Abstract
Ischemic stroke following coronary revascularization procedures remains one of the most potentially devastating complications. CHA2DS2-VASc score has been widely used for stroke risk stratification in AF patients. The aim of this nationwide study was to examine the association between the CHA2DS2-VASc score and ischemic stroke following coronary revascularization procedures. We identified patients undergoing coronary revascularization procedures, coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI), using the electronic Hospitalization Summary Reports. Logistic regression models were applied to evaluate the association of CHA2DS2-VASc score with the risk of post-procedural ischemic stroke. We identified 54,714 patients undergoing CABG and 263,063 patients undergoing PCI from 2013 to 2015. The CHA2DS2-VASc score had a positive graded association with the risk of post-procedural ischemic stroke in both CABG and PCI (P for trend <0.001). The adjusted risk of post-procedural ischemic stroke increased by an estimated 122.4% (odds ratio [OR], 2.22; 95% confidence interval [CI], 2.11–2.35) and 34.7% (OR, 1.35; 95% CI, 1.31–1.39) for each additional 1 point in the CHA2DS2-VASc score in CABG and PCI, respectively. In conclusion, these findings suggested that CHA2DS2-VASc score was an independent predictor of the development of post-procedural ischemic stroke in patients undergoing CABG and PCI.
Similar content being viewed by others
Introduction
Cardiovascular disease is the leading cause of death in the world, as well as in China1, 2. Coronary heart disease (CHD) accounts for the greatest proportion of cardiovascular disease. Coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) is the mainstay of revascularization procedures for patients with CHD. It was estimated that more than 1 million coronary revascularization procedures are performed annually in the United States3. The prognoses of patients undergoing revascularization procedures have aroused increasingly more attention4,5,6. Despite the continuous improvements in operating skills, peri-procedural care and service and management ability, post-procedural ischemic stroke remains one of the most potentially devastating complications. A large randomized, controlled trial involving 4,752 patients undergoing CABG surgery recruited from 79 hospitals in 19 countries reported that 30-day incidence rate of stroke after off-pump and on-pump CABG was 1.0% and 1.1%, respectively7. A recent meta-analysis of 6 contemporary randomized control trials including 5,673 patients with stable coronary artery disease who underwent PCI indicated that the incidence rate of post-procedural stroke was 2.0% at a weighted mean follow up of 55 months8. Prevailing evidence demonstrated that post-procedural ischemic stroke is an important cause of increased length of hospital stay, significant morbidity and mortality, and increased health care costs9,10,11. Stroke prevention in cardiac procedure has been an initiative championed by national societies as an overall effort to improve quality of clinical care12. Pre-procedural stratification of patients has significant clinical implications in individual decision-making, treatment selection, and post-procedural care.
CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75, diabetes mellitus, prior stroke or transient ischemic attack (TIA), vascular disease, age 65–74, female) score has been widely used for stroke risk stratification in patients with atrial fibrillation13. A handful of studies have linked higher CHA2DS2-VASc score with increased risk of postoperative stroke in patients undergoing CABG surgery14,15,16,17,18, while data is limited in its application in PCI. Patients undergoing PCI have been demonstrated to be at elevated risk for ischemic stroke19. A high CHA2DS2-VASc score has been reported to be predictive of thrombotic outcomes in patients with AF undergoing PCI20. However, the specific association between CHA2DS2-VASc score and ischemic stroke following PCI has, to our knowledge, not been studied before. Better understanding of this association is important because PCI and CABG have different risk profiles and survival trajectories6, 21.
The aim of the present study, which was based on a multicenter national database, was to determine the association between CHA2DS2-VASc score and post-procedural ischemic stroke in patients undergoing CABG and PCI.
Methods
Data collection
Data used in this study were obtained from the electronic Hospitalization Summary Reports (HSRs) in the top-ranked public hospitals in care safety and quality as evaluated by the National Hospital Performance Evaluation Project in the National Healthcare Data Center. The hospital ranking system considers several aspects, including hospital infrastructure, medical service and management, technical level and efficiency, and quality and safety of clinical care. The information recorded on the HSR includes basic demographics, dates of admission and discharge, hospitalization and discharge diagnoses in Chinese and their corresponding International Classification of Diseases, 10th Revision (ICD-10) codes, treatment procedures and their corresponding International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, discharge status (survival status, drug allergy, and hospitalization infection), and financial costs. The present study is considered exempt from institutional review board approval since the data used was collected for administrative purpose without any personal identifiers.
An updated electronic HSR was implemented in 2012. The updated HSR contains up to 11 listed ICD-10 coding discharge diagnoses with the first one recorded designated as the principal diagnosis or primary illness, while the others are available for comorbid conditions and complications. The updated HSR also contains a maximum of 10 ICD-9-CM coding procedures. A new variable is assigned next to each listed diagnosis to specify the timing of diagnosis.
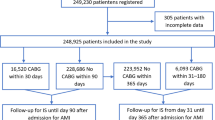
In the present study, we identified patients aged ≥18 years undergoing CABG (ICD-9-CM procedure codes: 36.10–36.19) and PCI (ICD-9-CM procedure codes: 36.06, 36.07 and 00.66) between January 1, 2013 and December 31, 2015. Patients with urgent/emergent CABG or PCI were not included in our study. Patients with atrial fibrillation were excluded from this study. We also excluded procedures for valvular disease. To minimize the influence of coding inaccuracy, we used the corresponding Chinese terms to check the identified cases. The flow chart of the enrollment of the study population is shown in Fig. 1. In total, we identified 54,714 patients undergoing CABG and 263,063 patients undergoing PCI in 172 hospitals in 24 provinces across China.
Measurements
The primary outcome in this study, post-procedural ischemic stroke, was defined as any ischemic stroke occurring in the period between the beginning of the procedure and the patient’s death or discharge from the hospital. Ischemic stroke was diagnosed according to the WHO criteria22 combined with brain computed tomography or magnetic resonance imaging confirmation.
The key independent variable in the present study was the CHA2DS2-VASc score. We calculated CHA2DS2-VASc score for each patient and 1 point was assigned for heart failure, hypertension, diabetes mellitus, vascular disease, age between 65 and 74 years and female gender whereas 2 points were assigned for ischemic stroke/TIA and age ≥75 years. Patients were classified into six groups according the following CHA2DS2-VASc scores: 0, 1, 2, 3, 4, and ≥5.
Statistical analysis
In this study, categorical variables are reported as proportion (%) whereas numerical data are reported as mean ± standard deviation (SD). The incidence rates of post-procedural ischemic stroke between different CHA2DS2-VASc score groups were compared using Pearson’s χ2 test. We applied the Cochran–Armitage test for trend to analyze the association between the occurrence of post-procedural ischemic stroke and CHA2DS2-VASc score. To allow the adjustment for other comorbid conditions, association rule mining model was applied to identify comorbidities associated with post-procedural ischemic stroke23. Finally, a history of hyperlipidemia, chronic obstructive pulmonary disease (COPD), ischemic heart disease, AF and previous cardiac and vascular implants and grafts were significantly associated with post-procedural ischemic stroke (Figure S1 and S2). Univariable and multivariable logistic regression models were used to measure the odds ratios (ORs) and 95% confidence intervals (95% CIs) for post-procedural ischemic stroke associated with CHA2DS2-VASc score. The multivariable model was simultaneously adjusted for hyperlipidemia, COPD, ischemic heart disease, AF and previous cardiac and vascular implants and grafts. The C-statistic, which measures the area under the receiver operating characteristic (ROC), was used to evaluate the predictive ability of CHA2DS2-VASc score in the risk of postoperative stroke. All reported P values were nominal and two-sided. All statistical analyses were performed by R software.
Results
Table 1 shows the demographic characteristics of the 54,714 patients undergoing CABG and 263,063 patients undergoing PCI by the presence of post-procedural ischemic stroke. Patients with post-procedural ischemic stroke had a higher prevalence of comorbid conditions and a higher CHA2DS2-VASc score.
Table 2 presents the post-procedural ischemic stroke occurrences according to CHA2DS2-VASc score. There were 612 (1.1%) and 1,874 (0.7%) ischemic stroke cases following CABG and PCI, respectively. Among patients undergoing CABG, the prevalence rate of post-procedural ischemic stroke increased steadily across CHA2DS2-VASc score groups, ranging from 0.2% among patients with CHA2DS2-VASc score of 0 to 0.3% among patients with CHA2DS2-VASc score of 1, 0.4% among patients with CHA2DS2-VASc score of 2, 1.3% among patients with CHA2DS2-VASc score of 3, 2.8% among patients with CHA2DS2-VASc score of 4, and 8.3% among patients with CHA2DS2-VASc score ≥ 5. The prevalence rates of post-procedural ischemic stroke in patients undergoing PCI with CHA2DS2-VASc score of 0, 1, 2, 3, 4 and ≥5 were 0.2%, 0.3%, 0.7%, 1.0%, 1.1% and 1.4%, respectively. The values of rho Spearman of correlation of CHA2DS2-VASc and ischemic stroke in CABG and PCI were 0.111 (P < 0.001) and 0.074 (P < 0.001), respectively.
Table 3 shows the association between CHA2DS2-VASc score and the risk of post-procedural ischemic stroke. There was a positive graded association between CHA2DS2-VASc score and post-procedural ischemic stroke following both CABG and PCI (P for trend <0.001). The adjusted risk of post-procedural ischemic stroke increased by an estimated 122.4% (odds ratio [OR], 2.22; 95% confidence interval [CI], 2.11–2.35) and 34.7% (OR, 1.35; 95% CI, 1.31–1.39) for each additional 1 point in the CHA2DS2-VASc score in CABG and PCI, respectively (data was not shown in tables). As compared to patients undergoing CABG with a CHA2DS2-VASc score of 0, the corresponding adjusted ORs of post-procedural ischemic stroke were 1.03 (95% CI: 0.56–1.91) and 6.28 (95% CI: 3.69–10.68) for those with a CHA2DS2-VASc score of 1, and ≥2, respectively. The corresponding values in patients undergoing PCI were 1.42 (95% CI: 1.09–1.85) and 4.04 (95% CI: 3.19–5.11), respectively (Table S1). The C-statistic values estimated by CHA2DS2-VASc score in CABG and PCI were 0.796 (95% CI: 0.778–0.815, P < 0.001) and 0.640 (95% CI: 0.628–0.651, P < 0.001), respectively (Figure S3).
Table 4 shows the association between post-procedural ischemic stroke and the risk of in-hospital mortality. In CABG, the average days between the CABG and the mortality events for patients with and without post-procedural stroke were 48.1 and 17.3 days, respectively. In PCI, the corresponding values were 10.7 and 6.8 days, respectively. Post-procedural ischemic stroke was significantly associated with in-hospital mortality in PCI, but not in CABG after adjustment for CHA2DS2-VASc score and other potential confounders.
Discussion
In this study from a national database identifying 54,714 CABGs and 263,063 PCIs between 2013 and 2015 in China, CHA2DS2-VASc score was positively associated with the risk of post-procedural ischemic stroke. This is the first study, to our knowledge, to simultaneously evaluate the CHA2DS2-VASc score in both CABG and PCI. Epidemiologic, quality evaluation, and health services studies aimed at improving the health outcomes of patients undergoing CABG and PCI are gaining increasingly more attention4,5,6, CHA2DS2-VASc score promises to be a highly useful tool in such research.
The prevention of post-procedural ischemic stroke has significant clinical implications because of its significant impacts on prognoses of patients after primary treatment9,10,11. Several risk stratification schemes have been proposed to stratify risk of post-procedural stroke, such as the Multicenter Study of Perioperative Ischemia Research Group (McSPI) and the Northern New England Cardiovascular Disease Study Group (NNECDSG) scores24, 25. However, none has been widely applied in routine clinical practice. CHA2DS2-VASc score, which was initially employed as a risk assessment tool for predicting stroke in patients with AF, has been extensively used in clinical practice and in some guidelines for treatment selection for stroke prevention13, 26. Recently, CHA2DS2-VASc score has been used to discriminate patients at high risk of stroke in patients undergoing CABG surgery14,15,16,17. In this study, we also observed a positive association between CHA2DS2-VASc score and post-procedural ischemic stroke following CABG. The simplicity and strong operability of CHA2DS2-VASc algorithm would be beneficial to ensure routine evaluation in the clinical setting. These findings suggest that the CHA2DS2-VASc score could be used as a complementary approach of stratifying the post-procedural ischemic stroke risk in patients undergoing CABG. The ability of CHA2DS2-VASc scores in predicting post-procedural ischemic stroke is of concern and provides a critical analysis for possible prevention strategies.
In this study, CHA2DS2-VASc score was significantly associated with the risk of post-procedural ischemic stroke following PCI. Although the predictive value of CHA2DS2-VASc score in predicting ischemic stroke following PCI is less established, the individual components of the CHA2DS2-VASc score have been demonstrated to represent significant risk factors for post-procedural ischemic stroke27, 28. For example, an analysis of 426,046 patients undergoing PCI in England and Wales between 2007 and 2012 in the British Cardiovascular Intervention Society (BCIS) database demonstrated that age, female gender, a history of stroke were significant predictors of post-procedural ischemic stroke/TIA, and ischemic stroke was independently associated with both 30-day mortality and in-hospital major adverse cardiovascular events (a composite of in-hospital mortality, myocardial infarction or reinfarction, and revascularization)27. An observational, multicenter, prospective study including 929 patients with AF indicated that a high CHA2DS2-VASc score was predictive of major adverse events (a composite of all-cause mortality, myocardial infarction, repeat revascularization, stent thrombosis, transient ischemic attack, stroke or other arterial thromboembolism) following PCI20. In our study, the use of ischemic stroke as the end point instead of a combined end point can better understand the association of CHA2DS2-VASc score with health outcomes following PCI.
The CHA2DS2-VASc score with risk estimates may possess some value in clinical practice. The CHA2DS2-VASc score could be used as a single index that reflect the overall burden of age, gender and comorbid conditions; this would diminish the confounding bias that results from these factors without necessitating the extremely large sample sizes that would be required to control for each condition separately29. A substantial confounding bias can be controlled if a strong risk factor could be accurately measured in health outcome studies30. Our study also illustrates the necessity to account for these factors. Patients undergoing CABG and PCI with a CHA2DS2-VASc score ≥5 had 40.5 and 6.0 times the risk of developing ischemic stroke, respectively, than those with a CHA2DS2-VASc score of 0. The CHA2DS2-VASc score can also be used to classify patients into risk categories or levels, leading to more tailored approaches for patient management. It may be useful in helping physicians to determine what treatment option would best suit a specific patient. In addition, using the CHA2DS2-VASc score as a screening tool, patients could be cared for in a suitable setting. Patients with a high CHA2DS2-VASc score suggesting a significant risk of post-procedural ischemic stroke would receive a high standard of medical care. Therefore, the CHA2DS2-VASc should be taken into consideration when planning individual treatment course. Research indicate that comorbid conditions had substantial influences on health outcomes and quality of life among cardiovascular patients after primary treatment31. Therefore, the CHA2DS2-VASc algorithm can be used as a supplementary factor to consider when managing follow-up care after primary treatment. Studies that examine these and other possible clinical applications of the CHA2DS2-VASc algorithm should be conducted to improve clinical practice.
Unique features of this study include the large sample size, national multicenter design, and real world data on both CABG and PCI procedures, all of which increase the generalizability of findings. However, our study has a few noteworthy limitations. First, the retrospective data collection and analysis may have introduced some confounding bias. Another limitation was our inability to account for the influences caused by patient- and procedure-related characteristics, such as smoking status, dietary habits and physical activity. In addition, each diagnosis of disease and procedure in this study was made by ICD codes, which may cause bias from coding errors. However, the validity and reliability of this database have been proved in prior studies32, 33. To minimize such potential confounding bias, we used the corresponding Chinese terms to check the identified diseases and procedures. Antithrombotic therapy may affect the development of post-procedural ischemic stroke. However, data on antithrombotic therapies was not available in our database. An additional analysis stratified on the use of oral anticoagulant drugs should be performed in the future study. Finally, it could be useful to compare with GRACE score or other surgical risk scores to predict stroke. However, as data on several components of these scores, such as creatinine level and left ventricular ejection fraction, was not available in our database, we cannot calculate these scores for each patient. Future studies are needed to evaluate the performance of these scores in the prediction of post-procedural ischemic stroke.
The present study is the first to simultaneously evaluate the CHA2DS2-VASc score in both CABG and PCI. The risk of post-procedural ischemic stroke increased with a high CHA2DS2-VASc score. Future studies should be conducted to test the performance and clinical application of the CHA2DS2-VASc score in various databases.
References
Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171 (2015).
Zhou, M. et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 387, 251–272 (2016).
Epstein, A. J., Polsky, D., Yang, F., Yang, L. & Groeneveld, P. W. Coronary revascularization trends in the United States, 2001–2008. Jama 305, 1769–1776 (2011).
Topaz, G. et al. Long term prognosis of atrial fibrillation in ST-elevation myocardial infarction patients undergoing percutaneous coronary intervention. Int J Cardiol 16, 32197–32190 (2017).
Lamy, A. et al. Five-Year Outcomes after Off-Pump or On-Pump Coronary-Artery Bypass Grafting. N Engl J Med 375, 2359–2368 (2016).
Cohen, D. J. et al. Quality of life after PCI with drug-eluting stents or coronary-artery bypass surgery. N Engl J Med 364, 1016–1026 (2011).
Lamy, A. et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 366, 1489–1497 (2012).
Taglieri, N. et al. Risk of Stroke in Patients with Stable Coronary Artery Disease Undergoing Percutaneous Coronary Intervention versus Optimal Medical Therapy: Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 11 (2016).
Selim, M. Perioperative stroke. New England Journal of Medicine 356, 706–713 (2007).
Stamou, S. C. et al. Stroke after coronary artery bypass: incidence, predictors, and clinical outcome. Stroke 32, 1508–1513 (2001).
Tarakji, K. G., Sabik, J. F. 3rd, Bhudia, S. K., Batizy, L. H. & Blackstone, E. H. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. Jama 305, 381–390 (2011).
Shahian, D. M. et al. The STS AVR+ CABG composite score: a report of the STS Quality Measurement Task Force. Ann Thorac Surg 97, 1604–1609 (2014).
Lip, G. Y., Nieuwlaat, R., Pisters, R., Lane, D. A. & Crijns, H. J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137, 263–272 (2010).
Biancari, F., Asim Mahar, M. A. & Kangasniemi, O. P. CHADS2 and CHA2DS2-VASc scores for prediction of immediate and late stroke after coronary artery bypass graft surgery. J Stroke Cerebrovasc Dis 22, 1304–1311 (2013).
Hornero, F. et al. Stroke after coronary artery bypass grafting: preoperative predictive accuracies of CHADS2 and CHA2DS2VASc stroke risk stratification schemes. J Thorac Cardiovasc Surg 144, 1428–1435 (2012).
Peguero, J. G. et al. Usefulness of the CHA2DS2VASc score to predict postoperative stroke in patients having cardiac surgery independent of atrial fibrillation. Am J Cardiol 115, 758–762 (2015).
Hu, W. S. & Lin, C. L. Postoperative ischemic stroke and death prediction with CHA2DS2-VASc score in patients having coronary artery bypass grafting surgery: A nationwide cohort study. Int J Cardiol 12, 34109–34102 (2017).
Barra, S. et al. Stroke prediction with an adjusted R-CHA2DS2VASc score in a cohort of patients with a Myocardial Infarction. Thromb Res 132, 293–299 (2013).
Varmdal, T. et al. Percutaneous Coronary Intervention as a Trigger for Stroke. Am J Cardiol 119, 35–39 (2017).
Puurunen, M. K. et al. CHADS2, CHA2DS2-VASc and HAS-BLED as predictors of outcome in patients with atrial fibrillation undergoing percutaneous coronary intervention. Thromb Res 133, 560–566 (2014).
Baber, U. et al. Comparative efficacy of coronary artery bypass surgery vs. percutaneous coronary intervention in patients with diabetes and multivessel coronary artery disease with or without chronic kidney disease. Eur Heart J 37, 3440–3447 (2016).
Rocca, W. A. et al. Stroke incidence and survival in three Sicilian municipalities. Sicilian Neuro-Epidemiologic Study (SNES) Group. Ital J Neurol Sci 19, 351–356 (1998).
Agrawal, R., Imieliński, T. & Swami, A. In Acm sigmod record. 207–216 (ACM).
Newman, M. F. et al. Multicenter preoperative stroke risk index for patients undergoing coronary artery bypass graft surgery. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Circulation 94, II74–80 (1996).
Charlesworth, D. C. et al. Development and validation of a prediction model for strokes after coronary artery bypass grafting. Ann Thorac Surg 76, 436–443 (2003).
Fuster, V. et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 123, 7 (2011).
Kwok, C. S. et al. Stroke following percutaneous coronary intervention: type-specific incidence, outcomes and determinants seen by the British Cardiovascular Intervention Society 2007–12. Eur Heart J 36, 1618–1628 (2015).
Myint, P. K. et al. Determinants and Outcomes of Stroke Following Percutaneous Coronary Intervention by Indication. Stroke 47, 1500–1507 (2016).
Charlson, M., Szatrowski, T. P., Peterson, J. & Gold, J. Validation of a combined comorbidity index. J Clin Epidemiol 47, 1245–1251 (1994).
Schneeweiss, S. & Maclure, M. Use of comorbidity scores for control of confounding in studies using administrative databases. Int J Epidemiol 29, 891–898 (2000).
Radovanovic, D. et al. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart 100, 288–294 (2014).
Tian, Y., Xu, B., Yu, G., Li, Y. & Liu, H. Age-adjusted charlson comorbidity index score as predictor of prolonged postoperative ileus in patients with colorectal cancer who underwent surgical resection. Oncotarget 11, 15285 (2017).
Tian, Y., Xu, B., Yu, G., Li, Y. & Liu, H. Comorbidity and the risk of anastomotic leak in Chinese patients with colorectal cancer undergoing colorectal surgery. Int J Colorectal Dis 23, 017–2798 (2017).
Acknowledgements
This work was supported by the National Natural Science Fund Projects of China [No. 81473043].
Author information
Authors and Affiliations
Contributions
H.L. contributed to the study concept. H.L. had full access to all the data in the study and take responsibility for the integrity of the data. Y.T., H.L. and C.Y. contributed to the statistical analysis and tables’ development of this article. Y.T., H.L. and C.Y. interpreted the findings and drafted the article. All the authors contributed to the critical revision of the article for important intellectual content.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, Y., Yang, C. & Liu, H. CHA2DS2-VASc score as predictor of ischemic stroke in patients undergoing coronary artery bypass grafting and percutaneous coronary intervention. Sci Rep 7, 11404 (2017). https://doi.org/10.1038/s41598-017-11923-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11923-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.