Abstract
The beneficial role of estrogen in the vascular system may be due, in part, through reduction of peripheral vascular resistance. The use of estrogen therapy to prevent cardiovascular disease in post-menopausal women remains contentious. This study investigated the influence of aging and the menopause on the acute vasodilatory effects of estrogen using ex vivo human and murine resistance arteries. Vessels were obtained from young (2.9 ± 0.1 months) and aged (24.2 ± 0.1 and 28.9 ± 0.3 months) female mice and pre- (42.3 ± 0.5 years) and post-menopausal (61.9 ± 0.9 years) women. Aging was associated with profound structural alterations of murine uterine arteries, including the occurrence of outward hypertrophic remodeling and increased stiffness. Endothelial and smooth muscle function were diminished in uterine (and tail) arteries from aged mice and post-menopausal women. The acute vasodilatory effects of 17β-estradiol (non-specific estrogen receptor (ER) agonist), PPT (ERα-specific agonist) and DPN (ERβ-specific agonist) on resistance arteries were attenuated by aging and the menopause. However, the impairment of estrogenic relaxation was evident after the occurrence of age-related endothelial dysfunction and diminished distensibility. The data indicate, therefore, that chronological resistance arterial aging is a prominent factor leading to weakened vasodilatory action of estrogenic compounds.
Similar content being viewed by others
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death worldwide1. Age is the biggest determinant of an individual’s cardiovascular health and since the number of older people is growing substantially, there is a need to develop a greater understanding of the influence of age on the cardiovascular system2. There are also marked differences in the prevalence of CVD in men and women; prior to 50 years of age, the average age of menopause onset, the risk is considerably lower in women but thereafter the incidence is similar3,4. Post-menopausal deprivation of female sex hormones, primarily estrogen, may be related to the increased CVD risk in aging women5.
Estrogen binds to two receptors, ERα and ERβ, which can then initiate ligand-activated transcription factor signaling6. However, estrogen can also induce acute arterial vasodilation separate from genomic-directed actions7,8,9,10,11,12,13. It has been proposed that estrogen activates endothelial nitric oxide synthase (and thus NO production), endothelial prostacyclin and endothelium-derived hyperpolarizing (EDH) factor, which act on adjacent smooth muscle cells to induce relaxation7,8,9,10,11,12,13. Indeed, it has been suggested that the contribution of EDH is greater in females due to the presence of estrogen14,15,16. Further to acting through the classical ERs, estrogen has been demonstrated to act through the unrelated G-protein-coupled estrogen receptor (GPER1) to induce vasodilation17,18,19,20,21. In large arteries, the effects of estrogen are considered to protect against inflammatory-related disorders such as atherosclerosis, whereas in the resistance vasculature, the acute vasodilatory actions may lower peripheral resistance and protect against hypertension22.
Consistent with the proposed cardiovascular protective role of estrogen, early observational studies, such as the Nurses’ Health Study, suggested estrogen therapy might reduce the risk of CVD in post-menopausal women23. However, randomized clinical trials largely failed to show any cardiovascular benefits of menopausal hormone therapy (MHT) in post-menopausal women, with guidelines concluding that MHT should not be used for CVD prevention24,25,26. However, subsequent re-analysis of the Women’s Health Initiative data revealed a trend for a lower risk of coronary heart disease in hormone-treated women from the youngest age group. This suggested the possibility of a ‘timing’ hypothesis wherein MHT initiated within 10 years of the menopause could be beneficial to CVD health27,28,29. Indeed, a recent study suggested there to be some beneficial cardiovascular effects of estradiol treatment in early (<6 years) menopausal compared to late (≥ 10 years) menopausal women30. Recent evidence has also highlighted the role for aging per se, independent of menopausal status, in female cardiovascular risk31,32. Thus, it remains an important objective to elucidate the relative contributions of aging and the menopause, and the influence of estrogenic compounds, on vascular function.
Therefore, in the present study we have sought to determine the contributions of age or the menopause on arterial function and estrogenic responsiveness in vitro. To do so we employed two experimental models of arterial vascular function; uterine and tail arteries from young (3 months) and old (24 and 29 months) mice, to determine the influence of age, and uterine arteries from non-pregnant women ranging in age from 32–75 years, to assess the influence of menopause and/or age.
Results
Aging negatively regulates murine uterine arterial function and passive structural characteristics
Prior to testing the influence of age on estrogenic responsiveness of murine resistance arteries, we sought to determine the role of aging on vasoactive and structural properties of these vessels. We utilized mice from the C57BL background (C57BL/Ircfa) from 3 separate age groups (Table 1). These mice remain generally healthy into old age with no strain-specific pathological conditions33,34,35,36. We chose to study two different aged groups (24 and 29 months) in order to assess the influence of advanced age on resistance artery structure and function, since it was not often possible to obtain samples from very old women. This 5-month difference is equivalent of human aging from 69 to 80 years of age37 and, therefore, from an observational standpoint one would expect an acceleration of age-related dysfunction. Indeed, percent survival of this mouse strain decreases considerably in this timespan36.
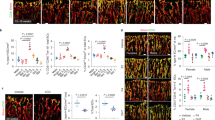
Vasoactive responses to the thromboxane agonist U46619 (U4) and the endothelium-dependent vasodilator acetylcholine (ACh), and the passive structural responses to increasing intravascular pressure (illustrated in Fig. 1) were assessed. Both U4-induced constriction (maximum contractions for 3-month-old: 20.7 ± 0.8 kPa, 24-month-old: 16.7 ± 0.9 kPa and 29-month-old: 15.4 ± 1.2 kPa, Fig. 1A) and endothelium-dependent vasodilation (maximum relaxations for 3-month-old: 55.1 ± 1.8%, 24-month-old: 42.1 ± 2.4% and 29-month-old: 42.9 ± 3.6%, Fig. 1B) were attenuated in aged mice.
Aging alters the vasoactive and passive structural characteristics of murine uterine arteries. For the vasoactive experiments, uterine arteries from 3- (n = 26), 24- (n = 10), and 29-month-old (n = 5) mice were mounted on a wire myograph and exposed to the thromboxane agonist U4 (10−6M, A). Following steady-state constriction, endothelium-dependent relaxation was assessed by the addition of acetylcholine (10−5M, B). *P < 0.05 from young mice (ordinary one-way ANOVA). For the assessment of passive structural characteristics, separate uterine arteries from 3- (n = 5), 24- (n = 7), and 29-month-old (n = 4) mice were mounted on a pressure myograph and measurements of (C) vessel diameter and wall thickness were used to calculate (D) wall: lumen ratio, (E) cross-sectional area (CSA), (F) vessel stress and (G) strain at each pressure step (0–120 mmHg) in calcium-free conditions. P < 0.05 from 24-month-old mice (Ø) or 29-month-old mice (∆) (repeated measures two-way ANOVA). Vessel stress was plotted against vessel strain to assess the stress: strain relationship, which is used to ascertain arterial distensibility (H). E = Elastic modulus. *P < 0.05 from young mice (unpaired t-test). Data are presented as mean ± SEM.
The effect of aging on passive structural characteristics was more complex. There was no difference in the passive lumen diameter of uterine arteries from 3- and 24-month-old mice. However, further aging to 29 months increased the passive diameter of uterine arteries (Fig. 1C), which was associated with an increase in cross-sectional area (CSA) (Fig. 1E). There was also a slight, but non-statistically significant, decrease in the wall-to-lumen ratio (Fig. 1D). Increases in passive diameter and CSA are consistent with outward hypertrophic remodeling. Aging was also associated with a decrease in the strain: pressure relationship (Fig. 1G) with no change in the stress: pressure relationship (Fig. 1F). Consequently, there was an increased elastic modulus in arteries from aged mice (Fig. 1H), indicating a reduced arterial distensibility (i.e. increased stiffness). This is supported by a leftward shift in the stress: strain curve in uterine arteries from aged mice (Fig. 1H).
Estrogenic responsiveness of murine uterine arteries is impaired in the oldest mice, after the occurrence of endothelial dysfunction and increased stiffness
We examined the effect of aging on the vasodilatory responses of resistance arteries to estrogenic compounds. Figure 2 demonstrates the vasorelaxant effects of 17β-estradiol (17β), PPT (ERα) and DPN (ERβ) on pre-constricted uterine arteries from 3-, 24- and 29-month-old mice. Aging was associated with attenuation of the acute vasodilatory properties of all three estrogenic compounds but the effects were agonist-specific. The acute vasodilatory effects of 17β and PPT were not different between 3 and 24 months but were reduced when comparing 3- and 29-month-old mice. In addition, the effects of both 17β-estradiol and PPT were greater in arteries from 24-month-old when compared to 29-month-old mice. Therefore, impaired relaxations to estrogenic compounds occurred after altered functional and elastic arterial properties of these vessels were demonstrable (see Fig. 1).
The acute responses of murine uterine arteries to estrogenic compounds are attenuated by aging. Acute relaxations to 17β (A), PPT (B) and DPN (C) were compared in uterine arteries from 3- (n = 26), 24- (n = 9) and 29-month-old (n = 5) female mice. P < 0.05 from young mice (*) or 24-month-old mice (+) (repeated measures two-way ANOVA). In a separate set of experiments, pre-constricted (U4, 10−6M) endothelium-intact (solid lines and open symbols, n = 26) and endothelium-abraded (dashed lines and closed symbols, n = 7) uterine arteries from young mice were evaluated for their responses to incremental doses (10−8M−10−4.5M) of 17β-estradiol (D), PPT (E) or DPN (F). # P < 0.05 vs. intact arteries (repeated measures two-way ANOVA). Data are presented as mean ± SEM.
Comparing Fig. 2A, B and C, it appears there are differences in the effects of the estrogenic compounds. Indeed, the acute vasodilatory effects of PPT were greater than those of 17β, which elicited a more pronounced response than DPN (repeated measures two-way ANOVA).
Having determined that endothelial dysfunction was associated with aging in murine resistance arteries, we hypothesized that the reduced vasorelaxant effects of estrogenic compounds may be attributable, in part, to altered endothelial-mediated relaxation. We therefore examined the relaxatory effects of each estrogenic compound in endothelium-intact and endothelium -abraded uterine vessels from young mice (demonstrated in Fig. 2D–F). The acute vasodilatory effects of 17β (intact: 17.2 ± 3.7% to 50.8 ± 3.9%; abraded: 15.4 ± 2.7% to 34.4 ± 4.8%, Fig. 2D) and PPT (intact: 16.7 ± 1.4% to 87.5 ± 4.0%; abraded: 24.8 ± 4.9% to 39.7 ± 8.2%, Fig. 2E) were blunted following endothelial abrasion. The acute vasodilatory effect of DPN did not differ between groups, possibly due to the minimal responses observed to this agonist (intact: 11.4 ± 3.0% to 36.1 ± 4.6%; abraded: 16.5 ± 3.1% to 29.4 ± 5.2%, Fig. 2F).
Estrogenic vasodilation is also impaired in non-uterine arteries from aged mice
In order to determine if the influence of aging on estrogenic vasodilation in uterine arteries was observable in other resistance vessels, we repeated studies on tail arteries from female mice. This is important, since there is considerable vascular bed-heterogeneity in the effects of estrogenic compounds9,22,38. Figure 3 illustrates tail arterial responses to U4, ACh, 17β, PPT and DPN from 3-, 24- and 29-month-old mice. Similar to uterine arteries, both U4-induced constriction (maximum contractions for 3-month-old: 22.8 ± 1.0 kPa, 24-month-old: 18.7 ± 0.7 kPa and 29-month-old: 17.9 ± 1.0 kPa, Fig. 3A) and endothelium-dependent vasodilation (maximum relaxations for 3-month-old: 57.7 ± 2.6%, 24-month-old: 43.5 ± 2.2% and 29-month-old: 40.4 ± 3.4%, Fig. 3B) were attenuated in aged mice. The acute relaxatory effects of 17β (Fig. 3C) and PPT (Fig. 3D) were greater than those of DPN (Fig. 3E) (repeated measures two-way ANOVA). Further, the acute estrogenic effects of 17β and PPT were attenuated with advanced aging to 29 months, similar to uterine arteries. However, there was a trend for a decrease in the responses of tail arteries from 24-month-old mice.
The acute responses of murine tail arteries to estrogenic compounds are impaired by aging. Tail arteries from 3- (n = 12), 24- (n = 10) and 29-month-old (n = 5) mice were mounted on a wire myograph and exposed to the thromboxane agonist U4 (10−6M, A). Following steady-state constriction, endothelium-dependent relaxation was assessed by the addition of acetylcholine (10−5M, B). *P < 0.05 from young mice (unpaired t-test). Acute relaxations to 17β (C), PPT (D) and DPN (E) were compared in tail arteries from 3- (n = 8), 24- (n = 9) and 29-month-old (n = 5) female mice. *P < 0.05 from young mice (repeated measures two-way ANOVA). Data are presented as mean ± SEM.
Human arterial function and estrogenic responsiveness is weakened in post-menopausal women
To determine whether the menopause influences vasoreactivity of resistance arteries from women, we studied the responses of uterine arteries from pre- and post-menopausal women from the ages of 32 to 75 years (Fig. 4). The menopause was associated with impaired U4-induced contractility (maximum contractions for pre-menopausal: 21.7 ± 0.9 kPa and post-menopausal: 10.1 ± 0.6 kPa, Fig. 4A) and endothelium-dependent relaxations (maximum relaxations for pre-menopausal: 61.5 ± 2.4% and post-menopausal: 40.7 ± 2.6%, Fig. 4B) to bradykinin.
Vasoactive properties and estrogenic responsiveness of human uterine arteries are attenuated in post-menopausal women. Uterine arteries were mounted on a wire myograph and exposed to the thromboxane agonist U4 (10−6M, A). Following steady-state constriction, endothelium-dependent relaxation was assessed by the addition of bradykinin (10−5M, B). Contractility and endothelial-dependent relaxations were compared in arteries from pre- (n = 26) and post-menopausal (n = 16) women. *P < 0.05 from pre-menopausal women (unpaired t-test). Acute relaxations to 17β (C), PPT (D) and DPN (E) were compared in uterine arteries from pre- (n = 20) and post-menopausal women (n = 13). *P < 0.05 from pre-menopausal women (repeated measures two-way ANOVA). Pre-MW = pre-menopausal women. Post-MW = post-menopausal women. Data are presented as mean ± SEM.
In contrast to murine resistance arteries, the relaxations induced by 17β-oestradiol (Fig. 4C) and DPN (Fig. 4E) were greater than those induced by PPT (Fig. 4D) in arteries from pre-menopausal women (repeated measures two-way ANOVA). Importantly, we found that the acute vasodilatory effects of all three compounds were attenuated in uterine arteries from post-menopausal women.
Human uterine arterial function is impaired with increasing age
The data presented in Fig. 4 were re-plotted to further examine the effect of aging on vasoreactivity of uterine resistance arteries from women. Figure 5 demonstrates U4-induced contractions (A) and endothelial-dependent relaxations (B) in women grouped into 4 age groups (years): 30–39 (n = 7), 40–49 (n = 18), 50–59 (n = 8) and 60–75 (n = 9). Maximal U4-induced contractions were similar in women under the age of 50, but were diminished in women over 50 (maximal contractions for 30–39: 19.5 ± 1.0 kPa, 40–49: 22.6 ± 1.1 kPa, 50–59: 13.0 ± 1.0 kPa and 60–75: 8.3 ± 0.6 kPa). Interestingly, maximal endothelial-dependent relaxations were strongest in the youngest group of women and diminished in all others (maximal relaxations for 30–39: 71.8 ± 2.9%, 40–49: 57.1 ± 3.2%, 50–59: 41.4 ± 3.6% and 60–75: 41.2 ± 3.3%). Increasing age negatively influenced both smooth muscle and endothelial function (Fig. 5C and D).
Human uterine arterial function is impaired with increasing age. Maximal U4-induced contractions (A) and endothelial-dependent relaxations (B) were compared in 4 age groups (30-39, 40-49, 50-59 and 60-75 years of age). P < 0.05 from; 30-39 (Ψ), 40-49 (θ), 50-59 (†) (ordinary one-way ANOVA). Scatter plots show (C) the mean maximal U4-induced constriction and (D) endothelial-dependent relaxation of uterine arteries from each individual patient plotted against age. *P < 0.05 from zero slope (F test). The slope and, r2 values are presented in each XY scatter graph.
Age-related endothelial dysfunction of human uterine arteries precedes changes in estrogenic responsivness
The effects of aging on the acute responses of human uterine arteries to estrogenic compounds were similarly assessed in female age groups (years): 30–39 (n = 5), 40–49 (n = 15), 50–59 (n = 8) and 60–75 (n = 6) (using data re-analyzed from Fig. 4). As shown in Fig. 6A–C, the maximal relaxatory effects of 17β (maximal relaxations for 30–39: 77.1 ± 4.2%, 40–49: 78.2 ± 4.3%, 50–59: 56.8 ± 7.1% and 60–75: 58.8 ± 4.4%) and DPN (maximal relaxations for 30–39: 63.8 ± 3.8%, 40–49: 69.5 ± 5.7%, 50–59: 58.9 ± 8.5% and 60–75: 25.2 ± 4.5%) were negatively influenced by increasing age. Interestingly, whereas the vasodilatory effect of 17β was impaired in women over the age of 50, a weakening of the effects of the ER-specific agonists appeared in women over the age of 60. Similar to murine arteries, the weakened responses of human arteries to these agonists were only apparent after the occurrence of endothelial dysfunction. In addition, there was a negative trend between vasodilatory responses of human uterine arteries to estrogenic compounds and aging (Fig. 6D and F).
Aging negatively regulates the acute vasodilatory effects of estrogenic compounds in women. Maximal acute relaxations to 17β (A), PPT (B) and DPN (C) were compared in 4 age groups (30-39, 40-49, 50-59 and 60-75 years of age). P < 0.05 from; 30-39 years old (Ψ), 40-49 years old (θ), 50-59 years old (†) (ordinary one-way ANOVA). Scatter plots show the maximal relaxations from each patient plotted against increasing age. *P < 0.05 from zero slope (F test). The slope and r2 values are presented in each XY scatter graph.
Note: due to the variable nature of myometrial samples provided, only a small number of vessels could be isolated from some biopsies. Therefore, in some experiments it was not possible to assess the effects of all three compounds. This is why there is a greater number of data points for 17β, which we included in every experiment.
Discussion
The acute vasodilatory effects of estrogen have previously been established but there is little information about how these arterial responses are influenced by the menopause and advancing age. This study investigated the influence of chronological age and the menopause on the acute vasodilatory effects of 17β and ER-specific agonists. Our data demonstrated that both aging and the menopause were associated with reduced vasodilatory effects of estrogenic compounds in murine and human uterine arteries, respectively. Interestingly, however, aging altered endothelial function prior to estrogenic responsiveness in human resistance arteries. Similarly, in mice, aging was associated with reduced resistance arterial compliance, occurring before the altered effects of estrogenic compounds became apparent. We therefore propose that age-related changes in arterial endothelial function and/or stiffness are prominent factors, and not just menopausal status per se, leading to impaired estrogenic actions in arteries from post-menopausal women.
We report that the acute vasodilatory effects of estrogenic compounds, 17β-estradiol and ER agonists, are impaired in resistance arteries from post-menopausal women. An important consideration is what contribution aging per se may make to these findings. Indeed, sub-group analysis of the data suggested that chronological aging negatively influenced the estrogenic responsiveness of human uterine arteries. In particular, endothelial-dependent relaxations were greater in women aged 30–39 than those aged 40–49. Therefore, endothelial dysfunction of human resistance arteries occurred prior to the defect in estrogenic responsiveness, and also the menopause. Due to the small study numbers in humans, we were not able to fully dissociate the influence of age and the menopause on human uterine vascular function. Therefore, we also examined the influence of chronological age on vascular responsiveness in female mice. The data, from two different vascular beds, also demonstrated age-related impairment of acute responses to 17β-estradiol, PPT and DPN. Similar reductions in vascular relaxation to 17β-estradiol have been reported for rat vessels21,39. Of note, the most pronounced effects of aging on 17β- and PPT-induced relaxations of murine uterine arteries were observed between 24 and 29 months rather than between 3 and 24 months. This impairment of estrogenic responsiveness occurred mostly after the endothelial dysfunction and altered distensibility, each evident at 24 months, were established. Therefore, in alliance with the literature21,31,32,39,40, our data supports the concept that the vascular aging phenotype is a continuum of many facets and that the marked structural and functional changes evident at 24 months impose extra strain that contributes to detrimental effects on estrogenic responsiveness.
It is important to note that the current study demonstrated the maximum vasodilatory effects of estrogenic compounds at micromolar concentrations. Similar ranges of concentrations have been used in previous studies7,10,11,38,39,41. It is also important to note that, since estrogen is a lipophilic molecule, the concentrations of oestradiol are possibly greater at the cellular site of action than those measured in the circulation.
We also demonstrated that outward hypertrophic remodeling of uterine arteries was evident in the oldest group of mice, suggesting changes to wall structure may be a response to worsened arterial compliance of resistance vessels. It has been suggested that hypertrophic remodelling is associated with a reduced myogenic response of resistance arteries42. Previous studies investigating the effect of age on contractile properties of resistance arteries have been conflicting, with the over-arching hypothesis that the effects are species-, tissue- and even agonist-specific40,43,44,45,46,47. However, a diminution in response to U46619 was a consistent feature of our data in resistance vessels from humans and mice.
The structural properties of human uterine arteries were not measured in the current study. Therefore, it is not known whether human uterine resistance arteries displayed outward hypertrophic remodelling with age. However, the functional studies indicate similar effects of age, and estrogen responsiveness, in human and mouse uterine arteries. As such it is tempting to suggest that the human arteries may evince similar structural changes to those measured in the mouse arteries from two separate vascular beds. However, others have shown that small arteries from gluteal biopsies display progressive eutrophic remodeling with age48,49.
Total peripheral resistance is an important determinant of blood pressure. The incidence of hypertension is increased with aging and further contributes to the risk of developing cardiovascular disease50,51. In addition, in mixed gender human studies using gluteal biopsies, eutrophic inward remodeling has been suggested to accompany chronological aging and essential hypertension48. However, none of the female human subjects in this study were reported to be hypertensive. Further, although we were unable to monitor blood pressure in our mouse studies, it is unlikely that hypertension was a contributory factor to the alterations in structure/function since we observed outward hypertrophic remodeling.
Previous studies have attributed age-related blunting of estrogenic vasodilation to a reduction in the expression of GPER1 and ERs21,39,52,53. In the current study, we found no difference in ER expression levels with age in human resistance arteries (supplementary Figure 2). In addition, we found that the GPER1 agonist G1 had no effect in human uterine arteries (supplementary Figure 1), supporting a previous study using uterine arteries from pregnant women9. Further, the data enables the speculation that estrogenic relaxation is mediated by predominantly ERα in mouse resistance arteries and ERβ in human uterine arteries. This contributes to the notion that similar estrogenic vascular responsiveness may be mediated in ER-specific manners38.
Alterations in arterial stiffness and endothelial dysfunction are often observed in unison and the former is a strong independent indicator for subsequent cardiovascular risk54. Endothelial dysfunction with aging has been associated with impaired nitric oxide (NO)- and EDH-mediated relaxation, each of which is a potential target of modulation by estrogenic compounds39,55,56. Either way, endothelial dysfunction, or deliberate removal of endothelial-dependent relaxation, resulted in a blunting of estrogenic responsiveness.
There is currently much debate as to the role of the menopause, and altered estrogen bioavailability, in determining risks for cardiovascular health and attenuation in estrogen-responsiveness of the resistance vasculature. The findings reported herein, have implications for the interpretation of clinical studies examining cardiovascular outcomes in aging females. Certainly, estrogen withdrawal has been reported to influence vascular gene expression, inflammation, oxidative stress, structure and function8,22,57,58,59,60,61,62,63,64. There is also some evidence that estrogen therapy administered to women soon after the menopause may be of more benefit than if treatment is delayed27,29,65,66. Recently, two clinical trials, the ‘Kronos Early Estrogen Prevention Study’ (KEEPS) and ‘Early versus Late Intervention Trial with Oestradiol’ (ELITE) trials, have been designed to assess if the length of time since menopause alters the effectiveness of estrogen therapy in post-menopausal women. Early data from the KEEPS trial has shown no significant improvement on cardiovascular mortality, endothelial function or carotid artery compliance of estrogen therapy in young post-menopausal women67,68,69. However, clinical data from the ELITE trial demonstrated that oestradiol treatment had a beneficial effect on carotid artery intima-medial thickness in recently menopausal women (<6 years) compared with distantly menopausal women (≥10 years), yet outcome measures of coronary atherosclerosis risk were unaffected30.
These studies necessitate a continued appraisal of the likely impact of changes in cardiovascular function related to age in women. For example, although long-term risks of adverse cardiovascular outcomes may be similar for post-menopausal women and males there is increasing evidence of key gender differences. These include: (i) first cardiovascular events occurring at older ages in females and the risk of coronary artery disease less than males3. (ii) apparent age-related gender differences in ischemic heart disease mortality in England, Wales and the United States being attributed to a deceleration of associated events in older males, rather than an acceleration in post-menopausal women32. (iii) in a large, cross-sectional, population-based cohort study, independent associations of age and menopausal transition being associated with cardiovascular risk factors31. These scenarios do not fit seamlessly with a notion that the menopause solely imparts enhanced risks to female cardiovascular health.
Taking our in vitro experimental data in context with the above clinical studies, it is reasonable to propose that chronological aging is a prominent factor in influencing the altered vasodilatory effectiveness of estrogenic compounds. However, since the numbers recruited to the human study were small, we were not able to fully dissociate the effects of the menopause and aging on the altered effects of estrogenic compounds. Therefore, there is merit in performing a study, with larger numbers of human participants to explore further the relationship of age-related vascular dysfunction and altered estrogenic responsiveness. This would require longer than the duration of the current study, and/or the co-operation of multiple sites. Access to other sources of resistance vasculature, such as from gluteal biopsies, would extend the interpretations to another human vascular bed and enable comparisons with previous mixed gender studies focussing on essential hypertension48,49. Such studies will be beneficial in determining the feasibility of developing new regimens of estrogenic agents to treat vascular disease in post-menopausal women.
Experimental procedures
Animals
Mouse strains from the C57BL background (C57BL/Ircfa) were housed in the Comparative Biology Centre, Newcastle University, UK. All animal experimentation was performed under UK Home Office License (PIL 60/13278) and complied with the United Kingdom Animals (Scientific Procedures) Act 1986. Female mice from three separate age groups (3-, 24-, and 29-month-old) were utilized in the study. Mice were anaesthetized in an induction chamber using a rising concentration of 3% isoflurane/3 L oxygen per min. Once anesthesia was achieved the animal was culled by cervical dislocation and death confirmed as required by Animals (Scientific Procedures) Act 1986. The estrous stage of the mice was determined by vaginal smear70. Vaginal smears from aged mice were characterized by persistent di-estrous as described previously71. Small uterine and tail arteries were obtained from mice immediately after culling and placed in ice-cold tissue collection buffer (TCB) (in mM: 154 NaCl, 5.4 KCl, 1.2 MgSO4, 10 MOPS, 5.5 glucose, 1.6 CaCl2; pH 7.4). The experimental protocol was approved by Newcastle University Animal Welfare Ethical Review Body.
Human subjects
Ethical approval was obtained from Newcastle and North Tyneside Research Ethics Committee 1 (10/H0906/71) to perform research on samples collected as part of the Uteroplacental Tissue Bank. Women who accepted to take part in the study gave written informed consent in compliance with the Helsinki Declaration and all experiments were performed in accordance with relevant guidelines and regulations. Patient characteristics are detailed in Table 2. In order to be considered postmenopausal, women had to report in the questionnaire that they had entered menopause in addition to reporting an amenorrhea of at least a year, which makes large-scale misclassification in this category less likely. Uterine arteries (identified on the myometrial surface under a dissecting stereomicroscope, at 1.5x magnification, Leica Microsystems, UK) from pre- and post-menopausal women were obtained from lower myometrial biopsies (~1 cm3) taken at the time of total hysterectomy, and placed directly into ice-cold tissue TCB. All experiments involving human samples were carried out in accordance with the relevant guidelines and regulations.
Isometric force measurements
Arteries were cut into 2–4 mm segments and mounted on a small vessel wire myograph (610 M; Danish Myotechnologies, Denmark) by inserting two tungsten wires (25 μm for mouse arteries and 40 μm for human arteries) through the lumen of the vessel. The first wire was attached to a micrometer (allowing adjustment of the lumen diameter during the normalization process) and the second wire was attached to a force transducer (which recorded changes in vessel wall tension). Vessels were normalized to a passive diameter equivalent to 0.9 of L13.3 kPa, as described previously72,73,74, and equilibrated in physiological salt solution (PSS) (in mM: 127 NaCl, 4.7 KCl, 2.4 MgSO4, 25 NaHCO3, 1.18 KH2PO4, 0.07 EDTA, 1.6 CaCl2, 6.05 glucose bubbled with 95% air/5% CO2; pH 7.4) at 37 °C for at least 20 minutes.
Vessels were pre-constricted with the thromboxane agonist 9,11-dideoxy-9a,11a- methanoepoxy prostaglandin F2α (U4, Merck Millipore, UK) and allowed to reach a steady-state contraction. To assess endothelial function, human and mouse arteries were exposed to the endothelium-dependent vasodilators, bradykinin and acetylcholine, respectively. Arteries were then washed 3 times with PSS, pre-constricted with U4 (10−6M), and exposed to increasing doses (5 min duration each, of 10−8, 10−7, 10−6.5, 10−6, 10−5.7, 10−5.3, 10−5, 10−4.5M) of either 1,3,5-Estratriene-3, 17β-diol (17β-estradiol, non-specific ER agonist) (Sigma-Aldrich, USA), 4,4′,4″-(4-Propyl-[1 H]-pyrazole-1,3,5-triyl)-trisphenol (PPT, ERα-specific agonist), 2,3-bis(4-Hydroxyphenyl)-propionitrile (DPN, ERβ-specific agonist), (±)-1-[(3aR*, 4S*, 9bS*)-4-(6-Bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone (G1, GPER-specific agonist) (Tocris Biosciences, UK) or vehicle control (ethanol). This concentration range has been used in previous studies9,10,12,41,75. In separate experiments, the endothelium of mouse uterine arteries was rendered dysfunctional by gently abrading the intimal surface with human hair. Endothelial functional integrity, determined by the relaxant effect of acetylcholine (10−5M) on pre-constricted arteries, was 62.4 ± 4.2% for intact and 6.4 ± 1.0% for abraded arteries indicating that abrasion had evoked endothelial vasodilatory dysfunction. Cumulative concentration response curves were then elicited with 17β, PPT, DPN and vehicle control.
Data recorded (Myodaq; Danish Myotechnologies, Denmark) as active wall tension (ΔT in mN/mm) was transformed to active effective pressure (ΔT/(diameter/2000)) denoted by kPa.
Passive structural characteristics
Murine arteries were mounted in a pressure myography system (Living Systems Instrumentation, USA) and allowed to equilibrate for 1 hour in calcium-free PSS (PSS with no added CaCl2 plus 1 mM EGTA) at 60 mmHg. Intravascular pressure was then reduced to 5 mmHg and subsequently increased to 10 mmHg, 20 mmHg and then in 20 mmHg steps to 120 mmHg. Diameters were allowed to stabilize for 5 minutes before proceeding to the next pressure step. Measurements of stable intraluminal diameter and left and right wall thickness (taken from 3 separate positions in the field of view, and averaged) were obtained at each pressure step.
Measurements of intraluminal diameter and wall thickness were used to calculate the following76:
-
Area of the lumen (μm2): πr2 (where r is the radius)
-
Area of the whole artery (μm2): π (lumen radius + one wall thickness)2
-
Cross-sectional area of the vessel wall (μm2): vessel area − lumen area
-
Stress (dyne/cm2): Pressure (1 mmHg = 1334 dyne/cm2) × radius/wall thickness
-
Strain (ΔD/D0): Change in diameter from 5 mmHg/diameter at 5 mmHg
-
Elastic modulus (E): E = β from the equation y = aeβx used to fit an exponential trend-line to the stress plotted against strain.
Western blotting
Arteries were cut into 5–10 mm segments and immediately snap frozen with 2-methylbutane (isopentane) cooled by liquid nitrogen and stored at −80 °C for further analysis of protein expression. Frozen samples were homogenized in cell lysis buffer (62.5 mM Tris – HCl, 2% SDS, 10% Sucrose) with 2% (vol/vol) protease inhibitor and 0.5% (vol/vol) phosphatase inhibitor at 5 µl per mg of tissue (minimum of 60 µl) and prepared for western blotting as previously described77. Thirty μg of protein was resolved by SDS-PAGE, transferred to a PVDF membrane for western blotting with ERα (Vector Labs #E-613 mouse monoclonal 1:200) or ERβ (Abcam #288 mouse monoclonal 1:500) and viewed chemiluminescently following incubation with HRP-goat anti-mouse-IgG (1:2000 Dakocytomation #PO447). Films were scanned (Umax powerlook scanner, Dallas, TX, USA) and bands were analyzed using the Intelligent Quantifier (Bio image systems, Jackson, MI, USA) software. Optical density (O.D.) of each band was measured relative to the O.D. of the positive control for each western blot. The background noise was subtracted from the O.D. of each band. PVDF membranes were stained with naphthol blue black in order to visualize actin expression and assess for equal protein loading between lanes.
Statistics
All values were presented as mean ± SEM. Throughout the study, n refers to the numbers of women or mice. The passive structural characteristics of mouse arteries were compared using repeated measures two-way ANOVA (Fig. 1C–G). The parameters that showed significant differences with two-way ANOVA were then analyzed with a Bonferroni test for the comparison between age groups. The elastic modulus (E) was compared between murine age groups using an unpaired t-test (Fig. 1H). The vasodilatory effects of increasing concentrations of estrogenic compounds were compared using repeated measures two-way ANOVA. All maximum relaxations/contractions displayed in bar graphs were compared using an ordinary one-way ANOVA, with the exception of Fig. 4, in which an unpaired t-test was used to compare arteries from pre- and post-menopausal women. For the human studies, the trend between aging and the vasodilatory responses of estrogenic compounds was also examined using a scatter plot. A linear regression line was plotted and significant deviation from zero slope was determined by an F-test. Analysis was carried out using the GraphPad Prism (6.0) software (La Jolla, CA, USA). Significance was assumed at P < 0.05.
References
Mozaffarian, D. et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131, e29–322, https://doi.org/10.1161/CIR.0000000000000152 (2015).
North, B. J. & Sinclair, D. A. The intersection between aging and cardiovascular disease. Circ Res 110, 1097–1108, https://doi.org/10.1161/CIRCRESAHA.111.246876 (2012).
Leening, M. J. et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ 349, g5992, https://doi.org/10.1136/bmj.g5992 (2014).
Lerner, D. J. & Kannel, W. B. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J 111, 383–390 (1986).
Kuhl, H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 8(Suppl 1), 3–63, https://doi.org/10.1080/13697130500148875 (2005).
Miller, V. M. & Duckles, S. P. Vascular actions of estrogens: functional implications. Pharmacol Rev 60, 210–241, https://doi.org/10.1124/pr.107.08002 (2008).
Al Zubair, K., Razak, A., Bexis, S. & Docherty, J. R. Relaxations to oestrogen receptor subtype selective agonists in rat and mouse arteries. Eur J Pharmacol 513, 101–108, https://doi.org/10.1016/j.ejphar.2005.03.006 (2005).
Bolego, C. et al. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor-alpha agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther 313, 1203–1208, https://doi.org/10.1124/jpet.104.082867 (2005).
Corcoran, J. J. et al. Human uterine and placental arteries exhibit tissue-specific acute responses to 17beta-estradiol and estrogen-receptor-specific agonists. Mol Hum Reprod. https://doi.org/10.1093/molehr/gat095 (2014).
Montgomery, S., Shaw, L., Pantelides, N., Taggart, M. & Austin, C. Acute effects of oestrogen receptor subtype-specific agonists on vascular contractility. Br J Pharmacol 139, 1249–1253, https://doi.org/10.1038/sj.bjp.0705368 (2003).
Patkar, S., Farr, T. D., Cooper, E., Dowell, F. J. & Carswell, H. V. Differential vasoactive effects of oestrogen, oestrogen receptor agonists and selective oestrogen receptor modulators in rat middle cerebral artery. Neurosci Res 71, 78–84, https://doi.org/10.1016/j.neures.2011.05.006 (2011).
Scott, P. A., Tremblay, A., Brochu, M. & St-Louis, J. Vasorelaxant action of 17 -estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol 293, H3713–3719, https://doi.org/10.1152/ajpheart.00736.2007 (2007).
Zhou, K. et al. 17beta-estradiol induces vasorelaxation by stimulating endothelial hydrogen sulfide release. Mol Hum Reprod 19, 169–176, https://doi.org/10.1093/molehr/gas044 (2013).
Kong, B. W., Vanhoutte, P. M., Man, R. Y. & Leung, S. W. 17beta-estradiol potentiates endothelium-dependent nitric oxide- and hyperpolarization-mediated relaxations in blood vessels of male but not female apolipoprotein-E deficient mice. Vascul Pharmacol 71, 166–173, https://doi.org/10.1016/j.vph.2015.02.009 (2015).
Leung, S. W. & Vanhoutte, P. M. Endothelium-dependent hyperpolarization: age, gender and blood pressure, do they matter? Acta Physiol (Oxf) 219, 108–123, https://doi.org/10.1111/apha.12628 (2017).
Wong, P. S., Roberts, R. E. & Randall, M. D. Sex differences in endothelial function in porcine coronary arteries: a role for H2O2 and gap junctions? Br J Pharmacol 171, 2751–2766, https://doi.org/10.1111/bph.12595 (2014).
Arefin, S. et al. Vasodilatory effects of the selective GPER agonist G-1 is maximal in arteries of postmenopausal women. Maturitas 78, 123–130, https://doi.org/10.1016/j.maturitas.2014.04.002 (2014).
Broughton, B. R., Miller, A. A. & Sobey, C. G. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol 298, H1055–1061, https://doi.org/10.1152/ajpheart.00878.2009 (2010).
Haas, E. et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104, 288–291, https://doi.org/10.1161/CIRCRESAHA.108.190892 (2009).
Lindsey, S. H., Cohen, J. A., Brosnihan, K. B., Gallagher, P. E. & Chappell, M. C. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology 150, 3753–3758, https://doi.org/10.1210/en.2008-1664 (2009).
Lindsey, S. H., da Silva, A. S., Silva, M. S. & Chappell, M. C. Reduced vasorelaxation to estradiol and G-1 in aged female and adult male rats is associated with GPR30 downregulation. Am J Physiol Endocrinol Metab 305, E113–118, https://doi.org/10.1152/ajpendo.00649.2012 (2013).
Kublickiene, K. et al. Small artery endothelial dysfunction in postmenopausal women: in vitro function, morphology, and modification by estrogen and selective estrogen receptor modulators. J Clin Endocrinol Metab 90, 6113–6122, https://doi.org/10.1210/jc.2005-0419 (2005).
Grodstein, F. et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 133, 933–941 (2000).
Duvernoy, C. S. & Mosca, L. Hormone replacement therapy trials: an update. Curr Atheroscler Rep 4, 156–160 (2002).
Grady, D. et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 288, 49–57 (2002).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Grodstein, F., Manson, J. E. & Stampfer, M. J. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt) 15, 35–44, https://doi.org/10.1089/jwh.2006.15.35 (2006).
Valdiviezo, C., Lawson, S. & Ouyang, P. An update on menopausal hormone replacement therapy in women and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes 20, 148–155, https://doi.org/10.1097/MED.0b013e32835ed58b (2013).
Rossouw, J. E. et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297, 1465–1477, https://doi.org/10.1001/jama.297.13.1465 (2007).
Hodis, H. N. et al. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N Engl J Med 374, 1221–1231, https://doi.org/10.1056/NEJMoa1505241 (2016).
de Kat, A. C. et al. Unraveling the associations of age and menopause with cardiovascular risk factors in a large population-based study. BMC Med 15, 2, https://doi.org/10.1186/s12916-016-0762-8 (2017).
Vaidya, D., Becker, D. M., Bittner, V., Mathias, R. A. & Ouyang, P. Ageing, menopause, and ischaemic heart disease mortality in England, Wales, and the United States: modelling study of national mortality data. BMJ 343, d5170, https://doi.org/10.1136/bmj.d5170 (2011).
Greaves, L. C., Barron, M. J., Campbell-Shiel, G., Kirkwood, T. B. & Turnbull, D. M. Differences in the accumulation of mitochondrial defects with age in mice and humans. Mech Ageing Dev 132, 588–591, https://doi.org/10.1016/j.mad.2011.10.004 (2011).
Lister, L. M. et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 20, 1511–1521, https://doi.org/10.1016/j.cub.2010.08.023 (2010).
Martin, R. M., Brady, J. L. & Lew, A. M. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods 212, 187–192 (1998).
Rowlatt, C., Chesterman, F. C. & Sheriff, M. U. Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab Anim 10, 419–442 (1976).
Flurkey K., C. J., Harrison, D. E. In The Mouse in Biomedical Research (ed Davisson, M. T., Fox, J. G., Quimby, F. W., Barthold, S. W., Newcomer, C. E., Smith, A. L.) 637–672 (2007).
Reslan, O. M., Yin, Z., do Nascimento, G. R. & Khalil, R. A. Subtype-specific estrogen receptor-mediated vasodilator activity in the cephalic, thoracic, and abdominal vasculature of female rat. J Cardiovasc Pharmacol 62, 26–40, https://doi.org/10.1097/FJC.0b013e31828bc88a (2013).
Wynne, F. L., Payne, J. A., Cain, A. E., Reckelhoff, J. F. & Khalil, R. A. Age-related reduction in estrogen receptor-mediated mechanisms of vascular relaxation in female spontaneously hypertensive rats. Hypertension 43, 405–412, https://doi.org/10.1161/01.HYP.0000111833.82664.0c (2004).
Hausman, N., Martin, J., Taggart, M. J. & Austin, C. Age-related changes in the contractile and passive arterial properties of murine mesenteric small arteries are altered by caveolin-1 knockout. J Cell Mol Med 16, 1720–1730, https://doi.org/10.1111/j.1582-4934.2011.01457.x (2012).
Shaw, L., Taggart, M. J. & Austin, C. Mechanisms of 17 beta-oestradiol induced vasodilatation in isolated pressurized rat small arteries. Br J Pharmacol 129, 555–565, https://doi.org/10.1038/sj.bjp.0703084 (2000).
Sonoyama, K., Greenstein, A., Price, A., Khavandi, K. & Heagerty, T. Vascular remodeling: implications for small artery function and target organ damage. Ther Adv Cardiovasc Dis 1, 129–137, https://doi.org/10.1177/1753944707086358 (2007).
Gros, R., Van Wert, R., You, X., Thorin, E. & Husain, M. Effects of age, gender, and blood pressure on myogenic responses of mesenteric arteries from C57BL/6 mice. Am J Physiol Heart Circ Physiol 282, H380–388 (2002).
Novella, S. et al. Aging enhances contraction to thromboxane A2 in aorta from female senescence-accelerated mice. Age (Dordr) 35, 117–128, https://doi.org/10.1007/s11357-011-9337-y (2013).
Moreau, P., d’Uscio, L. V. & Luscher, T. F. Structure and reactivity of small arteries in aging. Cardiovasc Res 37, 247–253 (1998).
Shipley, R. D. & Muller-Delp, J. M. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res 66, 374–383, https://doi.org/10.1016/j.cardiores.2004.11.005 (2005).
Vila, E., Vivas, N. M., Tabernero, A., Giraldo, J. & Arribas, S. M. Alpha 1-adrenoceptor vasoconstriction in the tail artery during ageing. Br J Pharmacol 121, 1017–1023, https://doi.org/10.1038/sj.bjp.0701193 (1997).
Bruno, R. M. et al. Different Impact of Essential Hypertension on Structural and Functional Age-Related Vascular Changes. Hypertension 69, 71–78, https://doi.org/10.1161/HYPERTENSIONAHA.116.08041 (2017).
Schofield, I., Malik, R., Izzard, A., Austin, C. & Heagerty, A. Vascular structural and functional changes in type 2 diabetes mellitus: evidence for the roles of abnormal myogenic responsiveness and dyslipidemia. Circulation 106, 3037–3043 (2002).
Lewington, S. et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360, 1903–1913 (2002).
Heagerty, A. M., Heerkens, E. H. & Izzard, A. S. Small artery structure and function in hypertension. J Cell Mol Med 14, 1037–1043, https://doi.org/10.1111/j.1582-4934.2010.01080.x (2010).
Novella, S., Dantas, A. P., Segarra, G., Medina, P. & Hermenegildo, C. Vascular Aging in Women: is Estrogen the Fountain of Youth? Front Physiol 3, 165, https://doi.org/10.3389/fphys.2012.00165 (2012).
Novensa, L. et al. Aging negatively affects estrogens-mediated effects on nitric oxide bioavailability by shifting ERalpha/ERbeta balance in female mice. PLoS One 6, e25335, https://doi.org/10.1371/journal.pone.0025335 (2011).
Laurent, S. & Boutouyrie, P. The structural factor of hypertension: large and small artery alterations. Circ Res 116, 1007–1021, https://doi.org/10.1161/CIRCRESAHA.116.303596 (2015).
Chan, M. V. et al. Distinct endothelial pathways underlie sexual dimorphism in vascular auto-regulation. Br J Pharmacol 167, 805–817, https://doi.org/10.1111/j.1476-5381.2012.02012.x (2012).
Kong, B. W., Man, R. Y., Gao, Y., Vanhoutte, P. M. & Leung, S. W. Reduced activity of SKC a and Na-K ATPase underlies the accelerated impairment of EDH-type relaxations in mesenteric arteries of aging spontaneously hypertensive rats. Pharmacol Res Perspect 3, e00150, https://doi.org/10.1002/prp2.150 (2015).
Arenas, I. A., Armstrong, S. J., Xu, Y. & Davidge, S. T. Chronic tumor necrosis factor-alpha inhibition enhances NO modulation of vascular function in estrogen-deficient rats. Hypertension 46, 76–81, https://doi.org/10.1161/01.HYP.0000168925.98963.ef (2005).
Sudoh, N. et al. Estrogen prevents oxidative stress-induced endothelial cell apoptosis in rats. Circulation 103, 724–729 (2001).
Keaney, J. F. Jr. et al. 17 beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation 89, 2251–2259 (1994).
Moreau, K. L. & Hildreth, K. L. Vascular Aging across the Menopause Transition in Healthy Women. Adv Vasc Med 2014, doi:https://doi.org/10.1155/2014/204390 (2014).
Moreau, K. L., Hildreth, K. L., Meditz, A. L., Deane, K. D. & Kohrt, W. M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97, 4692–4700, https://doi.org/10.1210/jc.2012-2244 (2012).
Kublickiene, K. et al. Effects in postmenopausal women of estradiol and medroxyprogesterone alone and combined on resistance artery function and endothelial morphology and movement. J Clin Endocrinol Metab 93, 1874–1883, https://doi.org/10.1210/jc.2007-2651 (2008).
Pinna, C., Cignarella, A., Sanvito, P., Pelosi, V. & Bolego, C. Prolonged ovarian hormone deprivation impairs the protective vascular actions of estrogen receptor alpha agonists. Hypertension 51, 1210–1217, https://doi.org/10.1161/HYPERTENSIONAHA.107.106807 (2008).
Tarhouni, K. et al. Key role of estrogens and endothelial estrogen receptor alpha in blood flow-mediated remodeling of resistance arteries. Arterioscler Thromb Vasc Biol 33, 605–611, https://doi.org/10.1161/ATVBAHA.112.300334 (2013).
Giordano, S. et al. Estrogen and Cardiovascular Disease: Is Timing Everything? Am J Med Sci 350, 27–35, https://doi.org/10.1097/MAJ.0000000000000512 (2015).
Schierbeck, L. L. et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 345, e6409, https://doi.org/10.1136/bmj.e6409 (2012).
Harman, S. M. Menopausal hormone treatment cardiovascular disease: another look at an unresolved conundrum. Fertil Steril 101, 887–897, https://doi.org/10.1016/j.fertnstert.2014.02.042 (2014).
Harman, S. M. et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med 161, 249–260, https://doi.org/10.7326/M14-0353 (2014).
Kling, J. M. et al. Endothelial function in women of the Kronos Early Estrogen Prevention Study. Climacteric 18, 187–197, https://doi.org/10.3109/13697137.2014.986719 (2015).
Evans, H. M. & Long, J. A. Characteristic Effects upon Growth, Oestrus and Ovulation Induced by the Intraperitoneal Administration of Fresh Anterior Hypophyseal Substance. Proc Natl Acad Sci USA 8, 38–39 (1922).
Joshi, D., Lekhtman, I., Billiar, R. B. & Miller, M. M. Gonadotropin hormone-releasing hormone induced luteinizing hormone responses in young and old female C57BL/6J mice. Proc Soc Exp Biol Med 204, 191–194 (1993).
Davis, M. J. & Gore, R. W. Length-tension relationship of vascular smooth muscle in single arterioles. Am J Physiol 256, H630–640 (1989).
Mulvany, M. J. & Halpern, W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41, 19–26 (1977).
Mulvany, M. J. & Nyborg, N. An increased calcium sensitivity of mesenteric resistance vessels in young and adult spontaneously hypertensive rats. Br J Pharmacol 71, 585–596 (1980).
Hisamoto, K. & Bender, J. R. Vascular cell signaling by membrane estrogen receptors. Steroids 70, 382–387, https://doi.org/10.1016/j.steroids.2005.02.011 (2005).
Izzard, A. S., Horton, S., Heerkens, E. H., Shaw, L. & Heagerty, A. M. Middle cerebral artery structure and distensibility during developing and established phases of hypertension in the spontaneously hypertensive rat. J Hypertens 24, 875–880, https://doi.org/10.1097/01.hjh.0000222757.54111.06 (2006).
Dordea, A. C. et al. Differential vasodilation of human placental and myometrial arteries related to myofilament Ca(2+)-desensitization and the expression of Hsp20 but not MYPT1. Mol Hum Reprod 19, 727–736, https://doi.org/10.1093/molehr/gat045 (2013).
Acknowledgements
This research was funded by the NIHR Biomedical Research Centre (UK). We thank Julie Taggart for the processing and cataloguing of human tissue and the clinical staff and patients of Newcastle Royal Victoria Infirmary.
Author information
Authors and Affiliations
Contributions
C.J.N. and M.S. performed the experiments and analyzed the data. S.C.R. facilitated human tissue provision. M.J.T., C.J.N. and S.C.R. conceived and designed the study. C.J.N. and M.J.T. prepared the manuscript. All authors approved the final version.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nicholson, C.J., Sweeney, M., Robson, S.C. et al. Estrogenic vascular effects are diminished by chronological aging. Sci Rep 7, 12153 (2017). https://doi.org/10.1038/s41598-017-12153-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12153-5
This article is cited by
-
Vessel size as a marker of survival in estrogen receptor positive breast cancer
Breast Cancer Research and Treatment (2023)
-
Mildly elevated diastolic blood pressure increases subsequent risk of breast cancer in postmenopausal women in the Health Examinees-Gem study
Scientific Reports (2022)
-
Is sex a predictor for delayed cerebral ischaemia (DCI) and hydrocephalus after aneurysmal subarachnoid haemorrhage (aSAH)? A systematic review and meta-analysis
Acta Neurochirurgica (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.