Abstract
Secretory phospholipase A2 Group-IIA (sPLA2-IIA) is involved in lipid catabolism and growth promoting activity. sPLA2-IIA is deregulated in many pathological conditions including various cancers. Here, we have studied the role of sPLA2-IIA in the development of cyclic alopecia and wound healing response in relation to complete loss of hair follicle stem cells (HFSCs). Our data showed that overexpression of sPLA2-IIA in homozygous mice results in hyperproliferation and terminal epidermal differentiation followed by hair follicle cycle being halted at anagen like stage. In addition, sPLA2-IIA induced hyperproliferation leads to complete exhaustion of hair follicle stem cell pool at PD28 (Postnatal day). Importantly, sPLA2-IIA overexpression affects the hair shaft differentiation leading to development of cyclic alopecia. Molecular investigation study showed aberrant expression of Sox21, Msx2 and signalling modulators necessary for proper differentiation of inner root sheath (IRS) and hair shaft formation. Further, full-thickness skin wounding on dorsal skin of K14-sPLA2-IIA homozygous mice displayed impaired initial healing response. Our results showed the involvement of sPLA2-IIA in regulation of matrix cells differentiation, hair shaft formation and complete loss of HFSCs mediated impaired wound healing response. These novel functions of sPLA2-IIA may have clinical implications in alopecia, cancer development and ageing.
Similar content being viewed by others
Introduction
Skin constantly renews itself throughout the adult life that acts as a protective barrier against pathogens, radiation etc.1. Adult skin is mainly composed of epidermis, dermis and hypodermis. Epidermal components mainly include interfollicular epidermis (IFE), hair follicle and sebaceous gland2. Dermis and hypodermis are mainly composed of collagen, elastic fibers and extrafibrillar matrix with various cell types including fibroblasts, adipose cells and macrophages. During embryogenesis, hair follicle is formed as an appendage of the epidermis by condensation of specialized mesenchymal cells (dermal papilla) in the dermis. The basal layer cells overlying mesenchymal cells get stimulated and subsequently form placode at E14.5 that proliferate and grow downward as a mature hair follicle at E16.5-E17.53. The hair follicle cycle comprises of distinct stages such as telogen (resting phase), anagen (growth phase) and catagen (regression phase)4. Initially, both the pulse-chase studies carried out using tritiated thymidine (3H) and BrdU showed the presence of infrequently dividing cells in the bulge of hair follicle5. Subsequently, pTre-H2BGFP/K5tTa (Tet off) double transgenic mice study showed that the hair follicle stem cells are highly dynamic, which divide infrequently and undergo random chromosome segregation to maintain tissue homeostasis6,7,8. During follicle regeneration, the dermal papilla at the base of hair follicle provides initiatory signalling cues generated from the mesenchymal niche9. The cyclic growth of hair follicle is coordinated by the stem cells residing at the base of the bulge, which proliferate and migrate to provide progeny required for the hair-follicle regeneration and hair growth10. Further, the temporal activity of hair follicle stem cells is strictly dependent on interplay between mesenchymal niche and bulge, which is governed by secretion of various signalling modulators such as Wnt, BMP, Shh, and FGF11. In particular, Wnt signalling is necessary for the hair follicle morphogenesis and required for stem cell proliferation and differentiation during hair follicle regeneration12,13,14. Additionally, EGF induced EGFR signalling is indispensable for the initiation of hair growth15. The EGF signalling modulator, secretory phospholipase A2 group IIA (sPLA2-IIA) is also known as enhancing factor (EF), which expressed by paneth cells in the small intestine16, 17. sPLA2-IIA has two independent activities, catalytic and non-catalytic (enhancing) and both these activities rely on the two different domains of this enzyme18. Also, various studies have reported the expression of sPLA2-IIA in mouse epidermis19, 20. Moreover, sPLA2-IIA has been implicated in various forms of cancer such as intestinal, colorectal, prostrate21 and overexpression of sPLA2-IIA in mice epidermis showed increased susceptibility towards chemical carcinogenesis22. We have recently reported that sPLA2-IIA enhances the expression of Hb-EGF, EPGN and downstream c-Jun and Fos-B23. However, the molecular insight, if sPLA2-IIA regulates the hair shaft differentiation and the development of alopecia is still unknown. Secondly, do cells of other epidermal components form hair in the complete absence of hair follicle stem cells? Thirdly, whether wound healing process is impaired in the absence of HFSCs is yet to be determined.
Notably, deregulation of various signalling modulators perturbs stem cells maintenance that may results in development and progression of cyclic alopecia. Impaired EGFR signalling by dominant negative mutant of epidermal growth factor receptor in the epidermis prevents the progression of the hair cycle to catagen stage and causes severe alopecia24. Further, knockout of Sox21 in mice alters differentiation of cuticle layer leading to development of cyclic alopecia25. Also, epidermal ablation of Smad4 resulted in hyperplasia of interfollicular epidermis (IFE) and sebaceous glands (SGs), that leads to exhaustion of the SC niche and progressive hair loss26. Expression of noggin in epidermis resulted in upregulated Wnt signalling with epidermal hyperplasia, progressive hair loss, and formation of trichofolliculoma-like tumors27. Msx2 deficiency exhibits abnormal structure of hair shafts and cycles of hair loss and regrowth28. Targeted disruption of Orai1 gene in mouse showed sporadic hair loss while inducible deletion of cnB1 gene in mice resulted in altered hair follicle structure and its mesenchyme adhesion, that causes cyclic alopecia29, 30. However, the molecular mechanism involved in cyclic alopecia is yet to be discovered.
In this report, we studied the effect of K14-sPLA2-IIA expression in homozygous mice on alopecia and wound healing. To our knowledge, for the first time our findings on K14-sPLA2-IIA homozygous mice showed hair loss that is associated with an increased proliferation and differentiation of hair follicle stem cells, which led to exhaustion of hair follicle stem cells and development of cyclic alopecia at an early age. Additionally, K14-sPLA2-IIA homozygous mice showed impaired healing response during full thickness epidermal wounding.
Results
Altered hair follicle development and hair cycling in K14-sPLA2-IIA homozygous mice
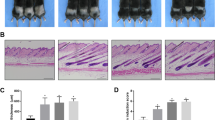
To check the expression pattern of sPLA2-IIA in mice epidermis, we performed the IHC staining of sPLA2-IIA on skin sections of wild type FVB mice at various postnatal ages. Our data showed that sPLA2-IIA expresses in basal layer, suprabasal layer and outer root sheath of hair follicle during morphogenesis, first hair cycle and one year old age (Supplementary Fig. S1). K14-sPLA2-IIA homozygous mice exhibited visible phenotypic growth abnormalities and are significantly smaller than the control littermates (Fig. 1a). Further, to confirm the nutritional status of the K14-sPLA2-IIA homozygous mice, we have quantified various nutritional parameters from the serum of the K14-sPLA2-IIA homozygous mice. Our data showed that there are no significant alterations in serum components such as serum albumin, Vitamin D 25-OH, Triglyceride, Sodium, Chloride, and marginal increase was observed in total protein, Vitamin B12, Calcium and Potassium (Supplementary Fig. S4). However, we have observed reduced serum glucose level after eight hours fasting in the K14-sPLA2-IIA homozygous mice (Supplementary Fig. S4). These results demonstrate that there are no significant alterations in the nutritional parameters of the K14-sPLA2-IIA homozygous mice. These K14-sPLA2-IIA homozygous mice showed progressive hair loss during hair follicle morphogenesis periods (PD15 and PD19). Further, haematoxylin and eosin staining (H&E) on the dorsal skin sections was performed during various postnatal days (PD15, 19, 21, 25, 28, 35, 41 and 49) (Fig. 1b). Our histological data revealed interfollicular epidermal cyst formation and abnormal thickening of the interfollicular epidermis (IFE) (Fig. 1c) as compared to wild type control littermate. Further, hair follicle cycling analysis on the dorsal skin at various postnatal days showed hair follicle is halted at anagen like stage in K14-sPLA2-IIA homozygous mice (Fig. 1d). This is due to the fact that we have not observed telogen at any postnatal day (PD15, PD17, PD21, PD25, PD28, PD30, PD35, PD41, PD45 and PD49). The change in morphology of the hair follicle is due to abnormal development of epidermal compartments. Further, we observed increased activation of β-catenin in K14-sPLA2-IIA homozygous mice skin as compared to hemizygous and wild type control littermate (Supplementary Fig. S2). These data indicate that the hair follicles of K14-sPLA2-IIA homozygous mice failed to progress into regression phase (Catagen) of the hair follicle cycle. Thus, epidermal overexpression of sPLA2-IIA results in morphological abnormalities of hair follicle with hair loss.
sPLA2-IIA overexpression altered the hair cycle with abnormal hair follicle morphology and hair loss. (a) Phenotypic appearance of WT, hemizygous and homozygous mice at PD21. (b) Images represents hematoxylin and eosin staining (H&E) of skin sections at various postnatal days of WT and K14-sPLA2-IIA homozygous mice to study hair follicle cycling. (c) Graphical representation of epidermal thickness measurements are in μm. Data are presented as mean ± SD. **P < 0.005, ***P < 0.001, ****P < 0.0001. (d) Comparative analysis of hair follicle progression to different stages of hair cycle (Anagen, Catagen and Telogen) at different postnatal days in WT and K14-sPLA2-IIA homozygous mice. (WT-Wild type, Homo- K14-sPLA2-IIA homozygous mice, n = 3 mice/genotype. A-Anagen, C-Catagen and T-Telogen, PD-Postnatal days).
Abnormal organization of epidermal components and stratification of epidermis
Morphological abnormalities of the hair follicle led us to investigate if there is any effect on proliferation and differentiation in different epidermal compartments. To evaluate the effect of sPLA2-IIA on cell proliferation, we have performed immunofluorescence staining of Ki67, a proliferation marker on wholemount of intact epidermal sheets (Fig. 2a). Our results showed increased number of Ki67 positive cells in the outer root sheath and dermal papillae of the hair follicles, suggesting enhanced proliferation in K14-sPLA2-IIA homozygous mice skin. Moreover, we observed enlarged width of infundibulum and junctional zone regions of the hair follicle in K14-sPLA2-IIA homozygous mice. Therefore, we sought to check the expression of Lrig1, the marker of junctional zone stem cells by immunofluorescence staining on skin section, which showed significant increase in the Lrig1 positive cells (Fig. 2b). Further, to study whether the hyper-proliferative cells of IFE also generates more differentiated progeny, the later stages of terminal differentiation was evaluated. Immunofluorescence staining of Loricrin showed enhanced expression of Loricrin in K14-sPLA2-IIA homozygous mice skin, which indicates that sPLA2-IIA markedly increased epidermal differentiation (Fig. 2c). This result was further confirmed by the Real time quantitative analysis of S100a9 mRNA expression that showed drastic upregulation, suggesting increased differentiation in K14-sPLA2-IIA homozygous mice (Supplementary Fig. S3).
sPLA2-IIA induced proliferation affects various epidermal compartments. (a) Immunofluorescence staining of Ki67 to assess cell proliferation in intact epidermal sheet of tail skin by wholemount assay at PD21. (b) Immunofluorescence labelling of Lrig1 in WT and K14-sPLA2-IIA homozygous mice skin sections at PD41. Dashed lines represent the boundary of junctional zone area. (c) Immunofluorescence staining of loricrin as a differentiation marker to label the cells of granular layers in WT and K14-sPLA2-IIA homozygous mice at PD49.
Complete exhaustion of hair follicle stem cells in K14-sPLA2-IIA homozygous mice
In K14-sPLA2-IIA homozygous mice, hair follicle was halted at anagen like stage and hair loss was observed with increase in proliferation and differentiation. Therefore, we attempted to understand the profile of hair follicle stem cells. We performed FACS analysis by using the mouse hair follicle stem cell markers such as CD34 and α6 integrin. Our results showed drastic depletion of CD34+/α6 integrin+ hair follicle stem cells at PD28 (Fig. 3a,b). Further, this data was validated by immunofluorescence staining (IFA) of CD34 and α6 integrin on the dorsal skin tissue sections at PD28 and PD49, which showed decrease in the number of CD34+/α6 integrin+ cells in hair follicle bulge of K14-sPLA2-IIA homozygous mice (Fig. 3c,d). Moreover, the counting of CD34/α6-integrin dual positive cells per hair follicle bulge showed complete loss of hair follicle stem cells at PD49 (Fig. 3e). In addition, to further confirm the loss of the hair follicle stem cells compartment, we have checked the Sox9 positive hair follicle stem cells in bulge of the hair follicle. Our data showed an absence of Sox9 positive cells in bulge region of hair follicle in the K14-sPLA2-IIA homozygous mice as compared to the wild type control littermate (Supplementary Fig. S5). This clearly demonstrates that the hair follicle stem cells pool is depleted. These data suggest that sPLA2-IIA induced hyper-proliferative response may lead to the subsequent complete exhaustion of hair follicle stem cell pool and aging-like skin phenotype in K14-sPLA2-IIA homozygous mice.
Overexpression of sPLA2-IIA leads to depletion of hair follicle stem cells. (a) Flow cytometry based analysis of hair follicle stem cells (CD34+/α-6 integrin+) in WT and K14-sPLA2-IIA homozygous mice at PD28. (b) Quantification of FACS analysis of CD34+/α6-integrin+ bulge HFSCs in wild type and K14-sPLA2-IIA homozygous mice at PD28. (c) Immunofluorescence analysis of CD34 and Ki67 expression in hair follicle at PD28. Scale bar: 50 µm. (d) Immunofluorescence analysis of CD34+/α6-integrin+ dual positive cells in hair follicle at PD49. Scale bar: 50 µm. (e) Quantification of CD34+/α6-integrin+ cells in the bulge of the dorsal skin in wild type and K14-sPLA2-IIA homozygous mice at PD49. PD-Postnatal days, HFSCs-Hair follicle stem cells. (WT-Wild type Homo- K14-sPLA2-IIA homozygous mice, n = 3 mice/genotype, PD-Postnatal days. Data are presented as mean ± SD. **P < 0.005, ***P < 0.001, ****P < 0.0001).
Development of cyclic alopecia in K14-sPLA2-IIA homozygous mice
The new-born K14-sPLA2-IIA homozygous pups could be easily distinguished at PD3 as compared to wild type control littermate by their short and wavy whiskers. We followed the K14-sPLA2-IIA homozygous mice from birth (PD1) to up to six months (PD180) and recorded (photographed) the hair growth patterns over time at every alternate day. The hair loss began from PD18 and complete hair loss was observed at PD22 (Fig. 4a). Further, the hair regrowth was started at PD27 that partially covered the body by PD33 (Fig. 4a). However, the hair of K14-sPLA2-IIA homozygous mice was short compared to smooth and shiny hair of wild type control littermate. This successive cycle of hair growth and loss was occurring repetitively after 18–22 days up to 6–8 months (Fig. 4b). This cyclic alopecia was observed in both male and female mice. In addition, we observed permanent alopecia in K14-sPLA2-IIA homozygous mice starting at six months age till their survival (One year, Data not shown).
Cyclic alopecia in K14-sPLA2-IIA homozygous mice during various post-natal days. (a) Phenotypic appearance of WT and K14-sPLA2-IIA homozygous mice at different postnatal days with cyclic loss of hair and regain followed till six months. (b) Graphical representation of alopecia time points with respect to postnatal days of the K14-sPLA2-IIA homozygous mice. (c) Real time PCR gene expression analysis of Sox21, Msx2, Foxn1. (d) Gene expression analysis by Real time PCR of Gata3, Krt82 and Krt71. (e) Gene expression profiling of Lef1, BMP4, Shh. The relative quantification is with respect to expression level of β-actin. (WT-Wild type Homo- K14-sPLA2-IIA homozygous mice, n = 3 mice/genotype, PD-Postnatal days, Data are presented as mean ± SD. **P < 0.005, ***P < 0.001, ****P < 0.0001).
Aberrant hair shaft differentiation mediated through deregulated expression of signalling modulators
We further investigated whether this cyclic phenomenon relies on the deregulation of known molecules or is there an unexplored novel mechanism. The expression levels of known genes such as Sox21, Msx2, Zdhcc13 and Foxn1 were analysed. Real time PCR data showed significant downregulation of Sox21, Msx2 and Foxn1 mRNA expression as compared to wild type control littermate (Fig. 4c). However, we did not observe any significant difference in expression level of Zdhcc13 and Gsdma3 mRNA (Supplementary Fig. S3). Importantly, Sox21 acts as a regulator of keratins expression, that is necessary for the formation of IRS (Inner root sheath) and anchoring of hair shaft. We examined the status of Gata3, Krt82 and Krt71 by real time PCR analysis. We found significant down-regulation of Gata3, Krt82 and Krt71 expression that suggest an aberrant differentiation of IRS and hair shaft precursor cells in K14-sPLA2-IIA homozygous mice (Fig. 4d). To understand the molecular mechanisms underlying impaired differentiation of the matrix cells, we checked the level of BMP4, Shh and Lef1, which are known to regulate the matrix cells proliferation and generation of precursors for IRS and hair shaft formation. We observed significant downregulation of BMP4, Lef1 and Shh in K14-sPLA2-IIA homozygous mice skin (Fig. 4e). These data suggested that deregulated signalling in matrix cells may lead to defects in differentiation of IRS and formation of hair shaft.
Delayed wound healing response in K14-sPLA2-IIA homozygous mice
Hair follicle stem cells contribute during epidermal regeneration after wounding. However, we observed drastic depletion of hair follicle stem cells in K14-sPLA2-IIA homozygous mice, which led us to evaluate wound healing response in K14-sPLA2-IIA homozygous mice. Scratch wounds were made on upper region at midline (yellow circle) (Fig. 5a) whereas, full thickness wounds (8 mm) were made on lower region of dorsal skin at PD49, that were monitored to assess the macroscopic healing defects (Fig. 5a). Our data showed impaired initial healing response in K14-sPLA2-IIA homozygous mice at day 5 (red circle) (Fig. 5b). However, the pace of wound recovery accelerated after 5 to 6 days and fully recovered at the same time of wild type control littermate (green circle) (Fig. 5b). To further investigate, whether the impaired wound healing response is due to the poor nutrition, we have checked the levels of serum albumin, Vitamin C, Vitamin D and Zinc from the serum. Our data showed that there is no significant difference in the levels of serum albumin, Vitamin C, Vitamin D and Zinc in the serum of the K14-sPLA2-IIA homozygous mice as compared to wild type control littermate (Supplementary Fig. S4). These results confirmed that the initial defects in the wound healing response may not be due to the poor nutrition. Thus, it demonstrates that overexpression of sPLA2-IIA delays initial response to wounding; however, the complete wound was filled at the same time point as compared to wild type control littermate.
Impaired initial response to wounding in K14-sPLA2-IIA homozygous mice skin. (a) Photographs of WT and K14-sPLA2-IIA homozygous mice at Day 0 (before wounding) and at Day 0 (after wounding). The healing process was recorded by photographs at various days and representative images are mentioned as Day 5, Day 10 and Day 15. (b) Graphical representation of comparative wound recovery during healing process. Red circle indicates defects in initial healing response in K14-sPLA2-IIA homozygous mice. Green circle indicates no significant difference in time periods for complete wound recovery during later stage. (WT-Wild type Homo- K14-sPLA2-IIA homozygous mice, n = 3 mice/genotype, PD-Postnatal days).
Discussion
The regenerative capability of adult tissue relies on activity of long-lived tissue specific stem cells. The cyclic activation of quiescent hair follicle stem cells depends on signalling cues present in the surrounding microenvironment. Involvement of different growth factors including EGF, TGFs and FGFs is well established in regulation of epidermal stem cells proliferation and fate determination11. As previously shown, deregulated release of Hb-EGF in mutant mice expressing soluble proHb-EGF induced epidermal hyperproliferation and displayed hyperplasia31. Overexpression of TGFα in transgenic mice epidermis demonstrates aberrant keratins expression, hyperproliferation and development of papillomas32. Further, expression of a dominant-negative FGF receptor mutant in transgenic mice under the control of Keratin 10 (K10) promoter induced epidermal hyper-thickening and altered keratinocytes differentiation33. Similarly, K14-sPLA2-IIA homozygous mice showed alterations in the hair follicle cycling, that are stuck in the anagen like phase of hair follicle and enhanced proliferation in various compartments of epidermis leading to epidermal hyperplasia. The sPLA2-IIA is known to enhance the EGF signalling in astrocytoma cells34 and continuous expression of epidermal growth factor prevents entry of hair follicle into the regression phase (Catagen) of hair cycle15. In agreement, our data of increased proliferation and hair follicle stuck at anagen like phase may be due to sPLA2-IIA mediated enhanced EGF signalling. Precisely, as EGF receptors are known to be expressed in the cells of outer root sheath of the hair follicle, and the proliferative response generated by upregulated mitogens (Hb-EGF and EPGN) directly suggest the involvement of catalytic independent activity of sPLA2-IIA in modulating EGFR signalling, which is not the case for the other group of secretory phospholipases. An aberrant signalling mediated hyperproliferation of the HFSCs resulted in loss of quiescence and exhaustion of stem cell pool. Smad4 deletion in mouse epidermis inhibits programmed regression of the hair follicles and exhibits progressive alopecia with depletion of hair follicle stem cells26, 35. Also, activation of mTOR by Wnt overexpression resulted in depletion of the hair follicle stem cells and accelerates hair loss and aging36. In agreement, our results showed hyperproliferation in various compartments of the hair follicle, that may lead to loss of quiescence and exhaustion of the hair follicle stem cells and development of alopecia.
Further, the contribution of the hair follicle stem cells in wound re-epithelialisation following injury is well established. Initially, the bulge-derived transient amplifying cells rapidly respond to the injury that contribute in wound repair and subsequently replaced by IFE derived cells over several weeks37. In contrast, partial ablation of the hair follicle stem cells by diphtheria toxin does not delay healing process following wounding thereby, suggesting that the bulge cells are dispensable for wound re-epithelialisation38. Epidermal hyperproliferation induced by deficiency of Jun-B resulted in delayed wound healing response39. Further, Runx1 deletion in mice showed delay in the activation of the HFSCs, which are further activated by skin injury40. Importantly, it has been shown that the bulge, hair follicle stem cells contribute to the process of wound healing and start migrating towards the wounded area within 24 hours, which suggests the involvement of the hair follicle stem cells in the initial process of wound healing6, 37, 41, 42. Our data of K14-sPLA2-IIA homozygous mice suggested that delay in initial response to wounding is likely due to the absence of immediate progenitor of the hair follicle stem cells, that sense the injury and effectively contributes towards initial healing response. Further, in agreement with the previous reports, IFE derived cells may take over the function and efficiently heal the wounded area at later time points.
Moreover, various studies have shown the phenomenon of permanent or cyclic alopecia in different genetic backgrounds of mice. The mice expressing dominant negative mutant of EGFR display severe alopecia24. Importantly, mice overexpressing human group II PLA2 displayed epidermal hyperplasia and complete alopecia43. Similarly overexpression of Pla2g2f in mice epidermis showed psoriasis like epidermal hyperplasia and alopecia44. Genetic disruption of the Sox21 and Msx2 resulted in defects in anchoring of hair shaft25 and aberrant hair shaft differentiation28 respectively. However, the level of Sox21 remained unaltered in Msx2 null background suggesting independent mode of action of both the molecules in the development of cyclic alopecia. We observed the downregulation of Sox21 and Msx2 in the K14-sPLA2-IIA homozygous mice suggesting that sPLA2-IIA may function upstream of both the factors and altering their expression. Further, mutation mediated downregulation of Zdhhc13 in mice exhibits epidermal hyperproliferation, abnormal hair follicle growth and cyclic alopecia at early telogen45, 46. However, we did not observe any alteration in Zdhhc13 level suggesting that the development of cyclic alopecia in K14-sPLA2-IIA homozygous mice is independent of cornifelin deficiency mediated cyclic alopecia as shown in Zdhhc13 mutant mice.
Importantly, the proliferation and fate determination of matrix cells to differentiate in the IRS and hair shaft is governed by various signalling modulators such as BMP4, Lef1 and Shh. Specifically, BMP4 is expressed in the hair matrix cells and dermal papillae and is known to be involved in differentiation of the hair matrix cells to precursors cells required for hair shaft formation. Our results of gene expression study are in agreement with the previous study of sPLA2-X overexpressing transgenic mice, that showed down regulation of BMP4, Shh, Lef1, Foxn1 and Gata3 during development of cyclic alopecia47. Together, we found that overexpression sPLA2-IIA enhances the terminal epidermal differentiation however, the differentiation of matrix cells to produce precursors of the IRS and hair shaft is severely affected. This is likely due to the deregulated expression of keratin gene regulators such as Sox21 and matrix cells differentiation to produce hair shaft. Overall, these results suggest that sPLA2-IIA may affect differentiation of matrix cells. With regards to our finding, we have proposed the model for comparative hair follicle cycling (Fig. 6). Altered hair follicle cycling in K14-sPLA2-IIA homozygous mice represented by abnormal morphology of hair follicle with the presence of affected hair shaft. The hair follicle cycling does not progress through the first hair cycle, as the hair follicles are being stuck in anagen like stage during the initiation of first hair follicle cyling process. Further at PD28, hair follicle is represented by presence of affected hair shaft due to downregulated expression of Sox21, Msx2 and Foxn1, which are known to be involved in the IRS differentiation. Moroever, downregulation of signalling modulators such as Bmp4, Shh and Lef1 is observed in K14-sPLA2-IIA homozygous mice. Together, downregulated expression of hair shaft differentiation regulators may lead to the development of alopecia in K14-sPLA2-IIA homozygous mice.
Proposed model of impaired hair follicle cycling in K14-sPLA2-IIA homozygous mice. WT- Graphical representation of hair follicle cycling in wild type mice from hair follicle morphogenesis to second telogen at PD49, which represented by presence of club hair and subsequent progression to the next hair cycle. Homozygous- Graphical representation of hair follicle cycling in K14-sPLA2-IIA homozygous mice represented by abnormal morphology of hair follicle with presence of affected hair shaft. (WT-Wild type Homo- K14-sPLA2-IIA homozygous IFE: inter follicular epidermis, SG: sebaceous gland, DP: dermal papilla, PD-Postnatal days).
In conclusion, we showed that sPLA2-IIA mediated increased proliferation results in the complete exhaustion of the HFSCs and development of alopecia due to aberrant expression of hair shaft differentiation regulators. The cyclic growth of hair and complete re-epithelialization of epidermis after wounding in absence of the HFSCs explains the functional involvement of stem cells from other compartments of the epidermis. This further opens a new avenue to explore the role of stem cells of other compartments of the epidermis and their ability to differentiate in hair shaft producing cells. This study provides a conceptual evidence of functional redundancy within stem cells population that reside in various compartments of epidermis. Further, the non-catalytic activity of sPLA2-IIA may provide information to develop potential sPLA2-IIA inhibitors for effective inhibition of sPLA2-IIA activity during various patho-physiological conditions such as arthritis, inflammation and cancer.
Materials and Methods
Transgenic mice and genotyping
K14-sPLA2-IIA mice were a gift from Dr. Rita Mulherkar22. The hemizygous K14-sPLA2-IIA was crossed to hemizygous K14-sPLA2-IIA mice to obtain the homozygous mice for the experiments. The K14-sPLA2-IIA hemizygous and homozygous transgenic mice were obtained based on phenotype and PCR genotyping as described previously22. Mice were sacrificed at various postnatal ages. Animal experimental study was approved by ACTREC’s Institutional Animal Ethics Committee (IAEC), and all the experiments were performed in accordance with the approved guidelines and regulations.
Histology, Immunostaining and tail whole mount assay
Mice skin tissue (dorsal and tail skin) at various postnatal day ages were collected and fixed by neutral buffered formalin (NBF) or directly embedded in OCT compound (Tissue-Tek) and frozen. Immunofluorescence assays (IF) and Immunohistochemical analysis (IHC-P) was performed as previously described7. Paraffin embedded tissue blocks were sectioned and Haematoxylin and Eosin staining (H&E) was performed to analyze the phase of the hair follicle cycle. Immunofluorescence assays (IF) and Immunohistochemical analysis (IHC-P) was performed on OCT frozen tissue and Paraffin embedded block respectively23. Tail whole mount was performed as described previously48. Briefly, tail skin was incubated in 5 mM EDTA followed by separation of epidermal sheet from dermis followed by fixing with 2% formaldehyde for 10 minutes. Nile red was used to stain the sebocytes of sebaceous gland and confocal microscopy was used for image acquisition. Primary antibodies used such as: CD34 (1:100, BD Pharmingen); α-6 integrin (1:100, BD Pharmingen); BrdU (1:250, Abcam); Ki67 (1:100; Novocastra); Filaggrin (1:1000, Abcam); Loricrin (1:1000, Abcam); K10 (1:1000, Abcam); Lrig1 (1:500, R&D systems) and Sox9 (1:500, Merck Millipore).
Fluorescence activated cell sorting analysis
Dorsal skin of wild type control littermate and K14-sPLA2-IIA homozygous mice at PD21 and PD49 was harvested and scrapped for fat removal, followed by overnight incubation in 0.25% trypsin at 4 °C. FACS experiments were performed as described previously7. Single cell suspension was obtained by first passing through 70 μm and then 40 μm cell strainers (BD Biosciences). Cells were stained with trypan blue and the hematocytometer chamber was used to count the cells. Further cells were stained by using the hair follicle stem cells markers: CD34-Biotin (eBiosciences), Streptavidin-APC (BD Pharmingen), and anti–α6-integrin-PE (BD Pharmingen). After washing cells were subjected to FACS acquisition using a FACS Aria and data was analyzed by using FACS DiVa software (BD Biosciences).
Real-time quantitative PCR
Total RNA was extracted from mouse epidermis by using the Absolutely RNA Miniprep Kit (Agilent Technologies). 2 μg of total RNA was reverse transcribed using cDNA synthesis kit (Invitrogen, Carlsbad, CA) as per the manufacturer instructions. Quantitative PCR (q-PCR) was performed by using the SYBR Green (Invitrogen) as per the manufacturer’s instructions. Gene expression was normalized to β-actin. The relative expression levels of mRNAs were calculated by relative quantification method with respect to β-actin. For primers sequences please refer supplementary information.
Wound healing assay
K14-sPLA2-IIA homozygous mice and control mice of 49 days were anaesthetized with isofluorane by inhalation for 30–60 seconds. After hair removal from the dorsal surface, single 8 mm full thickness excision skin wound on the midline was created. During the healing periods, recovery of the wounds was photographed. The wounded tissue was collected, OCT blocks and Paraffin blocks were prepared. Histological sections were stained with hematoxylin and eosin on paraffin blocks.
Quantification of nutritional parameters from serum
Wild type and K14-sPLA2-IIA homozygous mice were starved for eight hours before collecting the blood. Serum was separated by centrifugation at 6000 rpm for 10 mins. Further, ascorbic acid was quantified by using ascorbic acid assay kit (ab65656) and zinc was quantified by using zinc quantification kit (ab102507) as per the manufacturer’s instructions. The nutritional parameters such as serum albumin, serum glucose, triglyceride, Sodium, Phosphorus, Chloride, total protein, Calcium and potassium was quantified by using Siemens’s Dimension EXL with LM, automated biochem analyzer and Vitamin B12 and Vitamin D 25-OH was quantified by using Abbott’s architect plus i1000SR as per the manufacturer’s instructions.
Statistical analysis
Statistical significance was calculated to make the comparison between two groups by using the unpaired two-tailed student’s t-test with GraphPad Prism 5 for the data obtained from the measurement of IFE thickness, flow cytometry, Real time PCR, counting of hair follicle stem cells in bulge and nutritional parameters. Data represented with error bar indicating the mean ± SD of the mean: *P < 0.05, **P < 0.005, ***P < 0.0001.
Change history
13 March 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Fuchs, E. Skin stem cells: rising to the surface. J Cell Biol 180, 273–284, doi:https://doi.org/10.1083/jcb.200708185 (2008).
Blanpain, C. & Fuchs, E. Epidermal stem cells of the skin. Annual review of cell and developmental biology 22, 339–373, doi:https://doi.org/10.1146/annurev.cellbio.22.010305.104357 (2006).
Hardy, M. H. The secret life of the hair follicle. Trends Genet 8, 55–61 (1992).
Alonso, L. & Fuchs, E. The hair cycle. Journal of cell science 119, 391–393, doi:https://doi.org/10.1242/jcs02793 (2006).
Cotsarelis, G., Sun, T. T. & Lavker, R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004).
Waghmare, S. K. et al. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. Embo J 27, 1309–1320 (2008).
Waghmare, S. K. & Tumbar, T. Adult hair follicle stem cells do not retain the older DNA strands in vivo during normal tissue homeostasis. Chromosome research: an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology 21, 203–212, doi:https://doi.org/10.1007/s10577-013-9355-y (2013).
Rompolas, P. & Greco, V. Stem cell dynamics in the hair follicle niche. Seminars in cell & developmental biology 25–26, 34–42, doi:https://doi.org/10.1016/j.semcdb.2013.12.005 (2014).
Watt, F. M. & Jensen, K. B. Epidermal stem cell diversity and quiescence. EMBO Mol Med 1, 260–267, doi:https://doi.org/10.1002/emmm.200900033 (2009).
Lee, J. & Tumbar, T. Hairy tale of signaling in hair follicle development and cycling. Seminars in cell & developmental biology 23, 906–916, doi:https://doi.org/10.1016/j.semcdb.2012.08.003 (2012).
Huelsken, J., Vogel, R., Erdmann, B., Cotsarelis, G. & Birchmeier, W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105, 533–545 (2001).
Andl, T., Reddy, S. T., Gaddapara, T. & Millar, S. E. WNT signals are required for the initiation of hair follicle development. Dev Cell 2, 643–653 (2002).
Ito, M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320, doi:https://doi.org/10.1038/nature05766 (2007).
Mak, K. K. & Chan, S. Y. Epidermal growth factor as a biologic switch in hair growth cycle. J Biol Chem 278, 26120–26126, doi:https://doi.org/10.1074/jbc.M212082200 (2003).
Mulherkar, R. et al. Enhancing factor protein from mouse small intestines belongs to the phospholipase A2 family. FEBS Lett 317, 263–266 (1993).
Schewe, M. et al. Secreted Phospholipases A2 Are Intestinal Stem Cell Niche Factors with Distinct Roles in Homeostasis, Inflammation, and Cancer. Cell stem cell 19, 38–51, doi:https://doi.org/10.1016/j.stem.2016.05.023 (2016).
Kadam, S. & Mulherkar, R. Enhancing activity and phospholipase A2 activity: two independent activities present in the enhancing factor molecule. Biochem J 340(Pt 1), 237–243 (1999).
Gurrieri, S. et al. Differentiation-dependent regulation of secreted phospholipases A2 in murine epidermis. J Invest Dermatol 121, 156–164, doi:https://doi.org/10.1046/j.1523-1747.2003.12315.x (2003).
Ilic, D., Bollinger, J. M., Gelb, M. & Mauro, T. M. sPLA2 and the epidermal barrier. Biochim Biophys Acta 1841, 416–421, doi:https://doi.org/10.1016/j.bbalip.2013.11.002 (2014).
Scott, K. F. et al. Emerging roles for phospholipase A2 enzymes in cancer. Biochimie 92, 601–610, doi:https://doi.org/10.1016/j.biochi.2010.03.019 (2010).
Mulherkar, R. et al. Expression of enhancing factor/phospholipase A2 in skin results in abnormal epidermis and increased sensitivity to chemical carcinogenesis. Oncogene 22, 1936–1944, doi:https://doi.org/10.1038/sj.onc.1206229 (2003).
Sarate, R. M. et al. sPLA2 -IIA Overexpression in Mice Epidermis Depletes Hair Follicle Stem Cells and Induce Differentiation Mediated Through Enhanced JNK/c-Jun Activation. Stem Cells, doi:https://doi.org/10.1002/stem.2418 (2016).
Murillas, R. et al. Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure. Embo j 14, 5216–5223 (1995).
Kiso, M. et al. The disruption of Sox21-mediated hair shaft cuticle differentiation causes cyclic alopecia in mice. Proceedings of the National Academy of Sciences of the United States of America 106, 9292–9297, doi:https://doi.org/10.1073/pnas.0808324106 (2009).
Yang, L., Wang, L. & Yang, X. Disruption of Smad4 in mouse epidermis leads to depletion of follicle stem cells. Molecular biology of the cell 20, 882–890, doi:https://doi.org/10.1091/mbc.E08-07-0731 (2009).
Sharov, A. A. et al. Bone morphogenetic protein antagonist noggin promotes skin tumorigenesis via stimulation of the Wnt and Shh signaling pathways. Am J Pathol 175, 1303–1314, doi:https://doi.org/10.2353/ajpath.2009.090163 (2009).
Ma, L. et al. ‘Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development 130, 379–389 (2003).
Gwack, Y. et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol 28, 5209–5222, doi:https://doi.org/10.1128/mcb.00360-08 (2008).
Mammucari, C. et al. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell 8, 665–676, doi:https://doi.org/10.1016/j.devcel.2005.02.016 (2005).
Yamazaki, S. et al. Mice with defects in HB-EGF ectodomain shedding show severe developmental abnormalities. J Cell Biol 163, 469–475, doi:https://doi.org/10.1083/jcb.200307035 (2003).
Dominey, A. M. et al. Targeted overexpression of transforming growth factor alpha in the epidermis of transgenic mice elicits hyperplasia, hyperkeratosis, and spontaneous, squamous papillomas. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research 4, 1071–1082 (1993).
Werner, S. et al. Targeted expression of a dominant-negative FGF receptor mutant in the epidermis of transgenic mice reveals a role of FGF in keratinocyte organization and differentiation. Embo j 12, 2635–2643 (1993).
Hernandez, M., Martin, R., Garcia-Cubillas, M. D., Maeso-Hernandez, P. & Nieto, M. L. Secreted PLA2 induces proliferation in astrocytoma through the EGF receptor: another inflammation-cancer link. Neuro-oncology 12, 1014–1023, doi:https://doi.org/10.1093/neuonc/noq078 (2010).
Yang, L. et al. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res 65, 8671–8678, doi:https://doi.org/10.1158/0008-5472.can-05-0800 (2005).
Castilho, R. M., Squarize, C. H., Chodosh, L. A., Williams, B. O. & Gutkind, J. S. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell stem cell 5, 279–289, doi:https://doi.org/10.1016/j.stem.2009.06.017 (2009).
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature medicine 11, 1351–1354, doi:https://doi.org/10.1038/nm1328 (2005).
Garcin, C. L., Ansell, D. M., Headon, D. J., Paus, R. & Hardman, M. J. Hair Follicle Bulge Stem Cells Appear Dispensable for the Acute Phase of Wound Re-epithelialization. Stem Cells 34, 1377–1385, doi:https://doi.org/10.1002/stem.2289 (2016).
Florin, L. et al. Delayed wound healing and epidermal hyperproliferation in mice lacking JunB in the skin. J Invest Dermatol 126, 902–911, doi:https://doi.org/10.1038/sj.jid.5700123 (2006).
Osorio, K. M. et al. Runx1 modulates developmental, but not injury-driven, hair follicle stem cell activation. Development 135, 1059–1068 (2008).
Ito, M. & Cotsarelis, G. Is the hair follicle necessary for normal wound healing? J Invest Dermatol 128, 1059–1061, doi:https://doi.org/10.1038/jid.2008.86 (2008).
Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T. T. & Lavker, R. M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102, 451–461 (2000).
Grass, D. S. et al. II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J Clin Invest 97, 2233–2241, doi:https://doi.org/10.1172/jci118664 (1996). Expression of human group.
Yamamoto, K. et al. The role of group IIF-secreted phospholipase A2 in epidermal homeostasis and hyperplasia. The Journal of experimental medicine 212, 1901–1919, doi:https://doi.org/10.1084/jem.20141904 (2015).
Perez, C. J. et al. Increased Susceptibility to Skin Carcinogenesis Associated with a Spontaneous Mouse Mutation in the Palmitoyl Transferase Zdhhc13 Gene. J Invest Dermatol 135, 3133–3143, doi:https://doi.org/10.1038/jid.2015.314 (2015).
Liu, K. M. et al. Cyclic Alopecia and Abnormal Epidermal Cornification in Zdhhc13-Deficient Mice Reveal the Importance of Palmitoylation in Hair and Skin Differentiation. J Invest Dermatol 135, 2603–2610, doi:https://doi.org/10.1038/jid.2015.240 (2015).
Yamamoto, K. et al. Hair follicular expression and function of group X secreted phospholipase A2 in mouse skin. J Biol Chem 286, 11616–11631, doi:https://doi.org/10.1074/jbc.M110.206714 (2011).
Braun, K. M. et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development 130, 5241–5255, doi:https://doi.org/10.1242/dev.00703 (2003).
Acknowledgements
We thank Dr Rita Mulherkar for providing the K14-sPLA2-IIA mice and Dr Preeti Chavan for helping us in the quantification of nutritional parameters. We thank Vagisha Ravi, Priyanka Setia and Saloni Godbole for help in the Histology work. This work was partly supported by ACTREC ASF and ICMR Grant. We thank ACTREC Animal house, Flow cytometry and Microscopy facilities. GLC and RS supported by ACTREC fellowship, RMS supported by UGC fellowship.
Author information
Authors and Affiliations
Contributions
S.K.W. conceived and designed the project, analyzed and interpreted the data; G.L.C. and R.M.S. performed the experiments and analyzed; R.M.S. prepared the figures; V.K. performed the cyclic alopecia follow up; R.R.S. and N.P.G. helped in the Real time P.C.R.; G.L.C., R.M.S. and S.K.W. analyzed all the data; G.L.C. and S.K.W. wrote the manuscript; S.K.W. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chovatiya, G.L., Sarate, R.M., Sunkara, R.R. et al. Secretory phospholipase A2-IIA overexpressing mice exhibit cyclic alopecia mediated through aberrant hair shaft differentiation and impaired wound healing response. Sci Rep 7, 11619 (2017). https://doi.org/10.1038/s41598-017-11830-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11830-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.