Abstract
Maternal diabetes causes congenital malformations and delays embryonic growth in the offspring. We investigated effects of maternal diabetes on mouse embryos during gastrulation and early organogenesis (ED7.5–11.5). Female mice were made diabetic with streptozotocin, treated with controlled-release insulin implants, and mated. Maternal blood glucose concentrations increased up to embryonic day (ED) 8.5. Maternal hyperglycemia induced severe growth retardation (approx.1 day) in 53% of the embryos on ED8.5, death in most of these embryos on ED9.5, and the termination of pregnancy on ED10.5 in litters with >20% dead embryos. Due to this selection, developmental delays and reduction in litter size were no longer observed thereafter in diabetic pregnancies. Male and female embryos were equally sensitive. High-throughput mRNA sequencing and pathway analysis of differentially expressed genes showed that retarded embryos failed to mount the adaptive suppression of gene expression that characterized non-retarded embryos (cell proliferation, cytoskeletal remodeling, oxidative phosphorylation). We conclude that failure of perigastrulation embryos of diabetic mothers to grow and survive is associated with their failure to shut down pathways that are strongly down-regulated in otherwise similar non-retarded embryos. Embryos that survive the early and generalized adverse effect of maternal diabetes, therefore, appear the subset in which malformations become manifest.
Similar content being viewed by others
Introduction
The incidence of diabetes is increasing annually world-wide1, including that in pregnant young adults2. Preexisting diabetes affects over 1% of all pregnancies and is rising in prevalence. Diabetic pregnancies are associated with embryonic growth delay3 and increase the risk for congenital malformations in the offspring4,5,6.
Rodent models of pregestational diabetes are usually based on alloxan- or streptozotocin (STZ)-induced diabetes in females. Neural-tube and cardiovascular malformations are often seen7,8,9, and may result from an adverse effect of high glucose concentrations on the normal development of neural crest cells10,11. Neural-tube defects range from a wavy neural tube via spina bifida at various locations to exencephaly and craniorhachischisis12, while cardiovascular malformations typically display defects of the outflow tract, atrial septum and atrioventricular valves, and extensive apoptosis9,13. The frequency of these malformations in several mouse strains is 10–15% of live ED10.5–13.5 embryos7,9,13,14. In rats15, but possibly not in mice16, pregnancies in such preconceptionally diabetic females are accompanied by growth retardation, developmental delay, embryonic death, and increased perfusion of the affected implantation sites17.
The rodent embryo is most sensitive to the teratogenic effect of maternal hyperglycemia if exposed during the pre- and early somite stages of development (ED7.5–8.5 in the mouse)8,18,19. Accordingly, mutant mouse models suggest that perturbations of mesoderm specification and cell migration during and shortly after gastrulation can cause neural-tube and cardiovascular defects20. We reported recently that maternal hyperglycemia impaired expression of genes involved in cell proliferation in non-retarded ED8.5 mouse embryos, and genes involved in cytoskeletal remodeling and oxidative phosphorylation in non-retarded mouse ED9.5 embryos21. A quarter of the differentially regulated genes (mostly down-regulated) were associated with neural-tube or cardiovascular defects if deficient21. Studies of older mouse embryos (ED ≥ 10.5) with manifest diabetic embryopathy showed that maternal hyperglycemia affected the expression of genes involved in apoptosis, proliferation, migration and differentiation during organogenesis in the offspring9,12,22,23,24. This change in the spectrum of affected genes is compatible with developmental stage-specific adaptation of gene expression.
A major difference between our and earlier reported findings is that many ED8.5 and ED9.5 mouse embryos of diabetic mothers (“experimental” embryos) were developmentally retarded, whereas growth retardation and developmental delay were explicitly reported as not being observed in ED10.5 litters of diabetic dams23,25. In the present study, therefore, we investigated the developmental progress of embryos from diabetic mothers between ED7.5 and ED11.5 to determine if the manifestation of diabetic embryopathy at and after ED10.5 was preceded by a more general adverse effect on embryonic development. The study was performed in the FVB mouse strain, which is susceptible to malformations induced by maternal hyperglycemia26. Diabetes was induced with streptozotocin and treated with insulin implants, so that severe hyperglycemia only developed after implantation of the embryos7. Pathway analysis after high-throughput mRNA sequencing of one-day retarded experimental embryos revealed that these embryos typically failed to mount the adaptive and generally suppressive response in gene expression that characterized the ED8.5 and ED9.5 experimental embryos that were not retarded.
Results
Biometric data of the mouse model of diabetic pregnancy
The details about the efficacy of the STZ treatment protocol can be found in our previous study21. All mice treated with STZ became diabetic. Blood glucose concentrations reached steady-state levels (approx. 25 mmol/L) within 3 weeks after inducing diabetes with STZ. After administering the controlled-release insulin implants, glucose levels declined but remained higher (approx. 14 mmol/L) in diabetic than in nondiabetic mice. Due to the fixed release rate of insulin and the increasing demand for insulin with progressing pregnancy7, blood glucose concentrations increased again 1.7-fold in the first week of pregnancy and plateaued after ED8.5. Only pregnant females with blood glucose concentrations exceeding 17 mmol/L at sacrifice were included in the analysis.
Figure 1 shows that among 54 female diabetic mice, 18 (33%) did not become pregnant within 3 weeks after being exposed to a proven fertile male. Of these 18 mice, a vaginal plug was observed in 13 females (72%). At sacrifice, 5 of the 13 uteri (38%) were swollen and congested with blood, as occurs after death of all embryos. In the non-diabetic control groups, 7 out of 37 mice (19%) did not become pregnant within 3 weeks (no difference with the STZ group, P = 0.13). No swollen and congested uterus was observed in any of these mice. The average litter size of control mice was 9.4 ± 0.5.
Fecundity of female diabetic and control mice and the gestational age at which the mice were killed. “Plug” and “no plug” indicate mice with or without visible vaginal plug. “No embryos” indicates that a vaginal plug was seen, but a normal uterus without embryos was found at harvest (copulation, but no conception), whereas a “swollen” uterus indicates that a vaginal plug was seen and a uterus without embryos that was swollen and reddish with engorged blood vessels was found at harvest. All EDs represent the embryonic day when the mice were sacrificed. The numbers indicate the number of mice sacrificed.
An inventory of the visible congenital malformations in 10 litters of ED9.5–11.5 embryos of pregnant diabetic mice yielded 12 abnormal embryos among in total 96 embryos (13%; Supplemental Table 1), which is similar to that found in literature7,22. Among the abnormal embryos, neural-tube defects (“wavy” neural tube and spina bifida, sometimes at several locations) were prevalent, as reported12. Some embryos were also retarded in development. Figure 2A shows, for example, an ED9.5 embryo with a simple tubular heart and a short, straight tail, features which are typical for embryos at ED8.527. Additionally, the embryo in Fig. 2B has enlarged pericardial and neural-tube cavities, which is suggestive for cardiac failure. Other embryos had extensive fields of pycnotic nuclei in both embryonic atria (Fig. 2C–E) compared to control embryo (Fig. 2F). These morphological findings are consistent with those reported earlier in rat15 and mouse embryos7,11,12,14.
Growth retardation and apoptosis in embryos of diabetic mothers. Panel A shows a retarded experimental ED9.5 embryo with an incompletely turned caudal part of the body (“straight tail”) and a simple heart tube, which are compatible with a one-day delayed development. Panel B shows a hematoxylin and eosin-stained section of a delayed ED9.5 embryo. NT: dilated neural tube; OFT: outflow tract; V: ventricle, both in dilated pericardial cavity. Panels C and D (D is a magnification of the box in C) shows a hematoxylin and eosin-stained heart of an ED10.5 embryo from a diabetic mouse. Note the massive pycnotic fields in the dorsal wall of the embryonic atria. The 3D reconstruction (panel E; dorsal view of the heart region) identifies the pycnotic regions (yellow) in the left and right atria (green: the unaffected region; purple: ventricles and outflow tract). Panel F shows the hematoxylin and eosin-stained heart of a control ED10.5 embryo. Scale bars: 100 μm.
Maternal hyperglycemia causes embryonic growth retardation on ED8.5 and death on ED9.5
Since growth retardation appeared a common feature in embryos with maternal hyperglycemia and since ED7-8 was reported as the most sensitive period for the teratogenic effect of hyperglycemia in mice8, we investigated the frequency of delayed development in ED7.5-10.5 embryos. Criteria used to stage embryos under the dissecting microscope were the number of somites that had developed, the “turning” of the embryo for ED8.5 embryos and the development of the head and heart for ED9.5 embryos27. Table 1 shows the effect of maternal hyperglycemia on embryonic growth. In the control group, growth retardation occurred in 5% and 8% of the embryos on ED8.5 and ED9.5, respectively, without sex difference. In the diabetic group, however, 53% of the embryos had incurred a severe growth retardation, equivalent to approx. 1 day, on ED8.5 (P < 1E-9 vs non-diabetic group). On ED8.5, 3 litters consisted of retarded embryos only, in 2 litters 60–70% of the embryos were retarded, and in 4 litters 10–40% of the embryos were retarded. Intriguingly, only 14% of the retrieved embryos with maternal hyperglycemia were retarded on ED9.5 (P < 1E-9 vs ED8.5; 9 litters without retarded embryos, 4 litters with 1–2 retarded embryos, and 1 litter with 6 retarded embryos) and still fewer (3%) on ED10.5 (2 embryos in 2 litters; Table 1). We reanalyzed the data shown in Fig. 1 of our previous report21 to see if there was any correlation between retarded embryonic growth and maternal blood glucose at mating, on ED7.5, ED8.5 or ED9.5, but found none (not shown).
To track the fate of the retarded embryos, we determined the number of live embryos per litter for each age (Fig. 3 and Table 2). Maternal hyperglycemia resulted in growth retardation in 53% of ED8.5 embryos, but only few retarded ED9.5 or ED10.5 embryos were found (14% and 3%, respectively; Fig. 3A; P < 0.001). Coincident with the drop in the percentage of growth-retarded ED9.5 embryos in litters of diabetic mice, the number of live ED9.5 embryos decreased from 9.8 ± 0.3 on ED8.5 to 6.3 ± 0.9 on ED9.5 (Fig. 3B and Table 2; P = 0.021), indicating that many of the retarded embryos had died. More remarkably, the number of live embryos in litters of diabetic mice increased again to 9.7 ± 0.7 on ED10.5 (Fig. 3B and Table 2; P = 0.019). In conjunction with the apparent death of litters with a high percentage of retarded embryos (see previous section), we interpret the transient one-third reduction in the number of live embryos per litter in diabetic pregnancies on ED9.5 compared to ED8.5 and ED10.5 as showing that embryos that were severely growth-retarded on ED8.5 had died on ED9.5. We explain the apparent increase in the number of live embryos per litter on ED10.5 as a severe selection against and termination of litters of diabetic dams, in which >20% of the embryos were growth-retarded on ED8.5, because litters with few live embryos disappeared between ED9.5 and 10.5.
The effect of hyperglycemia on the prevalence of developmental retardation and litter size in pregnant diabetic mice. FVB females were made diabetic with streptozotocin and treated with insulin pellets. Embryos were harvested on ED8.5 (n = 9 litters), ED9.5 (n = 12 litters) and ED10.5 (n = 7 litters). Panel A: number of non-retarded and retarded embryos in a litter. “Non-retarded” indicates that the morphology of the embryos was appropriate for gestational age, whereas “retarded” indicates the morphology of the embryo resembled normal morphology one day earlier according to the criteria of Kaufman27. *P < 0.001. Panel B: number of live embryos in these diabetic litters. Gray symbol on ED8.5: one litter with only 1 embryo was considered an outlier (Grubb’s and Dixon’s outlier tests: P < 0.001 and <0.002, respectively). *P < 0.05. Note that approx. 50% of diabetic embryos were seriously retarded in development on ED8.5 (panel A), and that this was associated with a severe reduction in the number of live embryos per litter on ED9.5 (note “dumbbell” appearance of distribution) and the absence of these small litters on ED10.5 (panel B).
No sex difference in effect of hyperglycemia on embryonic growth
Because the sex of the embryo may determine its sensitivity to the teratogenic effects of maternal hyperglycemia28, we identified the male embryos by determining the presence of the Sry gene in the amniotic and chorionic membranes of ED7.5 and in the tails of older embryos. Table 1 shows that there was no significant difference in the percentage of male or female embryos that was growth-retarded on ED8.5 or ED9.5. In a small number of embryos, PCR amplification failed. Since this failure occurred as often in normal as in retarded embryos, it did not affect the interpretation of the data.
Gene expression profile and pathway analysis of one-day delayed embryos
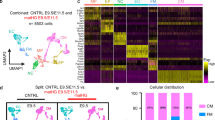
Our previous study showed that maternal hyperglycemia mainly suppressed the expression of genes involved in cell proliferation in ED8.5 embryos and the remodeling of the cytoskeleton and oxidative phosphorylation in ED9.5 embryos21. To investigate the effects of growth retardation on gene expression, we performed SOLiD SAGE sequencing (accession number: PRJNA328571; study accession: SRP078567). In total, 8 ED7.5 embryos and 5 ED8.5 embryos from non-diabetic control mothers, and 7 ED8.5 embryos, 8 ED8.5 one-day delayed embryos (ED8.5-1), and 4 ED9.5 one-day delayed embryos (ED9.5-1) from diabetic mothers were screened. Clusters were generated with generalized linear models (GLM) based on the expression of the top 100 genes ranked by P-value, which separated the retarded from the non-retarded embryos in diabetic pregnancies and from the corresponding embryos in control pregnancies (Fig. 4A–C).
Hierarchical cluster analysis of gene expression in control and experimental one-day delayed ED8.5 and ED9.5 embryos. Panel A: Control ED7.5 embryos were compared to diabetic 1-day retarded ED8.5 embryos. Panel B: Control ED8.5 embryos to diabetic 1-day retarded ED9.5 embryos; Panel C: diabetic non-retarded ED8.5 embryos to diabetic 1-day retarded ED9.5 embryos. Hierarchical clustering was performed by GLM. The tree diagram on the top of the panels reflects the correspondence in gene expression between embryos, with less height of the branches indicating a higher similarity. The dendrograms at the left margin indicate the correlation in the response of the respective genes. The intensity of the blue color represents the relative level of expression of a gene (after transforming the data for variance stabilization). “C” and “D” at the bottom margin of the panels indicate “control” and “diabetic” embryos, respectively. Panel D: Venn diagram of the number of differentially expressed genes (without RIKEN, EST, unassigned and hypothetical gene sequences) in control and non-retarded diabetic ED8.5 embryos (C8.5_D8.5; green), in non-retarded and 1-day retarded diabetic embryos (D8.5_D9.5-1; red) and in control and 1-day retarded diabetic embryos (C8.5_D9.5-1; blue). The numbers in the overlapping areas show the number of genes differentially regulated in the two or three overlapping comparisons. Note that the number of differentially expressed genes is high if control embryos are compared with non-retarded diabetic embryos, but very small if control embryos are compared with retarded diabetic embryos.
Differential gene expression was further analyzed with the Wald test in the DESeq2 package (available through Bioconductor)29. A Padj < 0.05 was considered statistically significant. As summarized in Fig. 4D, 3773 genes were differentially expressed in experimental non-retarded compared to control ED8.5 embryos, whereas this number was only 320 if experimental retarded ED9.5-1 embryos were compared to control ED8.5 embryos. When non-retarded ED8.5 embryos were compared to ED9.5-1 embryos (both offspring of diabetic dams), 3063 genes were differentially expressed (Fig. 4D). These data show that gene expression in retarded experimental embryos was more similar to that in control embryos of the same developmental stage than to that in non-retarded experimental embryos.
To find out whether the discrepancy between retarded and non-retarded embryos of diabetic dams converged on only a few metabolic or signaling pathways or, alternatively, indicated a failure of the retarded embryos to adapt to the maternal diabetic condition we performed pathway analysis of the differentially expressed genes. Figure 5 shows that, compared to control ED8.5 embryos, Integrin, Integrin-linked kinase (ILK), Rho-family GTPases and Ephrin-B signaling were most highly (down-)regulated in experimental ED8.5 embryos (see 3rd column). Only genes involved in antioxidant action of vitamin C were significantly upregulated. These pathways concur largely with those we identified earlier21 on the MetaCoreTM platform (GeneGo, Inc., St. Joseph, MI, USA; for a list of the embryos compared in our previous21 and present study, see Supplemental Table 2). Relative to control ED8.5 embryos, experimental ED9.5-1 embryos differed from experimental ED8.5 embryos in a similar, but much weaker downregulation of pathways, so that only few of these pathways were significantly regulated (compare 2nd and 3rd columns in Fig. 5). This weaker downregulation of gene expression is also seen in the direct comparison of experimental ED8.5 and experimental D9.5-1 embryos (reversed color in 1st column). The response in experimental ED8.5-1 of diabetic mothers compared to control ED7.5 embryos was more mixed: genes involved in ILK signaling and antioxidant action of vitamin C were e.g. highly upregulated, whereas NRF2-mediated oxidative stress and EIF2-mediated translation was significantly downregulated (4th column). Unfortunately, we do not have expression profiles of non-retarded experimental ED7.5 embryos. These data suggest that growth-retarded embryos in diabetic pregnancies fail to shut down many pathways that are strongly down-regulated in otherwise comparable non-retarded embryos and that this failure leads to their demise between ED8.5 and ED9.5.
Pathway analysis of differences in gene expression between control and retarded or non-retarded experimental embryos. Non-retarded ED8.5 experimental embryos were compared to 1-day retarded ED9.5 experimental embryos (D8.5_D9.5-1; left column), ED8.5 control embryos to 1-day retarded ED9.5 experimental embryos (C8.5_D9.5-1; 2nd column), ED8.5 control embryos to non-retarded ED8.5 experimental embryos (C8.5_D8.5; 3rd column), and ED7.5 control embryos to 1-day retarded ED8.5 experimental embryos (C7.5_D8.5-1; 4th column). Color intensity indicates stronger control, with blue and orange reflecting a negative and a positive Z-score, respectively, relative to the second term in the comparison. Note marked difference between 2nd and 3rd columns, which reflects the effect of growth retardation.
Discussion
In our experimental model, mouse embryos become sensitive to maternal hyperglycemia on ED7.5, as previously reported8, and develop visible malformations from ED10.5 onwards. Unexpectedly, we observed severe growth retardation in approx. 50% of the embryos of diabetic dams at ED8.5 and a approx. 30% decrease in litter size of these diabetic dams on ED9.5. Because we also observed diabetic dams with only dead embryos in their uterus, we interpret (for a graphical illustration of our reasoning, see Fig. 6) the transient decrease in litter size of diabetic dams on ED9.5 as showing that embryos that were severely growth-retarded on ED8.5, had died. The apparent normalization of the litter size of diabetic dams on ED10.5 can only be understood if diabetic pregnancies, in which >20% of the embryos had died from the sequels of maternal hyperglycemia, had terminated. As a result of this selection, no evidence (the percentage retarded embryos was not different from that in control pregnancies) of the deleterious effect of maternal hyperglycemia on embryonic growth was still demonstrable on ED10.5. Growth retardation was reported in ED11.5 rat embryos (26–29-somites, equivalent to ED10 mouse embryos30,31) when cultured in very high-glucose media15,32. Growth retardation and a temporary reduction in litter size were, however, not noted in diabetic mice16. However, in another study, a 0.5 day developmental delay on ED8.5 and a temporary reduction in litter size of diabetic pregnancies on ED9.5 were found, but the number of pregnancies studied was deemed too small to permit a conclusion33. These findings imply an early, rather general and possibly metabolic effect of maternal hyperglycemia during and immediately after gastrulation that leaves few traces due to the elimination of affected litters between ED9.5 and ED10.5, and a later, teratological effect that has thus far received most interest. If our finding can be extended to the human situation, the early effect would occur in the third week of gestation, so that the consequence, embryonic death, might not even be noted, except possibly for a perceived difficulty to become pregnant.
The fate of embryos in litters from diabetic mothers. Schematic representation of the fate of embryos in diabetic dams. This scheme, which is based on the data presented in Table 2 and Fig. 3, depicts the fate of 10 imaginary litters, with 10 embryos each, in the uterus of diabetic dams. Between ED7.5 and ED10.5, experimental embryos develop normally for gestational age (white), become delayed in development (gray), or die (black). The developmental delay in diabetic pregnancies typically developed between ED7.5 and ED8.5, and most embryos with a developmental delay died between ED8.5 and ED9.5. If >20% of the embryos in a litter were affected, the remaining embryos died between ED9.5 and ED10.5. Due to the demise of retarded embryos, average litter size declined from 10 on ED7.5 to 6 on ED9.5. As a result of selection against litters in which >20% of the embryos had died, small litters were no longer seen on ED10.5 and average litter size had increased again. The only trace of the affected litters was a lower percentage of pregnancies among diabetic relative to control females. The background rate of retarded development was approx. 5%.
Even a temporary increase in circulating glucose concentration on ED7-8, that is, during gastrulation, suffices to bring about neural tube defects8. Our data shows that blood glucose levels in diabetic dams had increased to a plateau by that time21. Euglycemia during gastrulation is apparently crucial for normal embryonic development and further organogenesis (cf ref.20), because nearly half of the embryos of diabetic mice had developed a growth delay on ED8.5. Glycolysis is still the prevailing energy source in ED9.5 mouse embryos21,34, although studies in rat35,36 and mouse embryos37 suggest that the production of lactate decreases and mitochondrial activity slowly increases during early organogenesis. We observed that glycolytic activity and capacity had declined substantially in ED9.5 mouse embryos from diabetic mothers, suggesting that embryos of diabetic mothers had an energy problem21. In agreement, exposure of such embryos to a supernormal glucose concentration did not increase glucose oxidation36,37, but caused swelling of mitochondria38 and a significant decrease in reduced glutathione39.
These findings raise the question how such a general disturbance of metabolism can result in malformations in a restricted range of organ systems, such as the neural tube and heart. Our present data may explain this paradox, because shortly after becoming sensitive to hyperglycemia, embryos do suffer from a generalized adverse effect. Because embryos that were most severely affected by maternal hyperglycemia did not survive, the embryos with malformations appear to be those that were only relatively mildly affected by the early toxic effect of maternal hyperglycemia. The difference between the 2 groups of embryos appears to reside in their adaptive response in gene expression: the growth-retarded embryos were unable to adapt to the maternal diabetic condition by a pronounced, generally downward change in gene expression that was found in the non-retarded ED8.5 embryos 21. It is tempting to speculate that this failure to adapt to maternal hyperglycemia represents or includes a causal factor for the failure to grow and survive. The differential sensitivity of the (mouse) embryos to glucose may develop if the complex downstream effects of maternal hyperglycemia, including glutathione synthesis and oxidation, hexosamine synthesis, p53 upregulation and apoptosis7,9,40,41, are not strictly deterministic, but vary stochastically, so that the outcome is not uniform.
Non-retarded ED8.5 embryos of diabetic dams suffer mainly from impaired cell proliferation, and ED9.5 embryos from impaired cytoskeletal remodeling and oxidative phosphorylation21. Pathway analysis on one-day delayed ED8.5 experimental embryos revealed a remarkably muted adaptive response in gene expression relative to the non-retarded experimental embryos. Integrin (ILK) signaling, antioxidant action of vitamin C and NRF2-mediated oxidative stress remained most strongly affected in growth-retarded embryos. ILK is the major regulator of Integrin-mediated signaling. Integrins are cell-adhesion molecules that bind to the extracellular matrix (ECM) and forge a connection between the ECM and the actin cytoskeleton42. Oxidative stress emerges as a more prominent factor in the present analysis on the Ingenuity platform than our previous analysis on the MetaCoreTM platform, but also this response was suppressed in the growth-retarded mouse embryos.
Conclusions
Our study showed that maternal hyperglycemia has an early and generalized adverse effect on embryonic development between ED7.5 and ED9.5 (equivalent to 18–28 days development in humans), whereas the characteristic malformations of the neural tube and cardiac outflow tract become apparent only thereafter. Hyperglycemia-mediated changes in gene expression were markedly absent in growth-retarded ED8.5 (mouse) embryos, while malformations developed in non-retarded, surviving embryos that were able to mount an adaptive response to hyperglycemia on ED8.5.
Materials and Methods
Animals
FVB mice (9–11 weeks old) were obtained from Harlan Sprague Dawley (Venray, The Netherlands) and fed an irradiated diet that was based on the Purina 9 F diet (http://labdiet.com/pdf/5020.pdf; production: ABDiets, Woerden, The Netherlands). Mice were kept on a 12-h light/12-h dark cycle at 22 °C with free access to water and food. The study was carried out in accordance with the Dutch Guidelines for the Care and Use of Laboratory Animals and approved by the Ethical Committee for Animal Research of the University of Amsterdam (ALC101225).
Induction of diabetes
Diabetes was induced in female FVB mice with streptozotocin as recommended by the Animal Models of Diabetic Complications Consortium (AMDCC)43; and as described in our previous study21. In brief, animals to be treated with streptozotocin were randomly chosen. The diabetic group included 54 and the control group 37 mice. 4–5 diabetic or control mice were housed in one cage. Sixty mg/kg streptozotocin (STZ; Sigma, Zwijndrecht, The Netherlands), freshly dissolved in sodium citrate (0.1 M, pH 4.5), was injected intraperitoneally for 5 consecutive days. Hyperglycemia was treated with subcutaneous sustained-release insulin implants (LinSHIN, Toronto, Canada) that released approx. 0.1 U/day per implant for at least 30 days. Mice were mated to proven fertile males. Pregnant mice were sacrificed by cervical dislocation and embryos recovered on ED7.5, ED8.5, ED9.5, ED10.5, ED11.5, or ED13.5 (one litter only). Embryos were considered alive when their heart was beating (>ED8.5) or if the RIN number of RNA isolated from them was >9.0. The age of the embryos was verified by comparison with the criteria of Kaufman27.
Sex identification
Because male human embryos were reported to be more sensitive to the diabetic state of their mothers than female embryos44, whereas the reverse was described for mouse embryos45, we studied male and female embryos separately. DNA from the visceral yolk sacs of ED8.5 embryos, or the posterior part (cut made just caudal to the heart) of ED9.5 embryos was isolated with TriPure Isolation Reagent (Roche, Almere, The Netherlands). The tissues were taken from the remaining parts of the embryos after harvesting the anterior part (ED8.5) and heart-containing segment (ED9.5) for mRNA quantification. The sex of the embryos was determined by a multiplex PCR amplification using primers to amplify the male-specific Sry gene and the autosomal Il3 gene for reference46.
Embryo morphology
ED8.5-ED10.5 embryos were fixed in Bouin’s fixative (saturated picric acid (15), formaldehyde (5), glacial acetic acid (1; v/v)) for 2 hours at 4 °C, stored in 70% ethanol, and embedded in paraffin. Serial transversely cut 7 μm-thick sections were stained with hematoxylin and eosin (HE), and photographed with a Leica DMRA2 microscope equipped with a Leica DM300 photo camera. Digital images from serial sections of mouse embryos were used to prepare three-dimensional reconstructions with Amira 5.2 software package (Visage Imaging, San Diego, CA, USA).
Quantification of mRNAs by SOLiD SAGE sequencing
In our previous study, we compared gene expression in non-retarded control and experimental ED8.5 or 9.5 embryos21. In the present study, we compared gene expression in control ED7.5 and ED8.5 embryos with one-day delayed ED8.5 and ED9.5 experimental embryos, respectively. Total RNA was extracted from whole ED7.5 and one-day delayed ED8.5 embryos, and from the anterior half of embryos (transverse cut made just caudal to the heart) control ED8.5 and one-day delayed experimental ED9.5 embryos with the TriPure Isolation Reagent (Roche, Almere, The Netherlands). Embryos were frozen and thawed 3 times using liquid nitrogen. After centrifugation, the aqueous phase of the TriPure extract was re-extracted 3 times with choloroform:isoamyl alcohol (24:1) and precipitated with ethanol after addition of 10 µg glycogen. The quantity of total RNA was determined with a Qubit 1.0 Fluorometer (Invitrogen, The Netherlands), while the quality was assessed with a Bioanalyser RNA 6000 Nano Chip (Agilent Technologies, Amstelveen, The Netherlands). The RNA integrity number (RIN) of all samples was between 9.0 and 10.0. The total RNA yield per embryo was 1.0 ± 0.1 µg for one-day delayed ED8.5 embryos and 0.9 ± 0.1 µg for one-day delayed ED9.5 embryos. The relative abundance of individual transcripts was determined by the SOLiD SAGE approach, as in our previous study21. Pathway analysis of the differentially expressed genes was performed using QIAGEN’s Ingenuity Pathway Analysis (IPA®, QIAGEN Redwood City, www.qiagen.com/ingenuity).
Statistical analyses
Blood glucose concentrations and litter size are expressed as mean ± SEM. Statistical analyses were performed using nonparametric Mann-Whitney U and Kruskal-Wallis tests. The number of normal and retarded embryos was compared with embryonic age and streptozotocin treatment as variables, using contingency-table statistics. Expected values were based on column and row totals or Mendelian expectation (gender). In case of a significant overall Chi-squared value, the contributions to the Chi-squared value were tested to determine the source of the observed difference. A P-value < 0.05 was considered statistically significant.
Data availability statement
The datasets generated during and/or analysed during the current study are available in NCBI (accession number: PRJNA328571; SRA study: SRP078567). The datasets generated in our earlier study21 are also available in NCBI (accession number: PRJNA275285; SRA study: SRP056150).
References
Jovanovic, L. et al. Trends in the incidence of diabetes, its clinical sequelae, and associated costs in pregnancy. Diabetes Metab Res Rev 31, 707–716 (2015).
Abell, S. K., Nankervis, A., Khan, K. S. & Teede, H. J. Type 1 and Type 2 diabetes preconception and in pregnancy: health impacts, influence of obesity and lifestyle, and principles of management. Semin Reprod Med 34, 110–120 (2016).
Pedersen, J. F. & Molsted-Pedersen, L. Early fetal growth delay detected by ultrasound marks increased risk of congenital malformation in diabetic pregnancy. Br Med J (Clin Res Ed) 283, 269–271 (1981).
Greene, M. F. Spontaneous abortions and major malformations in women with diabetes mellitus. Semin Reprod Endocrinol 17, 127–136 (1999).
Lisowski, L. A. et al. Congenital heart disease in pregnancies complicated by maternal diabetes mellitus. An international clinical collaboration, literature review, and meta-analysis. Herz 35, 19–26 (2010).
Ornoy, A., Reece, E. A., Pavlinkova, G., Kappen, C. & Miller, R. K. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res C Embryo Today 105, 53–72 (2015).
Phelan, S. A., Ito, M. & Loeken, M. R. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes 46, 1189–1197 (1997).
Fine, E. L., Horal, M., Chang, T. I., Fortin, G. & Loeken, M. R. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes 48, 2454–2462 (1999).
Kumar, S. D., Dheen, S. T. & Tay, S. S. Maternal diabetes induces congenital heart defects in mice by altering the expression of genes involved in cardiovascular development. Cardiovasc Diabetol 6, 34 (2007).
Sadler, T. W., Hunter, E. S. 3rd., Wynn, R. E. & Phillips, L. S. Evidence for multifactorial origin of diabetes-induced embryopathies. Diabetes 38, 70–74 (1989).
Chappell, J. H. Jr., Wang, X. D. & Loeken, M. R. Diabetes and apoptosis: neural crest cells and neural tube. Apoptosis 14, 1472–1483 (2009).
Salbaum, J. M. & Kappen, C. Neural tube defect genes and maternal diabetes during pregnancy. Birth Defects Res A Clin Mol Teratol 88, 601–611 (2010).
Morgan, S. C., Relaix, F., Sandell, L. L. & Loeken, M. R. Oxidative stress during diabetic pregnancy disrupts cardiac neural crest migration and causes outflow tract defects. Birth Defects Res A Clin Mol Teratol 82, 453–463 (2008).
Pavlinkova, G., Salbaum, J. M. & Kappen, C. Wnt signaling in caudal dysgenesis and diabetic embryopathy. Birth Defects Research A Clin Mol Teratol 82, 710–719 (2008).
Eriksson, U. J., Lewis, N. J. & Freinkel, N. Growth retardation during early organogenesis in embryos of experimentally diabetic rats. Diabetes 33, 281–284 (1984).
Chang, T. I. et al. Oxidant regulation of gene expression and neural tube development: Insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia 46, 538–545 (2003).
Zabihi, S., Wentzel, P. & Eriksson, U. J. Altered uterine perfusion is involved in fetal outcome of diabetic rats. Placenta 29, 413–421 (2008).
Freinkel, N. Diabetic embryopathy and fuel-mediated organ teratogenesis: lessons from animal models. Horm Metab Res 20, 463–475 (1988).
Eriksson, R. S., Thunberg, L. & Eriksson, U. J. Effects of interrupted insulin treatment on fetal outcome of pregnant diabetic rats. Diabetes 38, 764–772 (1989).
Herion, N. J., Salbaum, J. M. & Kappen, C. Traffic jam in the primitive streak: the role of defective mesoderm migration in birth defects. Birth Defects Res A Clin Mol Teratol 100, 608–622 (2014).
Zhao, J. et al. Effect of hyperglycemia on gene expression during early organogenesis in mice. PloS one 11, e0158035 (2016).
Jiang, B. et al. Global gene expression analysis of cranial neural tubes in embryos of diabetic mice. J Neurosci Res 86, 3481–3493 (2008).
Pavlinkova, G., Salbaum, J. M. & Kappen, C. Maternal diabetes alters transcriptional programs in the developing embryo. BMC genomics 10, 274 (2009).
Vijaya, M. et al. Differential gene expression profiles during embryonic heart development in diabetic mice pregnancy. Gene 516, 218–227 (2013).
Loeken, M. R. Advances in understanding the molecular causes of diabetes-induced birth defects. J Soc Gynecol Investig 13, 2–10 (2006).
Pani, L., Horal, M. & Loeken, M. R. Polymorphic susceptibility to the molecular causes of neural tube defects during diabetic embryopathy. Diabetes 51, 2871–2874 (2002).
Kaufman, M. H. The Atlas of Mouse Development, (Academic Press. 512p, 1992).
Evers, I. M., de Valk, H. W. & Visser, G. H. Male predominance of congenital malformations in infants of women with type 1 diabetes. Diabetes Care 32, 1194–1195 (2009).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014).
Theiler, K. The House Mouse: Atlas of Embryonlc Development, (Springer-Verlag. 178p, 1989).
R. Hebel, M. W. S. Anatomy and Embryology ofthe Laboratory Rat. Vol. 443 (BioMed-Verlag. 271p (1986).
Cockroft, D. L. & Coppola, P. T. Teratogenic effects of excess glucose on head-fold rat embryos in culture. Teratology 16, 141–146 (1977).
Kappen, C., Kruger, C., MacGowan, J. & Salbaum, J. M. Maternal diet modulates the risk for neural tube defects in a mouse model of diabetic pregnancy. Reprod Toxicol 31, 41–49 (2011).
Houghton, F. D., Thompson, J. G., Kennedy, C. J. & Leese, H. J. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev 44, 476–485 (1996).
Shepard, T. H., Tanimura, T. & Park, H. W. Glucose absorption and utilization by rat embryos. Int J Dev Biol 41, 307–314 (1997).
Svensson, A. M., Borg, L. A. & Eriksson, U. J. Glucose metabolism in embryos of normal and diabetic rats during organogenesis. Acta Endocrinol (Copenh) 127, 252–257 (1992).
Clough, J. R. & Whittingham, D. G. Metabolism of [14C]glucose by postimplantation mouse embryos in vitro. J Embryol Exp Morphol 74, 133–142 (1983).
Yang, X., Borg, L. A. & Eriksson, U. J. Altered mitochondrial morphology of rat embryos in diabetic pregnancy. Anat Rec 241, 255–267 (1995).
Sakamaki, H. et al. Significance of glutathione-dependent antioxidant system in diabetes-induced embryonic malformations. Diabetes 48, 1138–1144 (1999).
Horal, M., Zhang, Z., Stanton, R., Virkamaki, A. & Loeken, M. R. Activation of the hexosamine pathway causes oxidative stress and abnormal embryo gene expression: involvement in diabetic teratogenesis. Birth Defects Res A Clin Mol Teratol 70, 519–527 (2004).
Dong, D. et al. Maternal diabetes triggers DNA damage and DNA damage response in neurulation stage embryos through oxidative stress. Biochem Biophys Res Commun 467, 407–412 (2015).
Legate, K. R., Montanez, E., Kudlacek, O. & Fassler, R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 7, 20–31 (2006).
Breyer, M. D. et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16, 27–45 (2005).
Bracero, L. A., Cassidy, S. & Byrne, D. W. Effect of gender on perinatal outcome in pregnancies complicated by diabetes. Gynecol Obstet Invest 41, 10–14 (1996).
Machado, A. F., Zimmerman, E. F., Hovland, D. N. Jr., Weiss, R. & Collins, M. D. Diabetic embryopathy in C57BL/6J mice. Altered fetal sex ratio and impact of the splotch allele. Diabetes 50, 1193–1199 (2001).
Lambert, J. F. et al. Quick sex determination of mouse fetuses. J Neurosci Methods 95, 127–132 (2000).
Acknowledgements
We thank Ted Bradley, Anneloor L.M.A. ten Asbroek, and Frank Baas for advice and hospitality during the construction of the SAGE libraries. We are indebted to Antoon Moorman for including us in his EU CHeartED program.
Author information
Authors and Affiliations
Contributions
J.Z., T.B.M.H. and W.H.L. conceived the experiment(s), J.Z. conducted the experiment(s), T.B.M.H., J.M.R., A.J., J.K., S.M.A.S. and A.S. analyzed the results, J.Z., T.B.M.H. and W.H.L. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, J., Hakvoort, T.B.M., Ruijter, J.M. et al. Maternal diabetes causes developmental delay and death in early-somite mouse embryos. Sci Rep 7, 11714 (2017). https://doi.org/10.1038/s41598-017-11696-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11696-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.