Abstract
Childhood TB diagnosis is challenging. Studies in adults suggest Microscopic Observation Drug Susceptibility (MODS) culture or the Xpert MTB/RIF assay might be used to expand bacteriological diagnosis. However data from children are more limited. We prospectively compared MODS and Xpert MTB/RIF with standard microscopy and culture using the BD MGIT 960 system among 1442 Kenyan children with suspected TB. 97 specimens from 54 children were TB culture-positive: 91 (94%) by MGIT and 74 (76%) by MODS (p = 0.002). 72 (74%) culture-positive and 7 culture-negative specimens were Xpert MTB/RIF positive. Xpert MTB/RIF specificity was 100% (99.7–100%) among 1164 specimens from 892 children in whom TB was excluded, strongly suggesting all Xpert MTB/RIF positives are true positives. The sensitivity of MGIT, MODS and Xpert MTB/RIF was 88%, 71% and 76%, respectively, among all 104 true positive (culture and/or Xpert MTB/RIF positive) specimens. MGIT, MODS and Xpert MTB/RIF on the initial specimen identified 40/51 (78%), 33/51 (65%) and 33/51 (65%) culture-confirmed pulmonary TB cases, respectively; Xpert MTB/RIF detected 5 additional culture-negative cases. The high sensitivity and very high specificity of the Xpert MTB/RIF assay supports its inclusion in the reference standard for bacteriological diagnosis of childhood TB in research and clinical practice.
Similar content being viewed by others
Introduction
Definitive diagnosis of tuberculosis (TB) rests on detection of M. tuberculosis from clinical specimens, but is difficult in children. While sputum smear microscopy underpins the WHO DOTS and Stop TB strategies for TB diagnosis in adults1, obtaining sputum is more difficult in children, and the yield of microscopy alone is very poor due to small numbers of bacilli in clinical specimens. Bacteriological diagnosis therefore depends on mycobacterial culture2.
Strengthening laboratory capacity is a key component of the Stop TB Strategy and Global Plan to Stop TB1,3. Commercial liquid culture methods like the BACTEC Mycobacteria Growth Indicator Tube (MGIT) system (BD Diagnostics, Sparks, MD, USA) offer higher sensitivity and more rapid results than traditional solid media. However, barriers to their uptake include cost, a lack of technically trained personnel, and the need for biosafety level 3 facilities3.
Microcolony culture techniques such as the Microscopic Observation Drug Susceptibility (MODS) assay have several potential advantages over conventional culture methods in low resource settings, including high sensitivity; more rapid results that include drug susceptibility testing (DST); lower cost; and less stringent biosafety requirements making it applicable in biosafety level 2 facilities4. However only limited data exist on their use in children5,6,7, and no studies have compared MODS with MGIT for diagnosis of TB in young children; nor evaluated MODS yield from induced sputum samples, despite good evidence and international recommendations supporting sputum induction for childhood TB diagnosis2,8.
The Xpert MTB/RIF real-time PCR assay (Cepheid, Sunnyvale, CA, USA) offers a rapid and operationally simpler alternative to culture for detection of M. tuberculosis and rifampicin resistance9,10. A recent meta-analysis suggests modest sensitivity and high specificity for paediatric TB diagnosis11, and the latest WHO childhood TB guidelines (2014) recommend Xpert MTB/RIF as an alternative to conventional microscopy and culture - but acknowledge the “very low quality of evidence”2.
We compared microscopy, MGIT and MODS culture, and the Xpert MTB/RIF assay for diagnosis of childhood TB in Kenya.
Participants
We prospectively assessed each diagnostic method among children presenting to Coast Provincial General Hospital and Kilifi County Hospital in Coast Province, Kenya. Between July 2010 and December 2011 children aged <15 years were investigated for TB if one or more of the following features of suspected TB were present and they were not already on TB treatment: unexplained persistent cough for >2 weeks; pneumonia not responding to first line antibiotics; unexplained fever for >2 weeks; unexplained progressive weight loss or failure to thrive for >4 weeks; a history of close contact with a suspected or confirmed case of pulmonary TB; or clinical suspicion of TB for any other reason.
Clinical procedures
Each child underwent a structured history and examination, chest radiography and tuberculin skin testing (TST) according to WHO guidelines2. Severe malnutrition was defined as weight for age z-score of <−3 or the presence of nutritional oedema12. Provider initiated testing and counseling for HIV was performed according to Kenyan national guidelines, which recommend testing for all inpatients, and for all patients investigated for TB, on an opt-out basis.
Children who were able to expectorate provided up to three spontaneous sputum samples. Sputum induction was performed on the remainder. If sputum induction was contraindicated (e.g. due to severe respiratory distress), gastric aspiration was performed. Sputum induction and gastric aspiration were performed according to international recommendations2. Further investigations including extra-pulmonary or repeat sputum sampling were performed at the discretion of the clinical team caring for the patient.
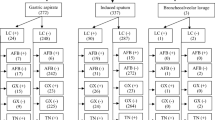
We classified children as Confirmed TB, Highly Probable TB, Possible TB or Not TB according to their clinical, radiological and microbiological findings. Categories were defined a priori and based closely on published definitions (Fig. 1)13,14. Treatment protocols followed Kenyan national guidelines. Children treated for TB were followed up until completion of treatment at 6 months. Other children were also followed up for 6 months, or until TB could be confidently excluded. Final diagnostic assignments were revised in the light of follow up data.
Laboratory procedures
Specimens were transported to the laboratory at 2–8 °C and processed the same day. Sputum specimens were decontaminated using the modified Petroff’s method and 4% sodium hydroxide15; centrifuged; and the sediment re-suspended in 2 ml culture broth containing Middlebrook 7H9 medium, oxalic acid, albumin, dextrose, and catalase (OADC, Becton Dickinson), and polymyxin, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (PANTA, Becton Dickinson)16. After vigorous vortexing to ensure sample homogenization, two drops were used to make a smear for Ziehl Neelsen staining, and the remainder divided equally for MGIT, MODS, and Xpert MTB/RIF. Samples for Xpert MTB/RIF analysis were promptly frozen at −80 °C for analysis at the end of the study.
Liquid mycobacterial culture was performed using the MGIT 960 system and MODS assay. A laboratory technician (RM) first received one month MODS training at the Universidad Peruana Cayetano Heredia in Lima, Peru. Standard protocols were followed15,16, except that MODS plates were examined 3 times a week rather than daily for logistic reasons.
Positive cultures from both methods were identified as M. tuberculosis complex (MTBC) or non-MTBC by the BD MGIT TBc Identification test (BD Diagnostics, Sparks, MD, USA). They were then further speciated and probed for isoniazid and rifampicin resistance mutations by PCR, using the Hain Genotype® line probe assay platform (Hain Lifescience GmbH, Nehren, Germany). The Xpert MTB/RIF assay (version G4) was performed at the end of the study on specimens from all children treated for confirmed, highly probable or possible TB, and from children in whom TB had been excluded. Laboratory procedures were externally monitored through the UK NEQAS quality assurance scheme and annual Good Clinical Laboratory Practice audits (Qualogy, UK).
In order to compare the performance of MGIT and MODS, and to prevent observer bias in the interpretation of either result, laboratory technologists were blinded to the identity of the MODS portion of each specimen. Specimens were instead identified by an electronically generated random numeric code, the key to which was held by the Principal Investigator.
Statistical analysis
Data were analyzed at both the patient and the specimen level. We first performed a per patient analysis to compare the sensitivity of smear microscopy, MGIT, MODS and Xpert MTB/RIF for identification of culture confirmed pulmonary TB cases. We then included all specimens (pulmonary and extra-pulmonary) in a per specimen analysis to calculate the sensitivity of each method against the existing reference standard of a positive M. tuberculosis culture (by MGIT and/or MODS).
To explore the specificity of Xpert MTB/RIF we calculated the proportion of specimens from Not TB cases that were Xpert MTB/RIF positive. Having established the very high specificity of the Xpert MTB/RIF assay, we then repeated the per specimen analysis using a composite reference standard incorporating Xpert MTB/RIF, such that a specimen was considered positive if either culture or Xpert MTB/RIF identified M. tuberculosis.
We used McNemar’s χ2 test to compare proportions between tests on paired aliquots of the same sample. To investigate any learning effect as laboratory staff became more experienced with MODS we performed the χ2 test for trend in the proportion of M. tuberculosis culture positive specimens identified by MODS in each quarter, and the Wilcoxon rank sum test to compare MODS time to detection (TTD) in the first and second years of the study. We used the Wilcoxon signed rank test to compare TTD of MTBC by MODS and MGIT. Associations with a positive Xpert MTB/RIF assay were explored using univariable and multivariable logistic regression models adjusted for clustering at the patient level.
Ethical approval and informed consent
The study was approved by the Kenya National Ethics Committee. All study procedures were performed in accordance with relevant guidelines and regulations. A parent or guardian provided written informed consent.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
We identified 1500 children with features of suspected TB, 1442 of whom were investigated for TB (Fig. 2). Active TB was diagnosed in 212 (14.7%) children, including 54 (25.5%) with Culture-confirmed TB, 63 (29.7%) with Highly Probable TB, and 95 (44.8%) with Possible TB. Most cases (185, 87%) had pulmonary TB (PTB). Baseline demographic and clinical characteristics are summarized in Table 1. In keeping with previous studies, the proportion of cases that were bacteriologically confirmed was higher among older children.
Per patient analysis of pulmonary TB cases
There were 51 cases of Culture-confirmed pulmonary TB. Smear microscopy, MGIT and MODS on the initial sputum specimen identified M. tuberculosis in 13 (25%), 40 (78%) and 33 (65%) cases, respectively (Table 2). Sensitivity was highest for each method among smear positive samples; Xpert MTB/RIF identified 100% of smear-positive cases and 53% of smear-negative cases on the first sputum specimen (Table 2).
Second and third sputum specimens were obtained from 237 (16%) and 102 (7%) children, respectively. Eight (16%) culture confirmed PTB cases were culture negative from the initial sample but grew M. tuberculosis from a subsequent sputum sample. MGIT, MODS and Xpert MTB/RIF each demonstrated a modest incremental yield from additional specimens (Table 3). None of the 8 initial culture-negative specimens from confirmed PTB cases were Xpert MTB/RIF positive. However Xpert MTB/RIF did detect M. tuberculosis in sputum from 5 additional children with culture negative pulmonary TB (3 with clinically Highly Probable TB and 2 who were treated for Possible TB).
Per specimen analysis of pulmonary and extra-pulmonary specimens combined
A total of 2085 specimens were obtained for mycobacterial culture. We were unable to do MODS on 182 samples due to holiday staffing shortages (169) or insufficient sample (13). A total of 1903 specimens were therefore included in the analysis, of which 1802 (95%) were sputum specimens, 33 (2%) were smear positive, and 97 (5%) were M. tuberculosis culture positive (Supplementary Appendix, Table S1). MGIT was more sensitive than MODS overall (93.8% vs 76.3%, p = 0.002), among all sputum specimens, among induced sputum specimens, and among specimens from both younger and older children (Table 4).
There was weak evidence of an increase in MODS sensitivity (χ2 test for trend p = 0.038) and a decrease in TTD over time, with a median TTD of 16 (IQR 7 to 21) days in 2010 compared with 10 (7 to 14) days in 2011 (p = 0.052; Supplementary Appendix, Fig. S1). Overall, TTD was slightly shorter for MODS than MGIT (11 [IQR 6 to 15] days versus 12 [7 to 17] days, p = 0.001).
Discordant culture results were obtained for 29 specimens. Independent bacteriological confirmation of M. tuberculosis was available for 21/29 of these specimens, either by the Xpert MTB/RIF assay on the original specimen (15/29) and/or by isolation of M. tuberculosis from an independent specimen from the same patient (Supplementary Appendix, Table S2). The clinical picture strongly supported the presence of M. tuberculosis complex in the remaining 8 culture-discordant samples: 5 MGIT+/MODS− specimens came from children with clinically Highly Probable TB prior to culture results; 2 MGIT−/MODS+ specimens came from children treated empirically for TB prior to the culture result although they did not meet the stringent definition of Highly Probable TB; and one MGIT+/MODS− specimen that grew M. bovis BCG came from a 4 month old HIV-infected child who had a clinical syndrome consistent with disseminated BCG disease (Supplementary Appendix, Table S2). All were the only positive cultures in their batch, arguing against cross-contamination causing the discordance. Even if cross-contamination were the cause and these specimens considered culture negative, MGIT would remain more sensitive than MODS (95.5% vs 80.9%, p = 0.005).
Xpert MTB/RIF identified 72/97M. tuberculosis culture positive specimens, giving a sensitivity compared to culture of 74.2% (95% CI 64.3 to 82.6). Xpert MTB/RIF was also positive on 5/86 (6%) specimens from patients with culture-negative but clinically highly probable TB, and 2/179 (1%) specimens from children treated for possible TB. No other specimens were Xpert MTB/RIF positive, including none of 1164 specimens obtained from children in whom TB was subsequently excluded - giving a specificity of 100% (1 sided 97.5% CI 99.7 to 100%) and strongly suggesting positive Xpert MTB/RIF results should be interpreted as true positives.
Against the composite reference standard of a positive TB culture and/or Xpert MTB/RIF result, the sensitivity of TB culture was 93.3% (86.6 to 97.3), compared with 76.0% (66.6 to 83.8) for Xpert MTB/RIF (p = 0.002; Table 5). The sensitivity of MGIT and MODS culture alone were 87.5% (79.6 to 93.2) and 71.2% (61.4 to 79.6), respectively.
Table 6 shows Xpert MTB/RIF sensitivity against the composite reference standard, broken down by smear status, specimen type, age and HIV status. Sensitivity was higher for smear positive specimens (100% compared with 65% for smear negative specimens, p < 0.001), with a trend towards higher sensitivity among sputum samples. Neither age nor HIV status were associated with Xpert MTB/RIF yield in univariable or multivariable analyses.
Xpert MTB/RIF identified rifampicin resistance in 4 specimens from the same child, all of which were confirmed resistant to rifampicin by MODS and the GenoType MTBDRplus assay. MODS did not identify any other rifampicin resistant isolates.
Discussion
The lack of reliable diagnostic tools is the single greatest obstacle to improved childhood TB case management, particularly in low resource settings where the disease burden is greatest. Expansion of TB laboratory diagnostic capacity provides an opportunity to improve both diagnosis and surveillance in many settings3. However good quality data on the performance of each method are required to optimize their use and interpretation.
Few studies have compared laboratory methods for childhood TB diagnosis. Limitations of existing studies include small numbers of confirmed TB cases5,6,7,14,17,18,19,20,21,22, particularly among young children in whom diagnosis is most challenging5,6,7,14,19,22, and exclusion of ‘gold standard’ methods for specimen collection and/or mycobacterial culture5,6,7,17,20,23,24,25,26. Strengths of this study include the use of both sputum induction and liquid mycobacterial culture in keeping with current international recommendations2, and rigorous diagnostic assignments based on detailed clinical assessment and follow up. Prospective recruitment of all children who met broad, pre-defined inclusion criteria also ensures generalizability to a wide range of settings and clinical syndromes, including among children under 5 years who accounted for 81% of those investigated and half of all confirmed cases.
While comparison of culture yield between studies is hampered by widely varying clinical definitions, specimen types and culture methods, the proportion of culture-confirmed cases in our study is consistent with other studies using liquid mycobacterial culture for paediatric diagnosis5,8,14,18,20,26,27,28,29,30.
In our study MGIT was more sensitive than MODS culture. This is in keeping with the only other published study comparing MGIT with MODS for paediatric TB diagnosis5. Importantly we were also able to demonstrate the higher yield of MGIT for culture of paediatric induced sputum samples. These results contrast with other studies in adults in which MODS compared more favourably with MGIT4. The paucibacillary nature of childhood TB may have a role, and variation between centres also suggests MODS performance is operator dependent4. In support of this, even after one month residential training with an experienced team, we were able to demonstrate a learning effect with increasing MODS yield and reducing time to detection during the two years of the study. We did not compare MODS with culture on solid media, however other studies have shown MODS to be superior to Lowenstein Jensen media for diagnosis of childhood TB6,7,26.
Against a reference standard of mycobacterial culture, Xpert MTB/RIF sensitivity was similar to other paediatric studies11, and comparable to MODS - in keeping with the only other study to compare the two methods29. Significantly, a combination of culture and Xpert MTB/RIF increased bacteriological yield without compromising specificity.
Importantly, we were able to confirm the very high specificity of Xpert MTB/RIF in a large sample of specimens from children in whom TB had been excluded by careful clinical, radiological and laboratory assessment and follow up. Positive Xpert MTB/RIF results have been reported in a small number of child TB suspects who did well at follow up without TB treatment11,24. However the very high precision with which we were able to demonstrate 100% specificity of Xpert MTB/RIF in a carefully characterized group of children without TB suggests that these apparent false positive results from TB suspects in very high burden settings may have represented self-limiting pulmonary tuberculosis. This is well recognized in the pre-chemotherapy literature31.
Our primary analysis compared Xpert MTB/RIF sensitivity with the current culture reference standard. Having demonstrated the very high specificity of Xpert MTB/RIF we then repeated the analysis using a composite reference standard of positive culture or Xpert MTB/RIF. Composite reference standards should be used with care32. However, given the equivalent high specificities of Xpert MTB/RIF and mycobacterial culture, incorporation bias in favour of Xpert MTB/RIF is unlikely. Furthermore, misclassification bias arising from the imperfect sensitivity of culture may bias estimates of the accuracy of Xpert MTB/RIF culture alone is used as the reference33,34. We believe the composite reference standard therefore provides a fairer comparison of the two methods, and a better estimate of the true sensitivity of the Xpert MTB/RIF assay.
One potential limitation of our study is that molecular typing was not performed on MGIT-MODS discordant culture isolates to exclude cross-contamination. Nevertheless there was strong microbiological and/or clinical evidence to corroborate the positive culture in every case. In addition, negative controls in every MGIT batch and MODS plate provided no evidence of cross-contamination throughout the study, consistent with the very low prevalence and concentrations of M. tuberculosis in these paediatric specimens.
Due to finite resources, most children only had a single sputum sample obtained. Although this and other studies show the incremental yield of additional specimens where obtained, analysis of a single specimen is likely to reflect practice in most low resource settings due to the incremental cost of processing additional specimens.
In conclusion, our results underline the imperfect sensitivity of all currently available methods for bacteriological diagnosis of childhood TB. The choice of method in any particular setting depends on several factors including prevalence of TB and drug resistance; resources available to risk stratify patients for testing; test sensitivity, cost35, time to detection; and the availability of trained staff and laboratory biosafety facilities. Our data confirm the superior sensitivity of MGIT compared with MODS and Xpert MTB/RIF. Although MODS may have a role in some settings, the operationally simpler Xpert MTB/RIF assay was as sensitive as MODS, demonstrated equivalent specificity to MGIT, and combined with MGIT it optimized bacteriological yield. Together these results strengthen the evidence base for inclusion of Xpert MTB/RIF in the reference standard for bacteriologically confirmed childhood TB in both WHO clinical guidelines35 and research.
Role of the funding source
The study was supported by the Wellcome Trust in the form of research fellowships to AJB (081697) and JAGS (098532), and a core grant to the KEMRI-Wellcome Trust research programme (077092); and by a project grant from the Pneumonia Etiology Research for Child Health (PERCH) project funded by the Bill & Melinda Gates Foundation at Johns Hopkins Bloomberg School of Public Health. The funders had no role in data analysis or in the decision to publish.
Change history
03 May 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
World Health Organization & Stop TB Partnership. The Global Plan to Stop TB 2011–2015: Transforming the fight towards elimination of tuberculosis. (WHO Press, 2010).
World Health Organisation. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Second edition edn, (World Health Organisation, 2014).
World Health Organization. A Roadmap for Ensuring Quality Tuberculosis Diagnostics Services within National Laboratory Strategic Plans (World Health Organization, Geneva, 2010).
Leung, E., Minion, J., Benedetti, A., Pai, M. & Menzies, D. Microcolony culture techniques for tuberculosis diagnosis: a systematic review. Int J Tuberc Lung Dis 16, 16–23, i–iii, https://doi.org/10.5588/ijtld.10.0065 (2012).
Ha, D. T. et al. Microscopic observation drug susceptibility assay (MODS) for early diagnosis of tuberculosis in children. PloS one 4, e8341 (2009).
Oberhelman, R. A. et al. Improved recovery of Mycobacterium tuberculosis from children using the microscopic observation drug susceptibility method. Pediatrics 118, e100–106 (2006).
Oberhelman, R. A. et al. Diagnostic approaches for paediatric tuberculosis by use of different specimen types, culture methods, and PCR: a prospective case-control study. The Lancet. Infectious diseases 10, 612–620, https://doi.org/10.1016/S1473-3099(10)70141-9 (2010).
Zar, H. J., Hanslo, D., Apolles, P., Swingler, G. & Hussey, G. Induced sputum versus gastric lavage for micorbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 2005, 130–134 (2005).
Boehme, C. C. et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363, 1005–1015, https://doi.org/10.1056/NEJMoa0907847 (2010).
Boehme, C. C. et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 377, 1495–1505 (2011).
Detjen, A. K. et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. The Lancet. Respiratory medicine. https://doi.org/10.1016/S2213-2600(15)00095-8 (2015).
World Health Organisation. Pocket book of hospital care for children: guidelines for the management of common illnesses with limted resources. (World Health Organisation, 2005).
Liebeschuetz, S. et al. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet 364, 2196–2203 (2004).
Rachow, A. et al. Increased and Expedited Case Detection by Xpert MTB/RIF Assay in Childhood Tuberculosis: A Prospective Cohort Study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, https://doi.org/10.1093/cid/cis190 (2012).
Siddiqi, S. H. & Ruesch-Gerdes, S. MGIT Procedure Manual. (Foundation for Innovative New Diagnostics (FIND), 2006).
Coronel, J., Roper, M., Caviedes, L. & Moore, D. A. MODS: A user guide. Microscopic observation drug susceptibility assay (2008).
Chisti, M. J. et al. A prospective study of the prevalence of tuberculosis and bacteraemia in Bangladeshi children with severe malnutrition and pneumonia including an evaluation of Xpert MTB/RIF assay. PloS one 9, e93776, https://doi.org/10.1371/journal.pone.0093776 (2014).
LaCourse, S. M. et al. Use of Xpert for the diagnosis of pulmonary tuberculosis in severely malnourished hospitalized Malawian children. The Pediatric infectious disease journal 33, 1200–1202, https://doi.org/10.1097/INF.0000000000000384 (2014).
Ntinginya, E. N. et al. Performance of the Xpert(R) MTB/RIF assay in an active case-finding strategy: a pilot study from Tanzania. Int J Tuberc Lung Dis 16, 1468–1470, https://doi.org/10.5588/ijtld.12.0127 (2012).
Pang, Y. et al. Evaluation of the Xpert MTB/RIF assay in gastric lavage aspirates for diagnosis of smear-negative childhood pulmonary tuberculosis. The Pediatric infectious disease journal 33, 1047–1051, https://doi.org/10.1097/INF.0000000000000403 (2014).
Walters, E., Goussard, P., Bosch, C., Hesseling, A. C. & Gie, R. P. GeneXpert MTB/RIF on bronchoalveolar lavage samples in children with suspected complicated intrathoracic tuberculosis: a pilot study. Pediatric pulmonology 49, 1133–1137, https://doi.org/10.1002/ppul.22970 (2014).
Sekadde, M. P. et al. Evaluation of the Xpert MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis in Uganda: a cross-sectional diagnostic study. BMC infectious diseases 13, 133, https://doi.org/10.1186/1471-2334-13-133 (2013).
Bates, M. et al. Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. The Lancet. Infectious diseases 13, 36–42, https://doi.org/10.1016/S1473-3099(12)70245-1 (2013).
Zar, H. J. et al. Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 55, 1088–1095, https://doi.org/10.1093/cid/cis598 (2012).
Raizada, N. et al. Enhancing TB case detection: experience in offering upfront Xpert MTB/RIF testing to pediatric presumptive TB and DR TB cases for early rapid diagnosis of drug sensitive and drug resistant TB. PloS one 9, e105346, https://doi.org/10.1371/journal.pone.0105346 (2014).
Tran, S. T. et al. Diagnostic accuracy of microscopic Observation Drug Susceptibility (MODS) assay for pediatric tuberculosis in Hanoi, Vietnam. PloS one 8, e72100, https://doi.org/10.1371/journal.pone.0072100 (2013).
Hatherill, M. et al. Induced sputum or gastric lavage for community-based diagnosis of childhood pulmonary tuberculosis? Arch Dis Child 94, 195–201, https://doi.org/10.1136/adc.2007.136929 (2009).
Marais, B. J. et al. The bacteriologic yield in children with intrathoracic tuberculosis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 42, e69–71 (2006).
Nhu, N. T. et al. Evaluation of Xpert MTB/RIF and MODS assay for the diagnosis of pediatric tuberculosis. BMC infectious diseases 13, 31, https://doi.org/10.1186/1471-2334-13-31 (2013).
Nicol, M. P. et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. The Lancet. Infectious diseases 11, 819–824, https://doi.org/10.1016/S1473-3099(11)70167-0 (2011).
Marais, B. J. et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 8, 392–402 (2004).
Naaktgeboren, C. A. et al. Value of composite reference standards in diagnostic research. BMJ 347, f5605, https://doi.org/10.1136/bmj.f5605 (2013).
Alonzo, T. A. & Pepe, M. S. Using a combination of reference tests to assess the accuracy of a new diagnostic test. Statistics in medicine 18, 2987–3003 (1999).
Togun, T. O. et al. Contribution of Xpert(R) MTB/RIF to the diagnosis of pulmonary tuberculosis among TB-exposed children in The Gambia. Int J Tuberc Lung Dis 19, 1091–1097, i–ii, https://doi.org/10.5588/ijtld.15.0228 (2015).
World Health Organization. Xpert MTB/RIF assay for the diagnosis of TB. Meeting Report., (World Health Organization, Geneva, 2016).
Acknowledgements
We would like to thank Dr. David Moore, Luz Caviedes and the team at Universidad Peruana Cayetano Heredia in Lima, Peru, for their helpful advice and practical support in MODS training and implementation; and the clinical, laboratory and administrative staff at Kilifi County Hospital and Coast Provincial General Hospital for their support of the study. We would also like to thank the following members of the Kilifi Improving Diagnosis & Surveillance of Childhood TB (KIDS TB) Study Group who contributed to patient recruitment, investigation and management: Victor Bandika, Jay Berkley, Joyce Langat, Kath Maitland, Carole Mulunda, Charles Newton, John Paul Odhiambo, Hemed Twahir, Joshua Wambua.
Author information
Authors and Affiliations
Contributions
A.J.B., J.A.G.S. and M.L. designed the study with input from D.M., R.M., A.M. and S.C.M. R.M. received residential training in MODS at Universidad Peruana Cayetano Heredia in Lima, Peru, following which he trained A.J.B., D.M. and A.M. in the technique. Laboratory work was performed by D.M., R.M., A.M., S.C.M. and A.J.B. A.J.B. had full access to all the data in the study and final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brent, A.J., Mugo, D., Musyimi, R. et al. Bacteriological diagnosis of childhood TB: a prospective observational study. Sci Rep 7, 11808 (2017). https://doi.org/10.1038/s41598-017-11969-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11969-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.