.0 g/kg ethanol. Values are mean ± SEM (n = 10). (A)*P < 0.05 or **P < 0.01 indicates significant differences assessed by one-way ANOVA followed by Fisher’s PLSD test. (B–E) *P < 0.05 or **P < 0.01 indicates significant differences performed using two-tailed unpaired Student’s t test.
Following pretreatment with vehicle or caffeine (5, 10, and 15 mg/kg), the latencies to REM sleep in mice treated with ethanol were 233.1, 204.7, 225.8, and 259.7 min, respectively, which were longer than the latency of 60.1 min after vehicle administration with vehicle pretreatment. And caffeine 10 mg/kg with vehicle also prolonged the latency to REM sleep compared with vehicle control (Fig. 4A, lower panel). These results clearly indicate that caffeine pretreatment does not alter the increase in REM sleep latency induced by ethanol.
Analysis of sleep architecture showed that pretreatment with 10 mg/kg caffeine 30 min before administration of 3.0 g/kg ethanol decreased the number of bouts of NREM sleep with durations of 60–110 s (P < 0.05) and increased the number of bouts of NREM sleep with durations of 480–950 s (Fig. 4B, P < 0.01). The number of episodes of NREM sleep was reduced by 20% (Fig. 4C, P = 0.054), and the mean duration of NREM sleep increased by 55% (Fig. 4D, P < 0.01). These factors resulted in no difference in the total amount of NREM sleep. Furthermore, there were no differences in the mean duration of REM sleep and wakefulness (Fig. 4D). In contrast, ethanol with caffeine pretreatment reduced the episode number of wakefulness (Fig. 4C, P < 0.05), and the episode number of REM sleep did not change (Fig. 4C). The stage transition numbers showed no significant change following administration of ethanol with caffeine pretreatment (Fig. 4E).These results show that pretreatment with 10 mg/kg caffeine completely abolishes the hypnotic effects caused by 3.0 g/kg ethanol. However, caffeine does not completely block ethanol-induced impairment of sleep architecture.
Ethanol still increased NREM sleep and decreased wakefulness with post-treatment of caffeine
To determine whether post-treatment with caffeine alters the hypnotic effects of ethanol, we administered ethanol (3.0 g/kg) at 20:30, followed by caffeine (10 mg/kg) administration at 21:00 into C57BL/6Slac mice. Ethanol with post-treatment of caffeine still increased NREM sleep (F1,158 = 12.6, P < 0.05) and decreased wakefulness (F1,158 = 10.5, P < 0.05) compared with their own control (Fig. 5A). Ethanol at 3.0 g/kg increased NREM sleep at 20:30–21:00 before administration of caffeine (P < 0.01, Fig. 5A). With post-treatment of caffeine at 10 mg/kg, ethanol at 3.0 g/kg still significantly increased NREM sleep by 2.0- and 2.1-fold (P < 0.05) during the fourth and sixth hours after injection, respectively, compared with control, with decreases in wakefulness by 30% and 47% (P < 0.05), respectively (Fig. 5A). There was no further disruption of sleep architecture during the subsequent period. In addition, we calculated the total time spent in NREM sleep, REM sleep, and wakefulness for the 6-h period following administration. Vehicle with post-treatment of caffeine decreased the total amount of NREM sleep by 37%, with a 20% increase in wakefulness (Fig. 5B). However, ethanol with or without post-treatment of caffeine increased the total amount of NREM sleep by 1.5-fold and 1.3-fold, with a 17% and 14% decrease in wakefulness, respectively (Fig. 5B). There were no statistical differences between the two groups. These results indicate that post-treatment of caffeine can not offset ethanol-induced hypnotic effects.
Sleep-wake profiles produced by administration of ethanol with post-treatment of caffeine in C57BL/6Slac mice. (A) Changes in NREM sleep, REM sleep, and wakefulness in mice treated with 3.0 g/kg ethanol and 10 mg/kg caffeine. (B) Total time spent in NREM sleep, REM sleep, and wakefulness for 6 h after administration of vehicle and drugs. Values are mean ± SEM (n = 4–8). *P < 0.05 or **P < 0.01 indicates significant differences compared with their own control as assessed by two-way ANOVA followed by Fisher’s PLSD test (A), or by two-tailed unpaired Student’s t test with their own control and one-way ANOVA among groups (B).
Deletion of A2AR abolished the hypnotic effects of ethanol
To clarify the importance of A2AR in the hypnotic effects of ethanol, we used A2AR KO mice and their WT littermates. Ethanol given to A2AR WT mice at 3 g/kg significantly increased NREM sleep for 5 h (F1,106 = 30.7, P < 0.01) compared with their own control (Fig. 6A), which was consistent with a reduction in wakefulness (F1,106 = 30, P < 0.01). However, A2AR KO mice given 3 g/kg ethanol did not exhibit any significant change in duration of NREM sleep compared with their own control (Fig. 6B). In A2AR WT mice, for the first 5 h after the ethanol injection, the total duration of NREM sleep increased by 1.7-fold, which was consistent with a 30% decrease in wakefulness, compared with their own control. However, there were no differences in the duration of NREM sleep and wakefulness in the A2AR KO mice (Fig. 6C). Compared with the A2AR KO mice, ethanol increased NREM sleep by 1.3-fold and decreased wakefulness by 21% in A2AR WT mice (Fig. 6C). These results clearly indicate that A2AR is a key receptor involved in ethanol-induced hypnotic effects.
Sleep-wake profiles produced by administration of ethanol in A2AR WT and A2AR KO mice. (A,B) Changes in NREM sleep, REM sleep, and wakefulness in A2AR WT (A) and KO (B) mice treated with 3.0 g/kg ethanol. (C) Effect of ethanol on total time spent in NREM sleep, REM sleep, and wakefulness for 5 h after administration of vehicle and ethanol in A2AR WT and KO mice. Values are mean ± SEM (n = 8–9). (A,B) *P < 0.05 or **P < 0.01 indicates significant differences compared with their own control as assessed by two-way ANOVA followed by Fisher’s PLSD test. (C) **P < 0.01 indicates significant differences compared with their own control as assessed by two-tailed unpaired Student’s t test. P < 0.01 indicates significant differences between A2AR WT and KO mice performed using two-tailed unpaired Student’s t test.
Ethanol altered the power spectra of NREM sleep in mice
The delta activity (0.5–4 Hz) during NREM sleep is a reliable indicator of sleep need41,42. We evaluated the EEG power spectra and compared the power densities of vehicle and treatment in mice during NREM sleep in each experiments. The power of each 0.5 Hz bin was first averaged across the sleep stages individually and then normalized by calculating the relative duration of each bin from the total power (0–24.5 Hz) for each individual animal. As shown in Fig. 7A, compared with their own control, ethanol (3.0 g/kg) elevated delta power density in the frequency ranges of 0.5–2, 2.5–3 and 3.5–4 Hz during NREM sleep. With caffeine pretreatment, ethanol (3.0 g/kg) still elevated delta power density in the frequency ranges of 0.5–2 Hz during NREM sleep (Fig. 7B). And ethanol elevated delta power density in A2AR WT and A2AR KO mice during NREM sleep (Fig. 7C, D). These results indicated that ethanol increased delta power density, which could not completely offset by caffeine or genetic knockout of A2AR.
Characteristics of EEG power density of NREM sleep after administration of vehicle or treatment. (A) EEG power density curves during NREM sleep after administration of vehicle or ethanol in C57BL/6Slac mice. (B) EEG power density curves during NREM sleep after administration of vehicle or caffeine and ethanol in C57BL/6Slac mice. (C,D) EEG power density curves during NREM sleep after administration of vehicle or ethanol in A2AR WT (C) and A2AR KO (D) mice. Horizontal bars indicate location of a statistically significant difference. Values are mean ± SEM (n = 8–10). *P < 0.05 indicates significant differences compared with their own control as assessed by two-tailed unpaired Student’s t test.
Discussion
Ethanol consumption is an integral part of daily life in many societies, as it can produce positive mood states and has stress-relieving effects43. Furthermore, ethanol is one of the most commonly used “over the counter” sleep aids, although consuming large amounts of alcohol clearly has the potential for enormous detrimental impacts, including sleep disruption. However, the molecular mechanisms that underlie the hypnotic effects of ethanol remain poorly identified. In the present study, we found that ethanol dose-dependently increased NREM sleep by increasing the mean duration of NREM sleep and shortening the latency to NREM sleep, which is consistent with other studies4,6,44. In addition, ethanol dose-dependently prolonged the latency to REM sleep and decreased the amount of REM sleep. Taken together, these results indicate that ethanol affects sleep-wake behaviors by altering sleep architecture.
Acute intoxicating effects of ethanol may be related to GABA facilitation and glutamate inhibition, which are also critically involved in sleep-wake regulation6. However, the pharmacological effects of ethanol involve multiple mechanisms, so other targets may be relevant to the effects of ethanol. In the present study, 3.0 g/kg ethanol significantly increased NREM sleep for 5 h in WT mice. However, these hypnotic effects were completely abolished in adenosine A2AR KO mice, indicating that adenosine A2AR is essential for the hypnotic effects of ethanol.
In the central nervous system, adenosine is an important endogenous purine neuromodulator that modulates many important cellular processes in neurons. Adenosine is proposed to act as a homeostatic regulator of sleep in which levels in the brain rise during waking and decline during NREM sleep24. Extracellular levels of adenosine depend on rates of formation, degradation, and diffusion between intracellular and extracellular spaces45. It has been reported that ethanol inhibits the type 1 equilibrative nucleoside transporter, which reduces adenosine reuptake and thereby increases extracellular adenosine22, and adenosine synthesis is increased during ethanol metabolism46. Furthermore, ethanol may act directly in the brain to increase extracellular adenosine20. Ethanol-increased extracellular levels of adenosine may contribute to its hypnotic effects. We found that caffeine, an adenosine receptor antagonist, can abolish the hypnotic effects of ethanol. Taken together, these results suggest that adenosine is a key mediator in the hypnotic effects of ethanol.
The delta activity (0.5–4 Hz) during NREM sleep is a reliable indicator of sleep depth. Compared with their own control, ethanol increased the delta power in the mice, which means the NREM sleep induced by ethanol was not physiological sleep. Pharmacological antagonism or genetic deletion of A2AR did not reverse the increased delta power density induced by ethanol. It has been reported that GABA facilitation and glutamate inhibition induced by ethanol are critically involved in sleep-wake regulation6. However, the mechanism of delta power change induced by ethanol remains to be elucidated.
There are 4 adenosine receptor subtypes, all of which are G protein-coupled receptors. A1R and A3R are primarily coupled to the Gi family of G proteins, whereas A2AR and A2BR are mostly coupled to the Gs type of G proteins47. A2BR is expressed widely but generally at very low levels, whereas A3R is expressed at intermediate levels in the hippocampus and cerebellum48. Little is known about the functional significance of A2BR and A3R in sleep24. Accumulated evidence has indicated that A2AR rather than A1R plays a key role in the effects of adenosine on sleep24,25,26,27. Several studies suggest that A2AR stimulation may be involved in the reinforcing effects of ethanol49,50. Caffeine is a non-specific adenosine A1R and A2AR antagonist. We found that pretreatment with 10 mg/kg caffeine completely blocked the hypnotic effects of ethanol, which was consistent with results observed in A2AR KO mice. These data further indicate that A2AR plays an important role in the hypnotic effects of ethanol.
A2ARs are abundantly expressed in the striatum51. In our previous study, global genetic knockout of A2AR, but not A1R, abolished arousal effect of caffeine39, and local deletion of A2AR in ventral striatum blocked caffeine-induced wakefulness38, indicating ventral striatum A2AR mediate caffeine-induced wakefulness. Optogenetic or chemogentic activation of A2AR expressing medium spiny neurons in the ventral striatum robustly induced NREM sleep52. Alcohol induces adenosine release and decreases adenosine uptake, resulting in an increase extracellular level of adenosine. Taken together, we thought that caffeine can block alcohol-induced adenosine combining with striatum A2A receptors.
Adenosine, as a non-classical neurotransmitter, mediates several other behavioral effects of ethanol including ataxia16. Ataxia appeared to be mediated by an abnormal balance between excitatory and inhibitory neurotransmitters induced by ethanol in the brain. Adenosine has been shown to modulate both GABAergic and glutamatergic transmission, and therefore adenosine maybe involved in the disruption of excitatory-inhibitory balance induced by ethanol. Pharmacological studies have shown that adenosine mediates ethanol-induced ataxia primarily through A1R in the whole brain53,54,55, striatum56, cerebellum57, and motor cortex58. But A2AR located mainly in the striatum maybe also involved in mediating ethanol-induced ataxia with A2AR agonist and antagonist altering the ethanol-induced motor incoordination53. However, the role of adenosine receptors in ataxia and other ethanol-induced molecular and behavioral effects need more research.
There is a popular belief that coffee can offset the debilitating effects of alcoholic intoxication59,60. However, several other studies could not demonstrate that the antagonistic effects of caffeine reduced performance deficits induced by ethanol59,61. In our experiment, pretreatment with caffeine at 10 mg/kg 30 min before injection of ethanol offset the hypnotic effects of ethanol. However, the antagonistic effects of caffeine were reduced when injected 30 min after administration of ethanol. With post-treatment of caffeine at 10 mg/kg, ethanol at 3.0 g/kg still increased NREM sleep for 2 h after administration, which was consistent with a reduction in wakefulness for 2 h. The different antagonistic effects following pretreatment or post-treatment with caffeine may result from alterations in sleep propensity. When caffeine was administered post-treatment, ethanol induced high sleep propensity in the mice. During the first 2 h, the high concentration of caffeine could offset the hypnotic effects of ethanol. However, as caffeine was metabolized, the antagonistic effects were reduced, which resulted in a resurgence of ethanol-induced hypnotic effects. In contrast, pretreatment with caffeine induced hyperarousal before ethanol injection. During low sleep propensity, the impact of caffeine metabolism may be weakened, so caffeine might completely offset the hypnotic effects of ethanol. These results indicate that the antagonistic effects of caffeine depend on whether caffeine is administered before or after ethanol intake.
The antagonistic effects of caffeine observed in this study are consistent with previous human experimental studies showing that caffeinated drinks reduce subjective feelings of ethanol intoxication, which is known as the “masking effect”36,62. Consumption of ethanol mixed with drinks may lead to subjective drunkenness induced by hypnotic effects, which can be delayed by caffeine during the early period. This results in an increase in ethanol consumption. Therefore, the intoxicating effects of ethanol mixed with caffeinated drinks are expected to be more severe, with a 3-fold greater risk of being legally intoxicated, in an accident, and injured during the subsequent period34,62. In this study, pretreatment with caffeine could reduce the hypnotic effects of ethanol, which suggests that combining ethanol and caffeinated drinks may be harmful. This is in agreement with an announcement by the U.S. Food and Drug Administration stating that caffeine is an unsafe food additive when combined with alcohol. Thus, the present results indicate that ingestion of caffeine-containing drinks with ethanol consumption is a high-risk behavior. Furthermore, A2AR is the most important target of caffeine63. A2AR gene KO reduced hypnotic effects of ethanol, which indicates that A2AR plays an important role in the mechanisms underlying the “masking effect”.
Our results indicate that ethanol dose-dependently promotes NREM sleep in mice, and A2AR mediates hypnotic effects of ethanol.
Methods
Animals
Male SPF inbred C57BL/6Slac mice (weighing 20–26 g, 11–13 weeks old) were obtained from Shanghai Laboratory Animal Center (SLAC), Chinese Academy of Sciences (Shanghai, China). C57BL/6Slac mouse is a substrain of C57BL/6J, which is got from the Jackson laboratory and inbred in SLAC laboratory. A2AR KO and WT littermates of the inbred C57BL/6 strain were generated from heterozygotes (provided by Boston University School of Medicine, Boston, MA). The animals were housed individually at a constant temperature (22 ± 0.5°C), with a relative humidity of 60 ± 2%, on an automatically controlled 12 h light/dark cycle (lights on at 07:00, illumination intensity ≈100 lux)64, and with free access to food and water. All efforts were made to minimize animal suffering and use only the number of animals required for production of reliable scientific data. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Fudan University Committee on Animal Care and Use.
Polygraphic recording and vigilance state analysis
Under pentobarbital anesthesia (50 mg/kg, i.p.), mice were chronically implanted with EEG and EMG electrodes for polysomnographic recordings as described previously65,66,67,68. One implant, which served as EEG electrodes, consisted of 2 stainless steel screws (1 mm diameter) inserted through the skull into the cortex (anteroposterior, +1.0 mm and left-right, −1.5 mm from bregma or lambda) according to the atlas of Franklin and Paxinos69. The other implant, which served as EMG electrodes, consisted of 2 insulated stainless steel, Teflon-coated wires bilaterally placed into both trapezius muscles. All electrodes were attached to a microconnector and fixed to the skull with dental cement.
After EEG/EMG implantation, mice were housed individually in transparent barrels for 7 d to eliminate the pain and stress from surgery. Then, mice were connected to cables and given 4 d to adapt in an insulated soundproof recording chamber before EEG/EMG recording. The animals then entered the pharmacological phase of the study. Sleep-wakefulness states were monitored for a period of 48 h, which comprised baseline and experimental days. Cortical EEG and EMG signals were amplified, filtered (EEG, 0.5–30 Hz; EMG, 20–200 Hz), digitized at a sampling rate of 128 Hz, and recorded using SLEEPSIGN (Kissei Comtec, Nagano, Japan) as described previously39,70. When completed, polygraphic recordings were automatically scored off-line in 10 s epochs as wakefulness, REM sleep, and NREM sleep by SLEEPSIGN according to standard criteria39,71. As a final step, defined sleep-wake stages were examined visually and corrected if necessary.
Pharmacological treatments
Ethanol (20% v/v, Sigma-Aldrich, Saint Louis, MO) was administered i.p. at 21:00 on the day of the experiment at doses of 2.1, 2.5, 3.0, or 3.6 g/kg. For baseline data, mice were injected i.p. with vehicle. To test the receptor mechanism, mice were pretreated with caffeine (Alfa Aesar, Ward Hill, MA) i.p. at 5, 10 or 15 mg/kg before ethanol injection. In a separate group, mice were post-treated with 10 mg/kg caffeine 30 min after ethanol administration. The drugs were freshly prepared prior to use, and injection volume (10 mL/kg) was kept constant.
Statistical analysis
Comparisons of time course changes in the hourly amounts of each stages in C57BL/6Slac mice treated with ethanol or vehicle, and the hourly amounts of each stages in C57BL/6Slac mice treated with combination of caffeine and ethanol or vehicle, and the hourly amounts of each stages in A2AR WT/KO mice treated with ethanol or vehicle were assessed using two-way ANOVA followed by Fisher’s least-significant difference (PLSD) test. Histograms of the amounts of sleep and wakefulness were assessed by one-way ANOVA followed by Duncan’s multiple range test. Significance of NREM and REM latency was assessed by one-way ANOVA followed by PLSD test. Comparisons of sleep amounts, as well as number of sleep/wake events, duration and transition of sleep/wake events between vehicle and treatment were performed using a non-paired, two-tailed Student’s t test. The EEG power spectra were compared using two-way ANOVA followed by PLSD.
References
El, Y. M. et al. Caffeine Reduces Hypnotic Effects of Alcohol through Adenosine a2a Receptor Blockade. Neuropharmacology. 45, 977–985 (2003).
Pesta, D. H., Angadi, S. S., Burtscher, M. & Roberts, C. K. The Effects of Caffeine, Nicotine, Ethanol, and Tetrahydrocannabinol On Exercise Performance. Nutr Metab (Lond). 10, 71 (2013).
Ferreira, M. P. & Willoughby, D. Alcohol Consumption: The Good, the Bad, and the Indifferent. Appl Physiol Nutr Metab. 33, 12–20 (2008).
Thakkar, M. M., Sharma, R. & Sahota, P. Alcohol Disrupts Sleep Homeostasis. Alcohol. 49, 299–310 (2015).
Rehm, J. et al. Global Burden of Disease and Injury and Economic Cost Attributable to Alcohol Use and Alcohol-Use Disorders. Lancet. 373, 2223–2233 (2009).
Roehrs, T. & Roth, T. Sleep, Sleepiness, Sleep Disorders and Alcohol Use and Abuse. Sleep Med Rev. 5, 287–297 (2001).
Brower, K. J. et al. Insomnia, Self-Medication, and Relapse to Alcoholism. Am J Psychiatry. 158, 399–404 (2001).
Colrain, I. M., Turlington, S. & Baker, F. C. Impact of Alcoholism On Sleep Architecture and Eeg Power Spectra in Men and Women. Sleep. 32, 1341–1352 (2009).
Brower, K. J. & Perron, B. E. Prevalence and Correlates of Withdrawal-Related Insomnia Among Adults with Alcohol Dependence: Results From a National Survey. Am J Addict. 19, 238–244 (2010).
Brower, K. J. Insomnia, Alcoholism and Relapse. Sleep Med Rev. 7, 523–539 (2003).
Porkka-Heiskanen, T. et al. Adenosine: A Mediator of the Sleep-Inducing Effects of Prolonged Wakefulness. Science. 276, 1265–1268 (1997).
Thakkar, M. M., Engemann, S. C., Sharma, R. & Sahota, P. Role of Wake-Promoting Basal Forebrain and Adenosinergic Mechanisms in Sleep-Promoting Effects of Ethanol. Alcohol Clin Exp Res. 34, 997–1005 (2010).
Sharma, R., Sahota, P. & Thakkar, M. M. Role of Adenosine and the Orexinergic Perifornical Hypothalamus in Sleep-Promoting Effects of Ethanol. Sleep. 37, 525–533 (2014).
Ruby, C. L. et al. Adenosinergic Regulation of Striatal Clock Gene Expression and Ethanol Intake During Constant Light. Neuropsychopharmacol (2014).
Nam, H. W. et al. Adenosine Transporter Ent1 Regulates the Acquisition of Goal-Directed Behavior and Ethanol Drinking through a2a Receptor in the Dorsomedial Striatum. J Neurosci. 33, 4329–4338 (2013).
Dunwiddie, T. V. & Masino, S. A. The Role and Regulation of Adenosine in the Central Nervous System. Annu Rev Neurosci. 24, 31–55 (2001).
Naassila, M., Ledent, C. & Daoust, M. Low Ethanol Sensitivity and Increased Ethanol Consumption in Mice Lacking Adenosine a2a Receptors. J Neurosci. 22, 10487–10493 (2002).
Clasadonte, J. et al. Chronic Sleep Restriction Disrupts Sleep Homeostasis and Behavioral Sensitivity to Alcohol by Reducing the Extracellular Accumulation of Adenosine. J Neurosci. 34, 1879–1891 (2014).
Fredholm, B. B. & Wallman-Johansson, A. Effects of Ethanol and Acetate On Adenosine Production in Rat Hippocampal Slices. Pharmacol Toxicol. 79, 120–123 (1996).
Sharma, R., Engemann, S. C., Sahota, P. & Thakkar, M. M. Effects of Ethanol On Extracellular Levels of Adenosine in the Basal Forebrain: An in Vivo Microdialysis Study in Freely Behaving Rats. Alcohol Clin Exp Res. 34, 813–818 (2010).
Krauss, S. W., Ghirnikar, R. B., Diamond, I. & Gordon, A. S. Inhibition of Adenosine Uptake by Ethanol is Specific for One Class of Nucleoside Transporters. Mol Pharmacol. 44, 1021–1026 (1993).
Nagy, L. E. et al. Ethanol Increases Extracellular Adenosine by Inhibiting Adenosine Uptake Via the Nucleoside Transporter. J Biol Chem. 265, 1946–1951 (1990).
Choi, D. S. et al. The Type 1 Equilibrative Nucleoside Transporter Regulates Ethanol Intoxication and Preference. Nat Neurosci. 7, 855–861 (2004).
Huang, Z. L., Urade, Y. & Hayaishi, O. The Role of Adenosine in the Regulation of Sleep. Curr Top Med Chem. 11, 1047–1057 (2011).
Huang, Z. L., Zhang, Z. & Qu, W. M. Roles of Adenosine and its Receptors in Sleep-Wake Regulation. Int Rev Neurobiol. 119, 349–371 (2014).
Satoh, S., Matsumura, H., Suzuki, F. & Hayaishi, O. Promotion of Sleep Mediated by the a2a-Adenosine Receptor and Possible Involvement of this Receptor in the Sleep Induced by Prostaglandin D2 in Rats. Proc Natl Acad Sci USA 93, 5980–5984 (1996).
Scammell, T. E. et al. An Adenosine a2a Agonist Increases Sleep and Induces Fos in Ventrolateral Preoptic Neurons. Neuroscience. 107, 653–663 (2001).
Urade, Y. et al. Sleep Regulation in Adenosine a2a Receptor-Deficient Mice. Neurology. 61, S94–S96 (2003).
Hong, Z. Y. et al. An Adenosine a2a Receptor Agonist Induces Sleep by Increasing Gaba Release in the Tuberomammillary Nucleus to Inhibit Histaminergic Systems in Rats. J Neurochem. 92, 1542–1549 (2005).
Stenberg, D. et al. Sleep and its Homeostatic Regulation in Mice Lacking the Adenosine a1 Receptor. J Sleep Res. 12, 283–290 (2003).
Fredholm, B. B. et al. Actions of Caffeine in the Brain with Special Reference to Factors that Contribute to its Widespread Use. Pharmacol Rev. 51, 83–133 (1999).
Azcona, O., Barbanoj, M. J., Torrent, J. & Jane, F. Evaluation of the Central Effects of Alcohol and Caffeine Interaction. Br J Clin Pharmacol. 40, 393–400 (1995).
Kunin, D. et al. Caffeine Promotes Ethanol Drinking in Rats. Examination Using a Limited-Access Free Choice Paradigm. Alcohol. 21, 271–277 (2000).
Ferre, S. & O’Brien, M. C. Alcohol and Caffeine: The Perfect Storm. J Caffeine Res. 1, 153–162 (2011).
Oteri, A., Salvo, F., Caputi, A. P. & Calapai, G. Intake of Energy Drinks in Association with Alcoholic Beverages in a Cohort of Students of the School of Medicine of the University of Messina. Alcohol Clin Exp Res. 31, 1677–1680 (2007).
Ulbrich, A. et al. Effects of Alcohol Mixed with Energy Drink and Alcohol Alone On Subjective Intoxication. Amino Acids. 45, 1385–1393 (2013).
Benson, S., Verster, J. C., Alford, C. & Scholey, A. Effects of Mixing Alcohol with Caffeinated Beverages On Subjective Intoxication: A Systematic Review and Meta-Analysis. Neurosci Biobehav Rev. 47, 16–21 (2014).
Lazarus, M. et al. Arousal Effect of Caffeine Depends On Adenosine a2a Receptors in the Shell of the Nucleus Accumbens. J Neurosci. 31, 10067–10075 (2011).
Huang, Z. L. et al. Adenosine a2a, but Nota1, Receptors Mediate the Arousal Effect of Caffeine. Nat Neurosci. 8, 858–859 (2005).
Xu, Q. et al. A Mouse Model Mimicking Human First Night Effect for the Evaluation of Hypnotics. Pharmacol Biochem Behav. 116, 129–136 (2014).
Vyazovskiy, V. V. & Tobler, I. The Temporal Structure of Behaviour and Sleep Homeostasis. Plos One. 7, e50677 (2012).
Anaclet, C. et al. The Gabaergic Parafacial Zone is a Medullary Slow Wave Sleep–Promoting Center. Nat Neurosci. 17, 1217–1224 (2014).
Spanagel, R. Alcoholism: A Systems Approach From Molecular Physiology to Addictive Behavior. Physiol Rev. 89, 649–705 (2009).
Ebrahim, I. O., Shapiro, C. M., Williams, A. J. & Fenwick, P. B. Alcohol and Sleep I: Effects On Normal Sleep. Alcohol Clin Exp Res. 37, 539–549 (2013).
Dunwiddie, T. V. The Physiological Role of Adenosine in the Central Nervous System. Int Rev Neurobiol. 27, 63–139 (1985).
López-Cruz, L., Salamone, J. D. & Correa, M. The Impact of Caffeine On the Behavioral Effects of Ethanol Related to Abuse and Addiction: A Review of Animal Studies. J Caffeine Res. 3, 9–21 (2013).
Chen, J. F., Eltzschig, H. K. & Fredholm, B. B. Adenosine Receptors as Drug Targets–What are the Challenges? Nat Rev Drug Discov. 12, 265–286 (2013).
Yaar, R., Jones, M. R., Chen, J. F. & Ravid, K. Animal Models for the Study of Adenosine Receptor Function. J Cell Physiol. 202, 9–20 (2005).
Mailliard, W. S. & Diamond, I. Recent Advances in the Neurobiology of Alcoholism: The Role of Adenosine. Pharmacol Ther. 101, 39–46 (2004).
Thorsell, A., Johnson, J. & Heilig, M. Effect of the Adenosine a2a Receptor Antagonist 3,7-Dimethyl-Propargylxanthine On Anxiety-Like and Depression-Like Behavior and Alcohol Consumption in Wistar Rats. Alcoholism Clin Exp Res. 31, 1302–1307 (2007).
Rosin, D. L. et al. Immunohistochemical Localization of Adenosine a2a Receptors in the Rat Central Nervous System. J Comp Neurol. 401, 163–186 (1998).
Oishi Y et al. Slow-Wave Sleep is Controlled by a Subset of Nucleus Accumbens Core Neurons in Mice. Nat Commun., in press, doi: 10.1038/s41467-017-00781-4 (2017).
Dar, M. S. Modulation of Ethanol-Induced Motor Incoordination by Mouse Striatal a(1) Adenosinergic Receptor. Brain Res Bull. 55, 513–520 (2001).
Connole, L., Harkin, A. & Maginn, M. Adenosine a1 Receptor Blockade Mimics Caffeine’s Attenuation of Ethanol‐Induced Motor Incoordination. Basic Clin Pharmacol. 95, 299–304 (2004).
Dar, M. S. & Mustafa, S. J. Acute Ethanol/Cannabinoid-Induced Ataxia and its Antagonism by Oral/Systemic/Intracerebellar a 1 Adenosine Receptor Antisense in Mice. Brain Res. 957, 53–60 (2002).
Meng, Z. & Dar, M. S. Intrastriatal Ro15-4513 Functionally Antagonizes Ethanol-Induced Motor Incoordination and Striatal Adenosinergic Modulation of Ethanol-Induced Motor Incoordination in Rats. J Pharmacol Exp Ther. 271, 524–534 (1994).
Dar, M. S. Ethanol-Induced Cerebellar Ataxia: Cellular and Molecular Mechanisms. The Cerebellum. 14, 447–465 (2015).
Barwick, V. S. & Dar, M. S. Adenosenergic Modulation of Ethanol-Induced Motor Incoordination in the Rat Motor Cortex. Prog Neuropsychopharmacol Biol Psych. 22, 587–607 (1998).
Drake, C. L. et al. Caffeine Reversal of Ethanol Effects On the Multiple Sleep Latency Test, Memory and Psychomotor Performance. Neuropsychopharmacol. 28, 371–378 (2003).
Hirvonen, J., Jaaskelainen, I. P., Naatanen, R. & Sillanaukee, P. Adenosine a1/a2a Receptors Mediate Suppression of Mismatch Negativity by Ethanol in Humans. Neurosci Lett. 278, 57–60 (2000).
Smith, A. P. Effects of Caffeine and Alcohol On Mood and Performance Changes Following Consumption of Lager. Psychopharmacology. 227, 595–604 (2013).
Marczinski, C. A. Can Energy Drinks Increase the Desire for More Alcohol? Adv Nutr. 6, 96–101 (2015).
Nam, H. W., Bruner, R. C. & Choi, D. Adenosine Signaling in Striatal Circuits and Alcohol Use Disorders. Mol Cells. 36, 195–202 (2013).
Zhang, Z. et al. Red Light at Intensities Above 10 Lx Alters Sleep–Wake Behavior in Mice. Light Sci. Appl. 6, e16231 (2017).
Chen, L. et al. Basal Forebrain Cholinergic Neurons Primarily Contribute to Inhibition of Electroencephalogram Delta Activity; Rather than Inducing Behavioral Wakefulness in Mice. Neuropsychopharmacol. 41, 2133–2146 (2016).
Qu, W. M. et al. Dopaminergic D1 and D2 Receptors are Essential for the Arousal Effect of Modafinil. J Neurosci. 28, 8462–8469 (2008).
Qu, W. M. et al. Essential Role of Dopamine D2 Receptor in the Maintenance of Wakefulness, but Not in Homeostatic Regulation of Sleep, in Mice. J Neurosci. 30, 4382–4389 (2010).
Wang, Q. et al. Morphine Inhibits Sleep-Promoting Neurons in the Ventrolateral Preoptic Area Via Mu Receptors and Induces Wakefulness in Rats. Neuropsychopharmacol. 38, 791–801 (2013).
Franklin, K. Paxinos G. The Mouse Brain in Stereotaxic Coordinates.: Academic Press, San Diego (1997).
Qu, W. M. et al. Lipocalin-Type Prostaglandin D Synthase Produces Prostaglandin D2 Involved in Regulation of Physiological Sleep. Proc Natl Acad Sci USA 103, 17949–17954 (2006).
Huang, Z. L. et al. Arousal Effect of Orexin a Depends On Activation of the Histaminergic System. Proc Natl Acad Sci USA 98, 9965–9970 (2001).
Acknowledgements
This study was supported in part by grants-in-aid for scientific research from the National Natural Science Foundation of China (31530035, 81420108015, 31671099, 31471064, 81471344, and 31421091); the National Basic Research Program of China (2015CB856401); and the Shanghai Committee of Science and Technology (14JC1400900).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Teng Fang, Zhi-Li Huang. Performed the experiments: Teng Fang, Hui Dong, Xin-Hong Xu. Analyzed the data: Teng Fang, Hui Dong, Xiang-Shan Yuan, Ze-Ka Chen. Contributed A2AR KO mice: Jiang-Fan Chen. Wrote the paper: Teng Fang, Wei-Min Qu, Zhi-Li Huang.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, T., Dong, H., Xu, XH. et al. Adenosine A2A receptor mediates hypnotic effects of ethanol in mice. Sci Rep 7, 12678 (2017). https://doi.org/10.1038/s41598-017-12689-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12689-6
This article is cited by
-
A cluster of mesopontine GABAergic neurons suppresses REM sleep and curbs cataplexy
Cell Discovery (2022)
-
Exploration of chalcones and related heterocycle compounds as ligands of adenosine receptors: therapeutics development
Molecular Diversity (2022)
-
Ethanol Induces Sedation and Hypnosis via Inhibiting Histamine Release in Mice
Neurochemical Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.