Abstract
Although many studies have addressed the prognostic value of programmed cell death-ligand 1 (PD-L1) expression in lung cancer, the results remain controversial. A systematic search of the PubMed, EMBASE, and Cochrane Library databases was performed to identify the correlation between PD-L1 expression and driver mutations and overall survival (OS). This meta-analysis enrolled a total of 11,444 patients for 47 studies, and the pooled results showed that increased PD-L1 expression was associated with poor prognosis (HR = 1.40, 95% CI: 1.19–1.65, P < 0.001). In subgroup analysis stratified according to histology types, the pooled results demonstrated that increased PD-L1 expression was an unfavorable prognostic factor for non-small cell lung cancer (NSCLC) (HR = 1.26, 95% CI: 1.05–1.52, P = 0.01) and pulmonary lymphoepithelioma-like carcinoma (LELC) (HR = 3.04, 95% CI: 1.19–7.77, P = 0.02), rather than small cell lung cancer (SCLC) (HR = 0.62, 95% CI: 0.27–1.39, P = 0.24). The pooled ORs indicated that PD-L1 expression was associated with gender, smoking status, histology, differentiation, tumour size, lymph nodal metastasis, TNM stage and EGFR mutation. However, PD-L1 expression was not correlated with ALK rearrangement and KRAS mutations.
Similar content being viewed by others
Introduction
Lung cancer, broadly divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), is deemed as the leading cause of cancer-related deaths in both the United States and China1, 2. More than 70% of patients are diagnosed with advanced disease, which are not amenable to curative therapy. Although much progress has recently been made for lung cancer such as low-dose spiral screening, minimally invasive techniques for diagnosis and treatment, advances in radiation therapy and molecularly targeted therapies, patients with lung cancer are still facing a relatively low 5-year survival rate, merely 17.4%3. Thus, immunotherapies have been considered as a very promising therapeutic strategy for different tumour types.
Programmed death 1 (PD-1), a member of the CD28 family, is a key immune checkpoint receptor expressing on the surface of the activated T, B and NK cells and plays a crucial role in tumour immune escape4. Programmed cell death ligand 1(PD-L1), the mainly ligand of PD-1, is upregulated in different types of tumours, including breast cancer5, NSCLC6, colorectal cancer7, gastric cancer8, testicular cancer9 and papillary thyroid cancer10. PD-L1 delivers negative costimulatory signals and binds PD-1 to reduce cellular immune responses by inducing T-cell apoptosis or exhaustion. Blocking the PD-1/PD-L1 pathway with monoclonal antibodies (MoAbs) is currently considered to be the most promising approach, offering durable activity and long-term survival outcomes11. Several meta-analyses have demonstrated that not only is PD-L1 expression associated with adverse clinical and pathologic features but an increased risk of death in many cancer types12,13,14,15. However, date regarding the prevalence and prognostic role of PD-L1 expression in NSCLC remains controversial, particularly in SCLC and other types of lung cancers.
NSCLC is a disease that is characterized by driver mutation-defined molecular subsets, and alterations in epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and KRAS are major oncogenic drivers in NSCLC16. However, the relationship between major driver mutations and PD-L1 expression remains unclear. A recent study showed that oncogenic EGFR mutations directly up-regulated PD-L1 protein expression on the surface of cells in NSCLC, and exposure to gefitinib also lead to PD-L1 up-regulation17. Another study showed the upregulation expression of PD-L1 in NSCLC as a result of an EGFR mutation and ALK rearrangement via common downstream signalling pathways mediated by PI3K-AKT and by MEK-ERK18, implicating driver mutations in the regulation of the expression of immunosuppressive molecules.
We therefore conducted a comprehensive meta-analysis to investigate the significance of PD-L1 expression as a prognostic marker and to determine the relation of PD-L1 expression to clinicopathological features and driver mutations in lung cancer patients.
Results
Search results and characteristics of studies
The literature review process is shown in Fig. 1. The initial search strategies retrieved a total of 2,402 potentially relevant articles. After screening the titles or abstracts, 1977 studies were excluded as irreverent, non-English, in vivo/in vitro studies, case reports, reviews, meta-analyses and comments. After reading the full texts of the remaining articles, 45 studies lacking sufficient data for further analysis were discarded. Forty-seven studies with 11,444 patients were finally included for meta-analysis.
Referring to Table 1 for the major characteristics included of the studies. Among the 47 studies, twenty-three investigated PD-L1 expression in NSCLC6, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40, thirteen in adenocarcinoma (ADC)41,42,43,44,45,46,47,48,49,50,51,52,53, six in squamous cell carcinoma (SCC)54,55,56,57,58,59, two in small cell lung cancer (SCLC)60, 61, and two investigated PD-L1 in pulmonary lymphoepithelioma-like carcinoma (LELC)62, 63, and one investigated PD-L1 in pulmonary pleomorphic carcinoma (PPC)64. Thirty-seven studies were conducted with Asian patients, and 10 studies were conducted with non-Asians patients. Twenty-three studies included non-metastatic lung cancer patients, while 5 studies involved metastatic disease, and 17 studies involved both non-metastatic and metastatic diseases. The Newcastle–Ottawa Quality Assessment Scale (NOS) scores of the studies ranged from 4 to 8, with a mean value of 6.92.
Correlation of PD-L1 expression with prognosis
The correlation between the expression of PD-L1 and overall survival (OS) in lung cancer is shown in Fig. 2. The meta-analysis indicated that PD-L1 expression is a correlative factor of OS, with the pooled hazard ratio (HR) values of 1.40 (95% CI: 1.19–1.65, P < 0.001) for OS using a random model with significant heterogeneity (I2 = 79%, P < 0.001).
To explore the sources of potential heterogeneity, subgroup analysis for OS was conducted according to histology type, TNM stage and ethnicity. Subgroup analyses based on histology types showed that PD-L1 expression significantly reduced the OS of NSCLC patients (HR = 1.26, 95% CI: 1.05–1.52, P = 0.01) and LELC patients (HR = 3.04, 95% CI: 1.19–7.77, P = 0.02), but not SCLC (HR = 0.62, 95% CI: 0.27–1.39, P = 0.24).To further examine the effects of different subtypes of NSCLC on survival, a subgroup analysis was conducted in patients with ADC and SCC. The results revealed that increased PD-L1 expression was associated with poor prognosis in patients with ADC (HR = 1.85, 95% CI: 1.30–2.63, P < 0.001), but not in SCC (HR = 1.49, 95% CI: 0.93–2.38, P = 0.10). In addition, subgroup analyses according to TNM stage showed that increased PD-L1 expression impacted OS negatively for lung cancer patients in stage I-III (HR = 1.61, 95% CI: 1.24–2.09, P < 0.001), but not in stage IV (HR = 0.66, 95% CI: 0.33–1.33, P = 0.25). When grouped according to ethnicity, the combined HRs of Asian studies and non-Asian studies were 1.64 (95% CI: 1.36–1.96, P < 0.001) and 0.85 (95% CI: 0.70–1.02, P = 0.07), respectively, indicating that PD-L1 is an indicator of the poor prognosis in Asian populations, but not in non-Asian populations (Fig. 3).
Correlation of PD-L1 with clinicopathological features
The correlation between PD-L1 expression and clinicopathological parameters of lung cancer is shown in Supplementary Figs 1–7. The pooled results showed that PD-L1 expression was increased in male (OR = 1.46, 95%CI: 1.24–1.71, P < 0.001), smoker (OR = 1.57, 95% CI: 1.28–1.93, P < 0.001), patients with SCC (OR = 1.59, 95% CI: 1.11–2.26, P = 0.01), a higher histological grade (OR = 2.55, 95% CI: 2.05–3.19, P < 0.001), larger tumor sizes (OR = 1.70, 95% CI: 1.29–2.25, P < 0.001), positive lymph nodal metastasis (OR = 1.34, 95% CI: 1.19–1.50, P < 0.001) and TNM stage (OR = 1.45, 95% CI: 1.18–1.78; P < 0.001). The analysis of the relation of PD-L1 expression to histological grade (P = 0.07; I2 = 39%), tumour size (P = 0.25; I2 = 24%), and lymph nodal metastasis status (P = 0.02; I2 = 42%) presented no heterogeneity; thus, a fixed effect model was used. The other analyses above were performed using the random effects model.
Correlation of PD-L1 with major driver mutations
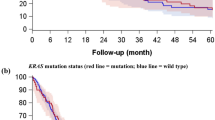
To further understand the role of PD-L1 expression as a biological marker, we investigated the relevance of increased PD-L1 expression and major driver mutations (EGFR/ALK/KRAS). As shown in Fig. 4, PD-L1 expression was associated with EGFR wild-type status (OR = 0.61, 95% CI: 0.42–0.90, P = 0.01), while no associations were identified between PD-L1 expression and ALK rearrangements (OR = 1.02, 95% CI: 0.61–1.71, P = 0.93) or KRAS mutations (OR = 1.34, 95% CI: 1.00–1.79, P = 0.05).
Publication bias and sensitivity analysis
Begg’s and Egger’s test were performed to evaluate the publication bias in the literature. And no indicator of publication bias among these studies was present. The P values for these tests were 0.237 and 0.120, respectively (Fig. 5). (Statistical significance was set at P < 0.05). Meanwhile, the sensitivity analysis was performed to assess the stability of the present meta-analysis by omitting one study. The results demonstrated that none of the studies influenced the overall HRs, suggesting that the results of the study are credible.
Discussion
High PD-L1 expression has been observed in various solid tumours, and a previous study demonstrated that the expression of PD-L1 contributes to poor prognosis65. Although heavily investigated; it remains controversial for the prognostic value of PD-L1 expression in lung cancer, reflecting the inconsistent results of previous studies. This meta-analysis included 47 studies with 11,444 patients to evaluate the significance of increased PD-L1 to the prognosis of lung cancer. The results of the present analysis showed increased PD-L1 expression was associated with poor prognosis in lung cancer patients.
According to subgroup analysis, high PD-L1 expression was an indicator of poor prognosis in Asian populations, but not in non-Asian populations, suggesting that the association between PD-L1 expression and prognosis is dependent on ethnicity. Different histological types of lung cancer process different biological characteristics. To reduce the heterogeneity of study, we performed a subgroup analysis on the basis of different histological types. The pooled results demonstrated that increased PD-L1 expression was an adverse prognostic factor for NSCLC and LELC, but not for SCLC. Our study analyzed the relationship between PD-L1 expression and prognoses of LELC and SCLC for the first time. This study provides important evidence on the prognostic value of the PD-L1 expression in LELC and SCLC patients. A potential correlation between PD-L1 expression and OS of patients with NSCLC was evaluated in previous meta-analyses66,67,68,69. The results of three meta-analyses revealed that NSCLC patients with increased PD-L1 expression had a poor OS66,67,68. Another meta-analysis did not indicate PD-L1 as a prognostic predictor for NSCLC69. However, the combined sample size of the four meta-analyses was relatively small. In addition, the four meta-analyses did not include SCLC and LELC, nor the investigation of the association between increased PD-L1 expression and driver mutations. Compared with those meta-analyses, more studies have been included in our research. Different thresholds to define positivity expression and particularly different baseline characteristics hinder the comparison of different studies reporting correlation of PD-L1 expression with OS in NSCLC. Standardized methods and definitions of PD-L1 positivity are clearly needed to facilitate studies of PD-L1 as a prognostic biomarker. Thus, a large multicenter study using the same antibody and cutoff of PD-L1 expression may be helpful to obtain more accurate results.
Several clinical trials using anti-PD-1 and anti-PD-L1 monoclonal antibodies, including nivolumab (BMS-936558)70, 71, pembrolizumab (MK-3475)72, and atezolizumab (MPDL3280A)73 have shown promising clinical activity in advanced NSCLC. In the era of precision medicine, it is particularly important to screen patients who are most likely to benefit from PD-1/PD-L1 antibody immunotherapy. Preliminary results suggested that high PD-L1 expression was associated with higher clinical activity of anti PD-1/PD-L1 monoclonal antibodies74. Therefore, the identification of patients with high PD-L1 expression is a vital question for anti-PD-1/PD-L1 therapy. In the present study, we investigated the relation of PD-L1 expression to clinicopathological factors. According to the pooled analysis, the expression of PD-L1 was increased in male, smoker, patients with SCC, a higher histological grade, larger tumour size, positive lymph nodal metastasis, and later clinical stage. These patients might benefit more from treatment targeting the PD-1/PD-L1 pathway. These data suggest that increased PD-L1 expression might promote lung cancer invasion and metastasis, leading to the poor prognosis of patients with lung cancer. It has been reported in several studies regarding the association of smoking status with PD-L1 expression in patients with lung cancer. Some studies have shown that the expression of PD-L1 was significantly higher in smokers28, 46, whereas other studies could not confirm this finding23, 24, 45, 54. In the present study, patients with high PD-L1 expression were associated with smoking status in lung cancer patients.
Accumulating evidence revealed the relationship between PD-L1 expression and driver mutations. EGFR mutations represent one of the most frequent driver mutations in NSCLC, particularly in ADC. Previous studies revealed that activating EGFR mutations induced PD-L1 expression in EGFR-driven NSCLC in cell lines and an animal model21, 75. Moreover, as observed in NSCLC cell lines, there was a high level of PD-L1 expression in NSCLC patients harboring EGFR mutations21, 24, 45. However, some studies have shown that PD-L1 positivity was more frequent in EGFR wild-type28, 44, 46, and other studies have shown no association between PD-L1 expression and EGFR mutations23, 26, 27.The present meta-analysis investigated the correlation of PD-L1 expression with EGFR mutations in lung cancers. The results of the present study showed that high PD-L1 expression was associated with EGFR mutations. The discrepancies among different studies might reflect the heterogeneous study population and variable definitions of PD-L1 expression. Additional studies are needed to further analyze this issue. In addition, we showed that increased PD-L1 is not associated with ALK rearrangements and KRAS mutations.
There are several limitations of the present study that should be acknowledged. First, the sample size of SCLC and LELC studies included in the present meta-analysis was relatively small; therefore, the pooled data might be less than the statistical power. Hence, additional well-designed studies with larger sample sizes are needed to provide a more comprehensive evaluation of the prognostic value of PD-L1 expression in patients with SCLC and LELC. Second, the HR values of some studies were extracted from survival curves, which is less reliable than direct data provided in the original literature. Third, the distinct antibodies and different cut-off levels of PD-L1 expression among diverse studies might also impact the accuracy of prognostic estimation for lung cancer. Moreover, some inevitable publication bias might exist in the present meta-analysis, as many negative studies could not be published. Furthermore, significant heterogeneity existed in the results, although we calculated the pooled subgroup data using random-effects models. The observed heterogeneity might reflect differences based on different baseline characteristics, study designs, or treatment protocols. Finally, all of included studies were retrospectively collected, which might have introduced heterogeneity from variable treatments.
In conclusion, despite the limitations described above, this study presents the first meta-analysis to systematically assess the association of PD-L1 expression with lung cancer survival and driver mutations. The results demonstrated that high PD-L1 expression represents an unfavorable biomarker in LELC and NSCLC, but not in SCLC. In addition, increased PD-L1 expression is correlated with EGFR wild-type status. To strengthen these findings, the validation of the prognostic value of PD-L1 expression in patients with lung cancer requires further studies.
Methods
This meta-analysis was performed according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement76. The present study was based on data from previously published studies, and therefore, ethical approval was not required.
Literature search
We conducted a systematic literature search for published articles in the PubMed, EMBASE, and Cochrane databases from January 1999 to July 2017. The search terms included the following keywords: (PD-L1 OR B7-H1 OR CD274 OR programmed cell death 1 ligand 1 protein) AND (lung cancer OR lung neoplasms OR pulmonary cancers). Furthermore, we manually searched the abstracts of the annual meetings of American Society of Clinical Oncology (ASCO), European Society of Medical Oncology c (ESMO) and the World Conference of Lung Cancer (WCLC) from 1999 to 2017. To explore additional studies, we also reviewed the reference lists of relevant articles.
Eligibility criteria
The following inclusion criteria were used: (1) All patients were histologically confirmed as having lung cancer; (2) PD-L1 expression was detected by immunohistochemistry (IHC) or quantitative immunofluorescence (QIF) in primary NSCLC tissue; (3) Studies showed a correlation between PD-L1 expression and overall survival; (4) Studies showed a correlation between PD-L1 expression and clinicopathological features; (5) Studies provided sufficient information to extract the HR and 95% CI date for OS; and (6) articles were published in English. Studies that did not meet the inclusion criteria were excluded. When several studies were conducted using the same cohort of patients, only the most recent study was included.
Data extraction
Two authors (ZMH and LGL) independently conducted the data extraction, and a third reviewer (WY) resolved any discrepancies. The following information was extracted: name of the author, year of publication, country, tumour type, number of patients, stage, detection method, PD-L1-positive expression, outcome, clinicopathological parameters and HRs and 95% CIs for OS. When the HR values were not directly reported, we obtained the additional data from the original authors. And the data was extracted from survival curves using the methods of Parmar under the circumstances of no response77. Two reviewers (ZS and WY) independently conducted the quality assessment for each study using the NOS, and any discrepancies were resolved after revisiting the original study and discussion until consensus was reached. The NOS maximum possible score was 9 points, and studies that receiving a score of 6 or higher were considered high quality78.
Statistical methods
The HR and its 95% CI values were used to evaluate the association between PD-L1 expression and survival, and the pooled OR and 95% CI values were used to determine the relationship between PD-L1 expression and clinicopathological features. Statistical heterogeneity between studies was assessed using the chi-squared test and I2. A P value <0.1 orI2 values of >50% were indicative of significant heterogeneity, with a random effects model being used; a fixed effects model otherwise. A subgroup analysis was conducted to explore the potential heterogeneity among studies. Potential publication bias was assessed using Egger’s and Begg’s tests. The meta-analysis was performed using Review Manager 5.3 (Revman the Cochrane Collaboration; Oxford, England) and STATA version12.0 (Stata Corporation; College Station, TX, USA). All statistical analyses were 2-sided, and P values < 0.05 were defined as statistically significant.
References
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66, 271–289 (2016).
Chen, W. et al. Cancer statistics in China, 2015. CA Cancer J Clin 66, 115–132 (2016).
Ettinger, D. S. et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J Natl Compr Canc Netw 14, 255–264 (2016).
Zou, W., Wolchok, J. D. & Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 8, 328rv324 (2016).
Qin, T. et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget 6, 33972–33981 (2015).
Ameratunga, M. et al. PD-L1 and Tumor Infiltrating Lymphocytes as Prognostic Markers in Resected NSCLC. PLoS One 11, e0153954 (2016).
Rosenbaum M. W., Bledsoe J. R., Morales-Oyarvide V., Huynh T. G., Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol (2016).
Zhang, M. et al. The clinicopathological and prognostic significance of PD-L1 expression in gastric cancer: a meta-analysis of 10 studies with 1,901 patients. Scientific reports 6, 37933 (2016).
Cierna, Z. et al. Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann Oncol 27, 300–305 (2016).
Chowdhury, S. et al. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget (2016).
Ma, W., Gilligan, B. M., Yuan, J. & Li, T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol 9, 47 (2016).
Guo Y. et al. Prognostic and Clinicopathological Value of Programmed Death Ligand-1 in Breast Cancer: A Meta-Analysis 11, e0156323 (2016).
Liu, Y. X. et al. Prognostic significance of PD-L1 expression in patients with gastric cancer in East Asia: a meta-analysis. Onco Targets Ther 9, 2649–2654 (2016).
Zhang, Y. et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: a meta-analysis. Medicine (Baltimore) 94, e515 (2015).
Iacovelli, R. et al. Prognostic Role of PD-L1 Expression in Renal Cell Carcinoma. A Systematic Review and Meta-Analysis. Target Oncol 11, 143–148 (2016).
Califano, R. et al. Beyond EGFR and ALK inhibition: unravelling and exploiting novel genetic alterations in advanced non small-cell lung cancer. Cancer Treat Rev 41, 401–411 (2015).
Han, J. J. et al. Change in PD-L1 Expression After Acquiring Resistance to Gefitinib in EGFR-Mutant Non-Small-Cell Lung Cancer. Clin Lung Cancer 17, 263–270.e262 (2016).
Ota, K. et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res 21, 4014–4021 (2015).
Mu, C. Y., Huang, J. A., Chen, Y., Chen, C. & Zhang, X. G. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 28, 682–688 (2011).
Chen, Y. B., Mu, C. Y. & Huang, J. A. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 98, 751–755 (2012).
Azuma, K. et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 25, 1935–1940 (2014).
Velcheti, V. et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 94, 107–116 (2014).
Cooper, W. A. et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 89, 181–188 (2015).
D’Incecco, A. et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 112, 95–102 (2015).
Mao, Y. et al. B7-H1 and B7-H3 are independent predictors of poor prognosis in patients with non-small cell lung cancer. Oncotarget 6, 3452–3461 (2015).
Schmidt, L. H. et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS One 10, e0136023 (2015).
Tang, Y. et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget 6, 14209–14219 (2015).
Inoue, Y. et al. Clinical significance of PDL1 and PDL2 copy number gains in nonsmallcell lung cancer. Oncotarget (2016).
Ji, M. et al. PD-1/PD-L1 expression in non-small-cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol Ther 17, 407–413 (2016).
Sorensen, S. F. et al. PD-L1 Expression and Survival among Patients with Advanced Non-Small Cell Lung Cancer Treated with Chemotherapy. Transl Oncol 9, 64–69 (2016).
Sun, J. M. et al. Prognostic Significance of PD-L1 in Patients with Non-Small Cell Lung Cancer: A Large Cohort Study of Surgically Resected Cases. J Thorac Oncol 11, 1003–1011 (2016).
Tokito, T. et al. Predictive relevance of PD-L1 expression combined with CD8 + TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer 55, 7–14 (2016).
Chen, Z. et al. PD-L1 expression is associated with advanced non-small cell lung cancer. Oncol Lett 12, 921–927 (2016).
Shimoji, M. et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung cancer 98, 69–75 (2016).
Teng, F. et al. Expressions of CD8 + TILs, PD-L1 and Foxp3 + TILs in stage I NSCLC guiding adjuvant chemotherapy decisions. Oncotarget 7, 64318–64329 (2016).
Igawa, S. et al. Impact of PD-L1 Expression in Patients with Surgically Resected Non-Small-Cell Lung Cancer. Oncology 92, 283–290 (2017).
Okita, R. et al. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol Immunother 66, 865–876 (2017).
Takada, K. et al. A Comprehensive Analysis of Programmed Cell Death Ligand-1 Expression With the Clone SP142 Antibody in Non-Small-Cell Lung Cancer Patients. Clin Lung Cancer, (2017).
Tsao, M. S. et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol 28, 882–889 (2017).
Zhou, C. et al. PD-L1 expression as poor prognostic factor in patients with non-squamous non-small cell lung cancer. Oncotarget, (2017).
Yang, C. Y., Lin, M. W., Chang, Y. L., Wu, C. T. & Yang, P. C. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 50, 1361–1369 (2014).
Zhang, Y. et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 7, 567–573 (2014).
Lin, C. et al. Programmed Death-Ligand 1 Expression Predicts Tyrosine Kinase Inhibitor Response and Better Prognosis in a Cohort of Patients With Epidermal Growth Factor Receptor Mutation-Positive Lung Adenocarcinoma. Clin Lung Cancer 16, e25–35 (2015).
Cha, Y. J., Kim, H. R., Lee, C. Y., Cho, B. C. & Shim, H. S. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer 97, 73–80 (2016).
Song, Z., Yu, X., Cheng, G. & Zhang, Y. Programmed death-ligand 1 expression associated with molecular characteristics in surgically resected lung adenocarcinoma. J Transl Med 14, 188 (2016).
Takada, K. et al. Clinical Significance of PD-L1 Protein Expression in Surgically Resected Primary Lung Adenocarcinoma. J Thorac Oncol (2016).
Huynh, T. G. et al. Programmed Cell Death Ligand 1 Expression in Resected Lung Adenocarcinomas: Association with Immune Microenvironment. J Thorac Oncol 11, 1869–1878 (2016).
Inamura, K. et al. Relationship of tumor PD-L1 expression with EGFR wild-type status and poor prognosis in lung adenocarcinoma. Jpn J Clin Oncol 46, 935–941 (2016).
Hirai, A. et al. Prognostic impact of programmed death-ligand 1 expression in correlation with human leukocyte antigen class I expression status in stage I adenocarcinoma of the lung. J Thorac Cardiovasc Surg, (2017).
Mori, S. et al. High expression of programmed cell death 1 ligand 1 in lung adenocarcinoma is a poor prognostic factor particularly in smokers and wild-type epidermal growth-factor receptor cases. Pathol Int 67, 37–44 (2017).
Toyokawa, G. et al. Relevance Between Programmed Death Ligand 1 and Radiologic Invasiveness in Pathologic Stage I Lung Adenocarcinoma. Ann Thorac Surg 103, 1750–1757 (2017).
Uruga, H. et al. Programmed Cell Death Ligand (PD-L1) Expression in Stage II and III Lung Adenocarcinomas and Nodal Metastases. J Thorac Oncol 12, 458–466 (2017).
Wu, S. et al. The significance of programmed cell death ligand 1 expression in resected lung adenocarcinoma. Oncotarget 8, 16421–16429 (2017).
Kim, M. Y. et al. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer 88, 24–33 (2015).
Yang, C. Y., Lin, M. W., Chang, Y. L., Wu, C. T. & Yang, P. C. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer 57, 91–103 (2016).
Ilie, M. et al. PD-L1 expression in basaloid squamous cell lung carcinoma: Relationship to PD-1 + and CD8 + tumor-infiltrating T cells and outcome. Mod Pathol 29, 1552–1564 (2016).
Guo, Q. et al. Programmed cell death-ligand 1 (PD-L1) expression and fibroblast growth factor receptor 1 (FGFR1) amplification in stage III/IV lung squamous cell carcinoma (SQC). Thorac Cancer 8, 73–79 (2017).
Takada, K. et al. The expression of PD-L1 protein as a prognostic factor in lung squamous cell carcinoma. Lung cancer 104, 7–15 (2017).
Zhang, M. et al. Prognostic significance of PD-L1 expression and 18F-FDG PET/CT in surgical pulmonary squamous cell carcinoma. Oncotarget, (2017).
Ishii, H. et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 10, 426–430 (2015).
Miao, L. et al. PD-L1 and c-MET expression and survival in patients with small cell lung cancer. Oncotarget (2016).
Fang, W. et al. PD-L1 is remarkably over-expressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. Oncotarget 6, 33019–33032 (2015).
Jiang, L. et al. Positive expression of programmed death ligand-1 correlates with superior outcomes and might be a therapeutic target in primary pulmonary lymphoepithelioma-like carcinoma. Onco Targets Ther 8, 1451–1457 (2015).
Chang, Y. L., Yang, C. Y., Lin, M. W., Wu, C. T. & Yang, P. C. High co-expression of PD-L1 and HIF-1alpha correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer 60, 125–135 (2016).
Wu, P., Wu, D., Li, L., Chai, Y. & Huang, J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One 10, e0131403 (2015).
Pan, Z. K., Ye, F., Wu, X., An, H. X. & Wu, J. X. Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis 7, 462–470 (2015).
Wang, A. et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 41, 450–456 (2015).
Xia, H. et al. PD-L1 over-expression is associated with a poor prognosis in Asian non-small cell lung cancer patients. Clin Chim Acta (2017).
Zhong, A., Xing, Y., Pan, X., Shi, M. & Xu, H. Prognostic value of programmed cell death-ligand 1 expression in patients with non-small-cell lung cancer: evidence from an updated meta-analysis. Onco Targets Ther 8, 3595–3601 (2015).
Borghaei, H. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 373, 1627–1639 (2015).
Brahmer, J. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 373, 123–135 (2015).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372, 2018–2028 (2015).
Fehrenbacher, L. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846 (2016).
Passiglia, F. et al. PD-L1 expression as predictive biomarker in patients with NSCLC: a pooled analysis. Oncotarget 7, 19738–19747 (2016).
Akbay, E. A. et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3, 1355–1363 (2013).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8, 336–341 (2010).
Parmar, M. K., Torri, V. & Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17, 2815–2834 (1998).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605 (2010).
Acknowledgements
This study was supported by grants from Project of Health Department of Heilongjiang Province [No. 2016-102], innovation fund of Harbin Medical University (2017LCZX95).
Author information
Authors and Affiliations
Contributions
M.H.Z. and Y.W. designed this study; H.L.Z. and G.L.L. searched databases and collected full-text papers; M.H.Z., G.L.L. and Y.B.W. extracted and analyzed data; Y.W., S.Z. and H.H.P. wrote the manuscript; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, M., Li, G., Wang, Y. et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep 7, 10255 (2017). https://doi.org/10.1038/s41598-017-10925-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10925-7
This article is cited by
-
PD-L1 expression and its significance in advanced NSCLC: real-world experience from a tertiary care center
Journal of the Egyptian National Cancer Institute (2024)
-
Expression of PD-L1 through evolution phase from pre-invasive to invasive lung adenocarcinoma
BMC Pulmonary Medicine (2023)
-
Common driver mutations and programmed death-ligand 1 expression in advanced non-small cell lung cancer in smokers and never smokers
BMC Cancer (2023)
-
Ezrin regulates the progression of NSCLC by YAP and PD-L1
Clinical and Translational Oncology (2023)
-
Cancer secretome: finding out hidden messages in extracellular secretions
Clinical and Translational Oncology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.