Abstract
Female to male sex reversal was achieved in an emerging agricultural insect pest, Drosophila suzukii, by creating a temperature-sensitive point mutation in the sex-determination gene, transformer-2 (tra-2), using CRISPR/Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated) homology-directed repair gene-editing. Ds-tra-2 ts2 mutants developed as normal fertile XX and XY adults at permissive temperatures below 20 °C, but at higher restrictive temperatures (26 to 29 °C) chromosomal XX females developed as sterile intersexuals with a predominant male phenotype, while XY males developed with normal morphology, but were sterile. The temperature-dependent function of the Ds-TRA-2ts2 protein was also evident by the up- and down-regulation of female-specific Ds-Yolk protein 1 (Ds-Yp1) gene expression by temperature shifts during adulthood. This study confirmed the temperature-dependent function of a gene-edited mutation and provides a new method for the more general creation of conditional mutations for functional genomic analysis in insects, and other organisms. Furthermore, it provides a temperature-dependent system for creating sterile male populations useful for enhancing the efficacy of biologically-based programs, such as the sterile insect technique (SIT), to control D. suzukii and other insect pest species of agricultural and medical importance.
Similar content being viewed by others
Introduction
The role of the transformer-2 (tra-2) sex determination gene in Drosophila melanogaster, and the temporal specificity of the hierarchal sex-determination gene pathway was elucidated in part by the selection of two tra-2 temperature-sensitive mutant alleles induced by chemical mutagenesis, which allowed its function and downstream effects to be controlled by ambient temperature1,2. Two missense point mutations, resulting in Ala151Val (GCC > GTC) for tra-2 ts1 and Pro181Ser (CCA > TCA) for tra-2 ts2, apparently result in temperature-labile conformational changes in the polypeptide at the elevated temperature of 29 °C that eliminate function, while normal function is maintained at 16 °C. The importance of these amino acids is also reflected in their conservation in tra-2 cognates found in other drosophilids, tephritids, a lepidopteran and two hymenopteran species3, that include the Ala161 and Pro191 peptides in the spotted winged drosophila, D. suzukii 4.
Notably, the ability to manipulate TRA-2 protein function during development provided the initial insight that its function, and thus, that of the sex-determination gene hierarchy, was required continuously for somatic female-specific differentiation, and in the transient absence of TRA-2, the default state of male differentiation would result1,2. The notion that this sex-specific control extended past metamorphosis, and was required throughout adulthood after completion of morphological differentiation, was supported by experiments using female-specific yolk protein (Yp) gene expression as a sex-specific biochemical and molecular marker during adulthood. By subjecting XX; tra-2 ts flies to shifts in ambient temperature it was discovered that Yp gene expression and protein synthesis could be diminished at 29 °C and re-initiated when shifted back to 16 °C. This showed that tra-2 function acted continuously in adult females to maintain Yp synthesis, and thus, female-specific processes throughout adulthood5.
In addition to the fundamental knowledge of how sex-determination genes control sex-specific development and behavior, the tra-2 ts2 studies, in particular, also presented an ideal strategy for generating a sterile male population that could be of use to the sterile insect technique (SIT)6, a highly effective biologically-based means of controlling the population size of a variety of insect pest species7,8,9. A cornerstone of SIT is the mass release of sterile males that, in sufficient numbers, outcompete wild males in the field for female mating thereby rendering them non-reproductive. Limitations to these programs has generally been the need for sexing in early development to eliminate the high cost of rearing females and the need to sterilize and release them with males in the absence of sexing, and the debilitating somatic effects of irradiation used for sterilization10.
The tra-2 ts2 mutation effectively addresses both of these limitations as demonstrated in D. melanogaster, where homozygous male and female tra-2 ts2 mutants reared at 16 °C develop normally and are fertile, but when reared at 29 °C, chromosomal XX females develop as sterile phenotypic (or pseudo) males that exhibit courtship behavior (observed in XX; tra-2 ts1/tra-2 def)11. XY chromosomal males develop normally at the restrictive temperature, but are sterile due to requisite TRA-2 function in the germ-line12,13. Thus, a tra-2 ts2 mutant strain could provide a normal breeding population at the permissive temperature, whose progeny reared at the restrictive temperature would all develop as sterile males for release. Two important benefits of this strategy are that: i) no other treatment other than elevated temperature would be required for both sexing and male sterility in drosophilid pests, such as D. suzukii (though other non-drosophilid species may require an independent sterility system for XY; tra-2 ts2 males), and ii) a smaller breeding population may be sufficient to generate the same number of phenotypic sterile males required for release, substantially increasing efficiency and cost effectiveness.
The ability to incorporate this strategy into insect pest species having an orthologous tra-2 gene, however, has been hindered by the need to generate the same mutation for a mass reared strain in the absence of the genetic tools available for D. melanogaster, including visible selectable markers and balancer chromosomes. Germ-line transformation could be used to introduce an in vitro mutagenized tra-2 ts allele, though targeted mutagenesis would be required to eliminate function of the native wild type alleles that would complement the mutant loss of function. These restrictions can be largely overcome by gene-editing techniques that allow a gene conversion or replacement of the native wild type tra-2 allele for a mutated tra-2 ts allele, in conjunction with incorporating a dominant fluorescent protein visible marker for ease of selection and rearing14. To demonstrate the feasibility of this approach in an insect pest species, we have created the Ds-tra-2 ts2 mutation by CRISPR/Cas9 editing in the spotted wing drosophila, D. suzukii. While this species is not an ideal subject for tra-2 ts-based sex-reversal, since it is not highly viable at optimal restrictive temperatures15, temperature-dependent phenotypes of morphological and biochemical sex reversal, and male and female sterility were demonstrated. Thus, this model for temperature-dependent sex reversal and sterility provides proof of concept for further testing in other species subject to control by SIT, and as a method for generating conditional alleles for functional genomic analysis in non-model species.
Results
Creation of the Ds-tra-2 ts2 mutation
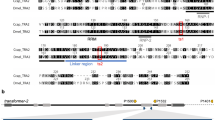
CRISPR/Cas9 gene editing was performed using a homology-directed repair (HDR) strategy (Fig. 1a). Two sgRNAs were used as scissors, and a donor construct provided a template including the putative Ds-tra-2 ts2 point mutation, the IE1hr5-DsRed marker gene, and two homologous arms upstream and downstream of the cutting site (~1 kb each). One sgRNA targeted Ds-tra-2 at a position 449 bp 5′ upstream of the mutation site and the other targeted the proximal downstream sequence to Ds-tra-2, predicted to be the biogenesis of lysosome-related organelles complex 1 subunit 1 gene (GenBank: XM_017072992). To bias editing towards HDR, the ligase 4 gene, a component of the non-homologous end joining (NHEJ) repair pathway, was suppressed using dsRNA-mediated RNA interference (RNAi). A mixture of sgRNAs, Cas9 protein, Ds-ligase-4 dsRNA and donor construct was injected into pre-blastoderm embryos of a D. suzukii wild strain, yielding 81 G0 adult flies (36 males and 45 females). Of these, 55 flies were fertile after individual backcrosses to wild type, with four G0 lines yielding G1 progeny expressing whole body red fluorescence, resulting in a germ-line transmission rate of 7.3% (4/55) based on marker expression. From each G0 line, two to five red fluorescent G1 flies (Fig. 1c) were selected to establish independent G1 lines. After being inbred to homozygosity, six lines were analyzed by PCR and sequencing showing that all lines had an insertion of the IE1hr5-DsRed marker gene, however, only three lines (F26m1, F26f4 and M36f1, from two G0 lines) carried the correct Ds-tra-2 ts2 (CCT > TCT) mutation while the other three lines could not be confirmed, resulting in a minimum mutation rate of 3.6% (2/55).
Strategy for generating the Ds-tra2 ts2 mutation and IE1hr5-DsRed marker insertion via CRISPR/Cas9 homology-directed repair. (a) Schematic (not to scale) showing the wild type Ds-tra-2 gene and downstream HA2 (homologous arm 2) sequence with expected recombination sites (X) for the Cas9/sgRNA complexes (top). Dotted lines from these sites extend to the donor construct recombination sites that include the HA1 Ds-tra-2 sequence mutagenized in vitro to create a Pro191Ser substitution (vertical red line) resulting in the mutated Ds-TRA2ts2 temperature sensitive protein, in addition to the IE1hr5-DsRed marker gene and HA2 sequence. The recombined donor construct resulting in the genomic insertion of the Ds-tra2 ts2 mutation and marker gene is shown below. (b) Ventral view of a homozygous Ds-tra2 ts2 adult XY male (left) and XX female (right) reared at 16 °C under brightfield, and (c) DsRed epifluorescence illumination. See Methods for further details.
Temperature sensitivity of Ds-TRA-2 protein function based on adult morphology and fertility
To determine the effect of temperature on the mutated Ds-TRA-2 protein in terms of morphological sexual differentiation in chromosomal females and males, the homozygous mutant line, M36f1, was first reared continuously at the expected permissive temperature of 16 °C, in addition to 20 °C. This resulted in normal development and fertility in all XX females and XY males at both temperatures (Fig. 1b; Table 1). However, when rearing was performed at the expected restrictive temperature of 29 °C, all mutants died during late pupation, as did wild type control flies. Rearing at 27 °C and 28 °C also resulted in inviability for the mutant line and a low survival rate for wild type (1–2%), with low levels of survival (5–10%) for both wild type and mutant flies first observed at 26 °C. At this temperature 80 surviving chromosomal female XX; Ds-tra-2 ts2 homozygotes exhibited an intersexual, though predominantly male (or pseudo-male), phenotype (Fig. 2c and f), but male-specific wing spots were not apparent (Fig. 2c). However, in a separate experiment where XX; Ds-tra-2 ts2 individuals (n = 21), were reared at 22 °C and shifted to 29 °C as late pupae, wing spot pigmentation was observed at 3 days after eclosion (Fig. 2c inset; Supplementary Table S1). Compared to mature ovaries from WT females (Fig. 2i), XX; Ds-tra-2 ts2 pseudo-males had a single poorly differentiated gonadal lobe (Fig. 2j) and no internal male reproductive organs, similar to XX; Dm-tra-2 ts2 flies reared at 29 °C1. At 26 °C pseudo-males were also sterile with fully differentiated male-specific foreleg sex combs (Fig. 3c) and male-specific pigmentation on tergite 6 (Fig. 3f), while tergite 5 lacked male-specific pigmentation and female-specific tergite 7 was observed. The differing sexual identity of the abdominal tergites, as well as other sex-specific tissues and structures, can most simply be attributed to a variable sensitivity to Ds-TRA-2 function that was diminished in pseudo-males reared at a sub-optimal restrictive temperature.
Phenotypic comparison of wild type and mutant flies. Ventral view of an adult D. suzukii wild type male (a), WT female (b), and a homozygous XX; Ds-tra2 ts2 mutant pseudo-male reared at 26 °C (red coloration is from the DsRed marker protein) (c). Lower right insets show an enlarged region of the posterior wing where male-specific wing spot pigmentation is present in the WT male (a), absent in the WT female (b), and is apparent in another XX mutant pseudo-male shifted from 26 °C to 29 °C at pharate adulthood (but absent when maintained at 26 °C) (c). An enlarged ventral posterior view of the WT male (d), WT female (e), and pseudo-male (f) showing the genital terminalia apparatus. Gonads dissected from adult flies reared at 26 °C including WT male testes (g), XY; Ds-tra2 ts2 male dysmorphic testes (h), WT female ovaries (i), and an XX; Ds-tra2 ts2 pseudo-male dysmorphic gonad (j).
Comparison of sex combs and abdominal pigmentation in wild type and mutant flies. Sex-specific differentiation of forelegs and dorsal abdominal cuticle in a wild type male (a and d), WT female (b and e), and an XX; Ds-tra2 ts2 pseudo-male (c and f) reared at 26 °C. The regions for foreleg sex comb bristle formation (a–c) are indicated by arrows, and abdominal tergite segments (d–f) are numbered. Orientation of the forelegs relative to the body are distal at the top and proximal at the bottom of the panels. Scale bars shown for (a–c) (50 µm) and (d–f) (200 µm).
A detailed examination of the external genitalia revealed that pseudo-males exhibited terminalia similar to WT males (Fig. 4), including the anterior paramere (clasper), posterior paramere, aedeagus and anal plates (Fig. 4c). However, the basal apodeme of the aedeagus was not apparent (Fig. 4a) and the female-specific sternite 8 was observed, while the female-specific spermatheca was not observed. At 26 °C, XY; Ds-tra-2 ts2 chromosomal males had normal external morphology, but were sterile consistent with poorly differentiated abnormal testes that had a small number of immotile sperm (n = 15, Fig. 2h, Supplementary Video S1), compared to the long spiral testes containing motile sperm found in WT males (Fig. 2g, Supplementary Video S2).
Comparison of genital apparatus in wild type and mutant flies. Genital terminalia of a wild type male (a), WT female (b) and an XX; Ds-tra2 ts2 pseudo-male (c) reared at 26 °C. Abbreviations for indicated structures in each panel: anterior paramere (clasper; A), aedeagus (E), anal plate (B), posterior paramere (P), basal apodeme of the aedeagus (O), vaginal plate (V), female-specific sternite 8 (N), and spermatheca (S). Sex specific structures are labeled in blue for male, red for female, and black for non-sex-specific. Scale bar: 100 µm.
To more precisely determine the temperature sensitivity of Ds-TRA-2 function in the mutant line, adult morphology and fertility were assessed in XY and XX; Ds-tra-2 ts2 adults that developed at rearing temperatures ranging from 16 °C to 26 °C (Table 1). As noted, at the permissive temperatures of 16–20 °C both XX females and XY males were morphologically normal (Fig. 1b) and fertile, but at 22 °C XX females first exhibited male sex combs and were sterile based on a failure to oviposit (n = 61), while WT females oviposited ≥78 fertile eggs/female (n ≥ 39) when reared at 20–24 °C (Table 1). Increased levels of male morphology occurred with increasing temperature, with nearly complete male morphology at 26 °C. XY males were fertile at 25 °C and below, with some exhibiting dysmorphic testes and sterility at 24–25 °C, that included all males above 25 °C. These results are consistent with Ds-TRA-2 function, or the quantity of functional protein, diminishing just above permissive temperature, with an increasing loss of Ds-TRA-2 activity with rising temperature. The sex-specificity of somatic tissue and germ-line function appeared to be more highly sensitive to loss of Ds-TRA-2 activity in XX females (i.e., onset of sex comb differentiation and sterility) than the influence on testis morphology and germ-line function (i.e., fertility) in XY males where the onset of defects were first detected at a higher temperature.
Complete sterility in XY; Ds-tra-2 ts2 males could be achieved by rearing at 25.5 °C or above, but viability at these temperatures was severely limited. Thus, XY; Ds-tra-2 ts2 males were reared at 22 °C until pharate adulthood or adult eclosion resulting in viability of 80 to 90%, and shifted to 26 °C and/or 29 °C to determine if these late shifts to restrictive temperature would result in sterility (Supplementary Table S1). Sterility was achieved in 90 to 100% of the males, with greater sterility in those that were shifted earlier and to the higher temperature, although this resulted in lower viability.
Mating behavior of mutant intersexes and pseudo-males
In D. melanogaster, temperature sensitive male courtship behavior and mating in XX individuals required a nearly null Dm-tra-2 genotype that used the stronger Dm-tra-2 ts1 allele over a Dm-tra-2 deficiency11. For XX; Ds-tra-2 ts2 pseudo-males, flies reared at 26 °C (n = 20), and shifted to 29 °C for 3d at eclosion from 26 °C (n = 15) or as late pupae (n = 23) did not attempt courtship or mating with wild type virgin females, while XY; Ds-tra-2 ts2 males reared at 26 °C (n = 22) did mate, but were sterile. At 22 °C, XX; Ds-tra-2 ts2 females developed female genital terminalia and did not attempt courtship with WT virgin females (n = 29), but were courted by WT and XY; Ds-tra-2 ts2 males who were rejected (n = 20).
Temperature sensitivity of Ds-TRA-2 function based on terminal sex-specific Yp1 expression during adulthood
Similar to studies on XX; Dm-tra-2 ts2 in D. melanogaster 5, the influence of Ds-TRA-2 activity on molecular and biochemical aspects of terminal sexual differentiation could be explored by applying temperature shifts to mutant adults. For D. suzukii in particular, the ability of adults to survive at 29 °C, after rearing at 26 °C or below, also allowed a more definitive test of the optimal restrictive temperature on Ds-TRA-2ts2 protein function. This was achieved by assessing the effect of temperature and temperature shifts on the sex specific expression of the D. suzukii yolk protein 1 (Ds-Yp1) gene in Ds-tra-2 ts2 mutants that, in D. melanogaster, is normally expressed only in female adult fat body and the ovarian follicular epithelium beginning at adult eclosion16,17.
To measure Ds-Yp1 transcript levels by qPCR, Ds-Yp1 was first identified by its similarity to D. melanogaster Yp1, having 94.3% amino acid sequence identity (Supplementary Fig. S1), providing primer sites for analysis. Initial qPCR tests for the primers showed that transcript levels were similar in XX WT and F23f1 control females, which were >20-fold higher than transcript in XX; Ds-tra-2 ts2 pseudo-male adults reared at 26 °C (Supplementary Fig. S2). For experimental tests, Ds-Yp1 transcripts were measured in XX; Ds-tra-2 ts2 (M36f1) phenotypic females and WT females reared and then maintained at 16 °C until 3 d, 6 d, and 9 d after eclosion (Fig. 5a; Supplementary Table S2). Transcript levels were not significantly different between the mutant and WT females at these time points, though transcript increased between days 3 and 6, and then decreased in both WT and XX; Ds-tra-2 ts2 females by day 9, though more so in M36f1 females (P > 0.05, two-tailed, no significant difference at all time points; Supplementary Table S2). In contrast, when mutant and WT females reared at 16 °C were shifted to 29 °C at eclosion, after 3 days WT females expressed >27-fold higher levels of Ds-Yp1 transcript compared to XX; Ds-tra-2 ts2 females (Fig. 5b). Decreasing levels of transcript occurred in both groups at 6 d and 9 d, with negligible transcript detected in mutant females after 9 d at 29 °C. To determine whether the basal transcript level observed in mutant females at 29 °C for 3 days could be reversed to a more normal female phenotype by turning on Ds-TRA-2 function, sibling flies were shifted to 16 °C at 3 d and assayed at days 6 and 9 after eclosion (Fig. 5c). At 6 d the Ds-Yp1 transcript level in WT females decreased ~40% (presumably due to a slowed metabolism), but increased ~9-fold in XX; Ds-tra-2 ts2 females, with similar levels maintained in both groups at 9 d (P > 0.05, two-tailed). However, if females at 6 d at 16 °C were shifted to 29 °C until 9 d, transcript levels decreased by half in WT females, but were barely detectable in the XX; Ds-tra-2 ts2 females, decreasing by >40-fold from day 6 (P < 0.05, two-tailed; Fig. 5d).
Response of Ds-Yp1 transcript expression to temperature. Ds-Yp1 transcript expression (relative to internal Ds-His3 controls) measured by qPCR from wild type females (WT-f, blue bars), XX; Ds-tra2 ts2 females (M36f1, red bars), and wild type males (WT-m, green bars) in response to indicated temperature and temperature shifts during adulthood. All flies were reared at 16 °C until adult eclosion, and time points indicate days from eclosion and temperatures maintained for the previous three days. Graphs show transcript levels from adults continuously reared at 16 °C (graph a) and 29 °C (graph b); adults reared from 0–3 days at 29 °C (3d-29 °C; from graph b) and then shifted to 16 °C for an additional 3 days (6d-16 °C) and 6 days (9d-16 °C) (graph c); or shifted to 29 °C at 6 days (9d-29 °C) (graph d; sibling samples from 3d and 6d in graph c shown for comparison). Each bar represents the mean fold change from three biological replicates ± SD relative to sample M36f1 at 3d-29 °C. Asterisks (*) above M36f1 samples indicate t-test significance (two-tailed) compared to WT-f at the same time point: ***P < 0.001; *0.01 < P < 0.05. Unmarked M36f1 data are not significantly different from WT-f. Transcript levels and P values are also presented in Supplementary Table S2.
These results showed that adult XX; Ds-tra-2 ts2 phenotypic females reared at 16 °C exhibit normal female expression of Ds-Yp1, but when sibling females were shifted from 16 °C to 29 °C, low diminished transcript levels resulted reaching non-detectable levels, normally expected in XY males, by day 9. Furthermore, Ds-Yp1 transcript levels increased and decreased according to shifts in temperature to permissive and restrictive conditions, respectively, indicating that the dynamic, and reversible, expression of Ds-Yp1 during adulthood is continuously regulated by Ds-TRA-2 function. Presumably this function is mediated by the more direct effect of Ds-TRA/Ds-TRA-2 interactions on the female-specific expression of Ds-dsx (GenBank: XM_017077018.1)4, as demonstrated in D. melanogaster 20.
Discussion
Here we report the use of CRISPR/Cas9 gene editing in D. suzukii to create a conditional temperature sensitive allele of the tra-2 sex determination gene, Ds-tra-2 ts2, analogous to the chemically induced Dm-tra-2 ts2 missense point mutation in D. melanogaster 1. This was achieved by comparing amino acid sequence identity for TRA-2 in both species (Supplementary Fig. S3), and identifying the common peptides that are substituted in Dm-tra-2 ts1 and Dm-tra-2 ts2 1,18. Since Dm-tra-2 ts2 was shown to exhibit better fertility than Dm-tra-2 ts1 at permissive temperatures1,18, its mutation site was the initial choice for editing in D. suzukii for this study, though the tra-2 ts1 allele might also present advantages for application, or additional insights into function.
Similar to Dm-tra-2 ts2, XX chromosomal females homozygous for Ds-tra-2 ts2 reared at permissive temperatures of 16–20 °C resulted in development of normal fertile females, while rearing at a restrictive temperature of 26 °C resulted in a sterile, though incomplete pseudo-male phenotype. The lack of full male phenotypic differentiation, unlike XX; Dm-tra-2 ts2 pseudo-males reared at 29 °C, was most likely the result of rearing D. suzukii at a lower sub-optimal restrictive temperature, since this species fails to complete pupal development when reared above 26 °C. Thus, complete loss of Ds-TRA-2 function at 26 °C was unlikely, though distinct male morphological differentiation was apparent based on complete foreleg sex comb development, posterior dorsal tergite 6 pigmentation, and wing spot pigmentation when shifted to 29 °C in early adulthood. The genital apparatus structures were intersexual, but predominantly male-like, and similar to XX; Dm-tra-2 ts2 pseudo-males, the gonads of XX; Ds-tra-2 ts2 pseudo-males were dysmorphic, possibly intersexual, and did not produce motile sperm1,19. Conversely, XY; Ds-tra-2 ts2 males reared at 26 °C exhibited normal male external morphology and mated, but were also sterile with dysmorphic testes consistent with the tra-2 null phenotype in D. melanogaster that is known to interfere with male germ-line development12,13. A notable difference between the tra-2 ts2 lines in the two species is that Dm-tra-2 ts2 XX females and XY males were highly sensitive to loss of Dm-TRA-2 activity, becoming sterile at 18 °C18, while Ds-tra-2 ts2 XX and XY individuals exhibited less sensitivity, becoming sterile at 22 °C and 26 °C, respectively.
While D. suzukii could not develop past late pupation at 29 °C, surviving adults at 26 °C or below could be reared at 29 °C, allowing the sex-specificity of downstream transcriptional products in adults to be assessed in response to Ds-TRA-2ts2 function at the optimal restrictive temperature. This allowed a more accurate comparison of TRA-2 function in D. suzukii and D. melanogaster and its downstream regulation of sexual differentiation. In D. melanogaster, functional TRA-2 acts in concert with the transformer gene product to facilitate the female-mode of doublesex alternative intron-splicing, resulting in DSX-Female function throughout adulthood20. The continuous need for control by the sex-determination gene pathway throughout female development was, indeed, first inferred by experiments with Dm-tra-2 ts2 that showed that female-specific Yp transcription and synthesis in adults could be up- and down-regulated by manipulating temperature-dependent Dm-TRA-2 function5. For Ds-TRA-2ts2, we showed that Yp gene expression could be similarly regulated by temperature such that XX; Ds-tra-2 ts2 pseudo-males reared at 16 °C and shifted to 29 °C at adult eclosion exhibited basal Yp1 transcription levels, that could be transiently up-regulated to levels comparable to WT females after a shift to 16 °C, and subsequently down-regulated to basal levels by a shift to 29 °C. Together, these studies confirmed the expressivity and temperature-dependent conditional function of the gene-edited Ds-tra-2 ts2 mutation that also provided support for the continuous, and reversible, requirement for Ds-TRA-2 in both females and males for adult sexual differentiation and fertility throughout development.
Beyond providing a conditional system to better understand how sex-determination genes control sexual differentiation and behavior, the Drosophila tra-2 ts studies also present a highly efficient strategy for generating a sterile male population for SIT6. For this, a tra-2 ts mutant strain reared at permissive temperature would result in a normal breeding population of fertile males and females, whose chromosomal male and female progeny all develop as sterile phenotypic males at the restrictive temperature. Thus, ideally, a tra-2 ts mutant strain could provide a complete sterile male population in response to a shift in temperature, and provide the significant efficiency of all zygotes being used for release, requiring a considerably smaller breeding population.
Current control of D. suzukii does not include SIT21, and the temperature-sensitive sexing system described is less than optimal for this species due to post-larval inviability at 29 °C required for complete XX female sex reversal. However, XY; Ds-tra-2 ts2 males are sterile but sexually active at ambient temperatures above 25 °C, and could be reared at lower temperatures for improved viability, yet sterilized when shifted to higher restrictive temperatures as pharate adults. These sterile males could directly compete with wild males in the field, though the ability of their sibling XX; Ds-tra-2 ts2 pseudo-males to provide mating competition remains uncertain. Regardless of their ability to mate with females in the field, chromosomal females that develop as phenotypic males or intersexuals would still preclude the need to eliminate or sterilize these individuals before release, unlike normal females, since they would not require sterilization, nor compete with females in the field for mating with released sterile males, or cause oviposition damage to host plants. Thus, the programmatic and cost advantages of Ds-tra-2 ts2-based sexing and sterility might still provide a level of efficiency that would support its incorporation into prospective SIT programs for D. suzukii. Nevertheless, the fertility and mating behavior of both XX and XY mutant adults would require further testing to ensure the efficacy of this system in response to temperature fluctuations under actual field conditions.
This demonstration of temperature-dependent sex reversal established by gene-editing in D. suzukii also supports the notion that this could be accomplished in other insect species having conserved tra-2 cognate function and sequence identity. This is especially so for several tephritid fruit fly species, such as Ceratitis capitata (medfly), Anastrepha ludens (mexfly), and A. suspensa (caribfly), which have conserved sites for the TRA-2 Alanine and Proline peptides substituted in the tra-2 ts1 and tra-2 ts2 mutations, respectively3,22. Importantly, sex reversal of chromosomal females by RNAi-based knock-down of the tra and tra-2 cognates, resulting in XX pseudo-males that copulate with wild females, has already been demonstrated for medfly and caribfly22,23. Given that these tropical insects thrive at ambient temperatures of 29 °C or above, it is likely that full male differentiation could be achieved in XX; tra-2 ts individuals with the potential for successful mating with females in the field. However, the RNAi studies also showed that while tra-2 knock-down in XX caribflies resulted in sterile pseudo-males, XY males remained fertile, and in medfly both XX pseudo-males and XY males remained fertile. Thus, for tephritid species tra-2 ts males reared at restrictive temperatures might remain fertile, requiring an independent conditional system for male sterility, such as the tetracycline-dependent embryonic lethality system described for medfly and caribfly24,25.
In summary, proof of principle has been demonstrated in D. suzukii for the creation of a conditional temperature-sensitive allele by gene-editing that most likely can be extended to other temperature-sensitive missense mutations, and in a wide range of species known to have an orthologous gene26. That a very broad range of genes can be edited in this way in insects is supported by D. melanogaster mutational studies that showed that greater than 10% of X-linked lethal and semi-lethal mutations are temperature-sensitive27. For the tra-2 gene in particular, that is conserved among several orders of insects, synthetic creation of temperature-sensitive mutations has significant potential for detailed comparative functional analyses and, for pest species, enhancing the efficacy of programs to control their population size. While this and other possible applications remain prospective, the ability to create conditional alleles in a large number of genes known to be important to many aspects of reproduction, development and behavior should have a highly significant impact on our understanding of genome function and evolution.
Methods
All data generated or analysed during this study are included in this published article (and its Supplementary Information files). All sequence files are available from the GenBank database (accession numbers MF142759, MF142760, MF142761 and MF142762) after publishing of this work.
Fly rearing, fertility and mating tests
A wild type strain of D. suzukii originally collected in Wimauma, Florida, was reared under standard laboratory conditions as described28. Gene-edited transgenic homozygous flies were reared at 16 °C ± 0.2 °C (EchoTherm™IN40 incubator, Torrey Pines Scientific, Inc.), and tested at indicated temperatures ( ± 0.2 °C) for temperature sensitivity after 24 h and 48 h of egg collection at 16 °C. Fertility tests were performed by mating each mutant male to five wild type virgin females (n ≥ 15) and each mutant female to three WT males (n ≥ 20) followed by egg collections for 12–15 days. Progeny were reared to adulthood and screened for red fluorescence. Mating tests were performed by combining previously unmated 3 d adult flies for observation of courtship behavior including body orientation, wing vibration, licking and copulation for 6 h at mid-day (1000–1600), and for 4 h beginning at dawn (approx. 0700) of the following day.
Mutation generation via CRISPR/Cas9
The Ds-tra-2 gene was identified by a Blast search using Dm-tra-2 mRNA (GenBank: NM_057416.3) as a query, and was identified in contig1010 from the D. suzukii genome (GenBank: XM_017071951)4. The putative Ds-tra-2 ts2 mutation site was localized to nt 571 in the CDS (GenBank: MF142759) based on alignment with the Dm-tra-2 ts2 amino acid sequence1,18, and confirmed with primers AH1001/AH1002 by RT-PCR and sequencing. Oligonucleotide primers were designed with Geneious® 8.0.5 (Biomatters Ltd.) software and listed in Supplementary Table S3 with annealing conditions. sgRNA targets were identified by a manual search for NGG proto-spacer adjacent motif (PAM) sequences. Two targets were identified having high GC content within the six PAM-proximal nucleotides (PAMPNs)29 and less similarity to other genomic sequences to improve efficiency and avoid off-target effects. sgRNAs were synthesized as previously described30 using primers AH992, AH1053 and AH1054 (Supplementary Table S3). Their specificities were confirmed by embryonic injections and Surveyor tests (Supplementary Fig. S2).
To create the donor construct, a 3.6 kb fragment from D. suzukii genomic DNA was cloned using Q5® High-Fidelity DNA Polymerase (New England Biolabs) with primers AH1020/AH1021, which included two homologous arms of more than 1 kb upstream and downstream from the two sgRNA target sites. The Ds-tra-2 ts2 point mutation was introduced by primers AH1027/AH1028. To avoid cleavage of the donor construct and mutagenesis after integration by CRISPR/Cas9, two single-nucleotide synonymous substitutions (G > C for sgRNA1 site; G > A for sgRNA2 site) were introduced into the two sgRNA target site PAM sequences, respectively, since an intact PAM sequence is necessary for successful double strand breakage31. Homologous arm 1 (HA1) was assembled using the GeneArt® Seamless Cloning and Assembly Kit (Thermo Fisher Scientific) from three fragments, which were amplified with primer pairs AH1026/AH1027, AH1028/AH1029, and AH1030/AH1031. HA2 was assembled from two fragments amplified with primer pairs AH1032/AH1033 and AH1034/AH1035 with the Ds-tra-2 ts2 mutation and PAM substitutions confirmed by sequencing. Next, a fragment containing the IE1hr5-DsRed/SV40 marker gene was amplified from the piggyBac transformation vector plasmid, pB{IE1hr5-DsRed/SV40} with primers AH1044/AH1045, and the complete HA1 and HA2 fragments were amplified using primer pairs AH1026/AH1043 and AH1035/AH1046. The DsRed marker, HA1 and HA2 were then assembled with the GeneArt® Kit and confirmed by sequencing.
The D. suzukii Ds-ligase4 gene (GenBank: XM_017079597)4 was identified by Blastn alignment similarity to Dm-ligase4 from D. melanogaster (GenBank: NM_132679.3; protein alignment of the conceptual translation of these sequences shown in Supplementary Fig. S4). The online SnapDragon tool (http://www.flyrnai.org/snapdragon) was used to design a 421 bp dsRNA fragment whose sequence was confirmed after PCR amplification with primers AH1003/AH1004 (GenBank: MF142760). The dsRNA was then synthesized as described previously22,32.
Embryo micro-injection
Pre-blastoderm embryos were injected with a mixture of 400 ng/µl Cas9 protein (PNA Bio Inc), 75 ng/µl sgRNA1, 75 ng/µl sgRNA2, 100 ng/µl donor plasmid and 350 ng/µl Ds-ligase4 dsRNA using D. suzukii germ-line transformation injection methods28. Putative G1 transformant adults were selected by DsRed epifluorescence (TxRed filter: ex: 560/40; em: 610 LP; Chroma) that were backcrossed to wild type with G2 homozygous lines established by successive inbreeding, selection by marker fluorescence intensity, and verification by outcrosses to wild type. Genomic DNA of homozygous flies was extracted with ZR Genomic DNA™-Tissue MicroPrep (Zymo Research) with the edited regions amplified with primer pairs AH1093/AH986 and AH987/AH1094 and sequenced for molecular confirmation.
Cuticle preparation
Flies were dissected in phosphate-buffered saline (PBS) solution with forelegs, abdominal and terminal cuticles incubated in 10% NaOH at 80 °C for 30 minutes to remove soft tissue, rinsed with PBS and mounted in Acrytol Mounting Media (Electron Microscopy Sciences) for imaging.
Real-time qPCR
Total RNA was isolated with the ZR Tissue & Insect RNA MicroPrep™ kit (Zymo Research), followed by cDNA synthesis from 1 μg RNA using the iScript™ cDNA Synthesis Kit (Bio-Rad). The Ds-Yp1 (GenBank: XM_017081211) and Ds-His3 (GenBank: XM_017082962) genes were identified by a Blastn search of the D. suzukii genome4 with their D. melanogaster cognates (GenBank: NM_078548.3 for Yp1 and Flybase: CG33866-RA for His3). Primer pairs AH1133/AH1134 and AH1135/AH1136 were used to amplify their partial CDS (GenBank: MF142761 for Ds-Yp1, and MF142762 for Ds-His3) whose sequence identities were verified by Blastx and ClustalW33 alignments (Supplementary Figs S1 and S5). Primers AH1139/AH1140 and AH1137/AH1138 were designed to compare the expression levels of Ds-Yp1 to the internal control Ds-His3 gene, respectively, using a CFX96 Touch™ Real-Time time PCR Detection System (Bio-Rad). 100 ng cDNA was added into iQ™ SYBR® Green Supermix (Bio-Rad). The 2−∆∆Ct method was used with three biological replicates for each sample at each time point34, with all data normalized to the relative Yp1/His3 expression of sample M36f1 at 3 d–29 °C (mean = 0.2), and the Student t-test was performed to compare differences.
References
Belote, J. M. & Baker, B. S. Sex determination in Drosophila melanogaster: analysis of transformer-2, a sex-transforming locus. Proc. Natl. Acad. Sci. USA 79, 1568–72 (1982).
Belote, J. M. & Lucchesi, J. C. Male-specific lethal mutations of Drosophila melanogaster. Genetics 96, 165–86 (1980).
Sarno, F. et al. The gene transformer-2 of Anastrepha fruit flies (Diptera, Tephritidae) and its evolution in insects. BMC Evol. Biol. 10, 140 (2010).
Chiu, J. C. et al. Genome of Drosophila suzukii, the Spotted Wing Drosophila. G 3 3, 2257–71 (2013).
Belote, J. M., Handler, A. M., Wolfner, M. F., Livak, K. J. & Baker, B. S. Sex-specific regulation of yolk protein gene expression in Drosophila. Cell 40, 339–48 (1985).
Handler, A. M. Prospects for using genetic transformation for improved SIT and new biocontrol methods. Genetica 116, 137–49 (2002).
Knipling, E. F. Possibilities of Insect Control or Eradication Through the Use of Sexually Sterile Males. J. Econ. Entomol. 48, 459–62 (1955).
Knipling, E. F. Sterile-male method of population control. Science 130, 902–4 (1959).
Vreysen, M., Robinson, A. & Hendrichs, J. Area-wide control of insect pests: from research to field implementation (Springer, 2007).
Hooper, G. H. Sterilization of the Mediterranean fruit fly with gamma radiation: effect on male competitiveness and change in fertility of females alternately mated with irradated and untreated males. J. Econ. Entomol. 65, 1–6 (1972).
Belote, J. M. & Baker, B. S. Sexual behavior: its genetic control during development and adulthood in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 84, 8026–30 (1987).
Belote, J. M. & Baker, B. S. The dual functions of a sex determination gene in Drosophila melanogaster. Dev. Biol. 95, 512–7 (1983).
Schüpbach, T. Autosomal mutations that interfere with sex determination in somatic cells of Drosophila have no direct effect on the germline. Dev. Biol. 89, 117–27 (1982).
Housden, B. E., Lin, S. & Perrimon, N. Cas9-based genome editing in Drosophila. Methods Enzymol. 546, 415–39 (2014).
Tochen, S. et al. Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ. Entomol. 43, 501–10 (2014).
Garabedian, M. J., Hung, M. C. & Wensink, P. C. Independent control elements that determine yolk protein gene expression in alternative Drosophila tissues. Proc. Natl. Acad. Sci. USA 82, 1396–400 (1985).
Handler, A. M. & Postlethwait, J. H. Endocrine control of vitellogenesis in Drosophila melanogaster: Effects of the brain and corpus allatum. J. Exp. Zool. 202, 389–402 (1977).
Amrein, H., Maniatis, T. & Nöthiger, R. Alternatively spliced transcripts of the sex-determining gene tra-2 of Drosophila encode functional proteins of different size. EMBO J. 9, 3619–29 (1990).
Fujihara, T., Kawabe, M. & Oishi, K. A sex-transformation gene in Drosophila melanogaster. J. Hered. 69, 229–36 (1978).
Tian, M. & Maniatis, T. A splicing enhancer complex controls alternative splicing of doublesex pre-mRNA. Cell 74, 105–14 (1993).
Haye, T. et al. Current SWD IPM tactics and their practical implementation in fruit crops across different regions around the world. J. Pest Sci. (2004). 89, 643–51 (2016).
Schetelig, M. F., Milano, A., Saccone, G. & Handler, A. M. Male only progeny in Anastrepha suspensa by RNAi-induced sex reversion of chromosomal females. Insect Biochem. Mol. Biol. 42, 51–7 (2012).
Salvemini, M. et al. Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. Int. J. Dev. Biol. 53, 109–20 (2009).
Schetelig, M. F., Caceres, C., Zacharopoulou, A., Franz, G. & Wimmer, E. A. Conditional embryonic lethality to improve the sterile insect technique in Ceratitis capitata (Diptera: Tephritidae). BMC Biol. 7, 4 (2009).
Schetelig, M. F. & Handler, A. M. Strategy for enhanced transgenic strain development for embryonic conditional lethality in Anastrepha suspensa. Proc. Natl. Acad. Sci. USA 109, 9348–53 (2012).
Housden, B. E. et al. Loss-of-function genetic tools for animal models: cross-species and cross-platform differences. Nat. Rev. Genet. 18, 24–40 (2017).
Suzuki, D. T. et al. Temperature-sensitive mutations in Drosophila melanogaster,I. Relative frequencies among gamma-ray and chemically induced sex-linked recessive lethals and semilethals. Proc. Natl. Acad. Sci. USA 57, 907–12 (1967).
Schetelig, M. F. & Handler, A. M. Germline transformation of the spotted wing drosophilid, Drosophila suzukii, with a piggyBac transposon vector. Genetica 141, 189–93 (2013).
Ren, X. et al. Enhanced Specificity and Efficiency of the CRISPR/Cas9 System with Optimized sgRNA Parameters in Drosophila. Cell Rep. 9, 1151–62 (2014).
Bassett, A. R., Tibbit, C., Ponting, C. P. & Liu, J. L. Highly Efficient Targeted Mutagenesis of Drosophila with the CRISPR/Cas9 System. Cell Rep. 4, 220–8 (2013).
Mojica, F. J. M., Díez-Villaseñor, C., García-Martínez, J. & Almendros, C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology 155, 733–40 (2009).
Posnien, N. et al. RNAi in the red flour beetle (Tribolium). Cold Spring Harb. Protoc. 2009, pdb.prot5256 (2009).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–80 (1994).
Livak, K. J. & Schmittgen, T. D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–8 (2001).
Acknowledgements
We thank Shelley Olson, Sara Brennan and G. Patrick Whitmer for technical assistance. This project was supported by the USDA-AFRI Foundational Program competitive grant no. 2016-67013-25087 from the USDA National Institute of Food and Agriculture (to A.M.H.).
Author information
Authors and Affiliations
Contributions
J.L. and A.M.H. designed the research, analyzed data and wrote the paper, and J.L. performed the research and contributed new reagents.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Handler, A.M. Temperature-dependent sex-reversal by a transformer-2 gene-edited mutation in the spotted wing drosophila, Drosophila suzukii . Sci Rep 7, 12363 (2017). https://doi.org/10.1038/s41598-017-12405-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12405-4
This article is cited by
-
Sequence and expression analysis of the spermatogenesis-specific gene cognates, wampa and Prosα6T, in Drosophila suzukii
Genetica (2023)
-
A transgenic female killing system for the genetic control of Drosophila suzukii
Scientific Reports (2021)
-
Identification and characterization of four Drosophila suzukii cellularization genes and their promoters
BMC Genetics (2020)
-
Precise single base substitution in the shibire gene by CRISPR/Cas9-mediated homology directed repair in Bactrocera tryoni
BMC Genetics (2020)
-
Near-chromosome level genome assembly of the fruit pest Drosophila suzukii using long-read sequencing
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.