Abstract
Cancer-associated fibroblasts (CAFs) contribute to the poor prognosis of ovarian cancer. Unlike in tumour cells, DNA mutations are rare in CAFs, raising the likelihood of other mechanisms that regulate gene expression such as long non-coding RNAs (lncRNAs). We aimed to identify lncRNAs that contribute to the tumour-promoting phenotype of CAFs. RNA expression from 67 ovarian CAF samples and 10 normal ovarian fibroblast (NOF) samples were analysed to identify differentially expressed lncRNAs and a functional network was constructed to predict those CAF-specific lncRNAs involved in metastasis. Of the 1,970 lncRNAs available for analysis on the gene expression array used, 39 unique lncRNAs were identified as differentially expressed in CAFs versus NOFs. The predictive power of differentially expressed lncRNAs in distinguishing CAFs from NOFs were assessed using multiple multivariate models. Interrogation of known transcription factor-lncRNA interactions, transcription factor-gene interactions and construction of a context-specific interaction network identified multiple lncRNAs predicted to play a role in metastasis. We have identified novel lncRNAs in ovarian cancer that are differentially expressed in CAFs compared to NOFs and are predicted to contribute to the metastasis-promoting phenotype of CAFs.
Similar content being viewed by others
Introduction
Ovarian cancer is the most lethal gynaecologic malignancy in women, of which high-grade serous ovarian cancer (HGSOC) is the most common and aggressive subtype1. The majority of women are diagnosed at an advanced stage when the tumour has metastasised throughout the peritoneal cavity, and while initial response to the currently used chemotherapies is high, the development of chemoresistance is common2. As such, the 5-year survival rate is reduced from 80% if diagnosed early to <30% for women diagnosed with advanced disease3. A greater understanding of the factors that promote metastasis and chemoresistance may improve the ability to provide new and more effective therapeutic targets for the treatment of HGSOC.
The tumour microenvironment is now established as an essential part of tumour progression4. Cancer-associated fibroblasts (CAFs) are the most abundant cell type in the tumour microenvironment and are responsible for the secretion of extracellular matrix components, growth and differentiation factors. It has long been acknowledged in multiple cancer types that CAFs interact with tumour cells and play a vital role in promoting cancer cell proliferation, migration and invasion, as well as contribute to angiogenesis and chemoresistance5,6,7,8,9. CAFs are also recognised to play a similar role in ovarian cancer10,11,12,13. Therefore, understanding the molecular characteristics of ovarian CAFs compared to normal ovarian fibroblasts (NOFs) is pivotal in designing effective therapies that target the tumour microenvironment.
Expression profiling studies have identified differential expression of several genes in CAFs or tumour-associated stroma compared to normal stroma in cancers including breast14, lung15 and ovarian16,17,18. In addition, genetic alterations in CAFs and tumour-associated stroma have been investigated in several cancer types, including ovarian cancer; however, these studies have concluded that somatic mutations are extremely rare19,20,21. This lack of somatic genetic alterations in the tumour stroma and CAFs raises the likelihood of alternative mechanisms of gene regulation. For example, differences in methylation between cancer-associated and normal stroma have been reported in breast, gastric and prostate cancer22,23,24,25. In addition, one study in ovarian cancer demonstrated that microRNAs differentially expressed in ovarian CAFs compared to normal omental fibroblasts can also regulate gene expression and play a role in the conversion of normal fibroblasts to CAFs26.
Long non-coding RNAs (lncRNAs) represent another potential candidate for regulating gene expression and function in CAFs that has yet to be explored. Once thought to be “transcriptional noise”, lncRNAs are non-protein coding RNAs >200 nucleotides in length that have relatively recently been shown to play vital roles in a large variety of biological functions such as genetic imprinting, chromatin modification and modulation of gene expression27. Increasingly, lncRNAs are being implicated in a variety of diseases, including cancer28. However, it has not been determined whether lncRNAs contribute to the phenotype and function of CAFs in any cancer. Given that the functions of many lncRNAs are still unknown, integrative computational approaches that discern different avenues of lncRNA functionality are highly desired29. Therefore, in this study we first sought to identify whether lncRNAs are differentially expressed in ovarian CAFs versus NOFs. We then developed a novel integrative network-based computational approach to identify those CAF-specific lncRNAs that may play a role in metastasis.
Results
Identification of lncRNAs differentially regulated in CAFs compared to NOFs
To identify lncRNAs that were differentially expressed in CAFs versus NOFs, we analysed gene expression data from microdissected CAF samples obtained from 67 HGSOC patients and compared them to NOFs obtained from 10 patients who underwent oophorectomy for benign conditions. A significance cutoff of |log2(fold-change)| > 1 and p-value < 0.05 (adjusted p-value < 0.1) were used to identify lncRNAs differentially expressed in CAFs versus NOFs. In total, 60 probes were identified reflecting 39 unique lncRNAs with known titles (Table 1). A heatmap depicting the expression profiles of the identified lncRNAs in CAFs and NOFs is shown in Fig. 1A. Figure 1B depicts box plots representing the distributions of expressions of each lncRNA across CAF and NOF samples based on multiple statistics, i.e., minimum, median, maximum, and the interquartile range. Overall, 17 lncRNAs were up-regulated in CAFs and 22 lncRNAs were downregulated with evidently distinct distributions as the box plots display. Of the 39 lncRNAs differentially expressed in CAFs compared to NOFs, 11 were also differentially expressed in the corresponding laser-capture microdissected tumor epithelium compared to normal ovarian surface epithelium (Supplementary Table S1).
Differentially expressed lncRNAs in ovarian CAFs vesus NOFs. (A) Heatmap illustrating the expression profile differences of the identified lncRNAs differentially expressed in CAFs compared to NOFs. (B) Box plots showing the distribution of expression of each lncRNA in CAF samples compared to NOF samples.
Predictive power of the differentially expressed lncRNAs to distinguish between CAFs and NOFs
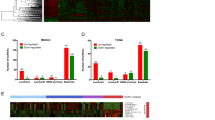
To accurately assess the predictive power of the differentially expressed lncRNAs to distinguish between CAFs and NOFs, the unbalanced class distribution was first adjusted using SMOTE algorithm30, a well-known oversampling machine learning technique. We oversampled NOFs, increased sample size by 50%, and undersampled CAFs, decreased samples by 50% which resulted in 15 NOFs and 33 CAFs for classification analyses. Supplementary Fig. S1 visualises the original and oversampled/undersampled dataset by plotting the samples across the first two principle components of DE lncRNAs. Highlighted NOFs are those generated using SMOTE oversampling. Samples were then randomly partitioned into discovery and validation sets and were analysed according to the workflow outlined in Fig. 2A. Logistic regression (LR), Random Forest (RF) and Support Vector Machine (SVM) multivariate models whose predictors are differentially expressed lncRNAs across the discovery samples were trained on the discovery set. The quality of the models in distinguishing CAFs and NOFs was then assessed by estimating accuracy, sensitivity, and specificity measures on the validation set. Boxplots in Fig. 2B show the distributions of these measures over 100 iterations of randomly partitioning samples to discovery and validation sets. The SVM model significantly outperformed the LR model in all measures and the RF model in accuracy and sensitivity measures (Wilcoxon p-value < 10E-5). The average specificity of SVM is 0.91 ± 0.13 confirming the ability and stability of this model in predicting underrepresented NOF samples. It also demonstrated the average accuracy of 0.92 ± 0.06 confirming the overall discriminatory power of the identified lncRNAs.
Predictive power of the identified lncRNAs. (A) Workflow used to assess the predictive power of differentially expressed lncRNAs (B) Box plots showing the distribution of accuracy, sensitivity and specificity of logistic regression (LR), random forest (RF), and Support Vector Machine (SVM) multivariate classification models across 100 iterations; for each box plot, the exact values of mean ± standard deviation are displayed (C) Bar plots representing the ‘feature stability’ of 34 lncRNAs identified to be differentially expressed in 50 iterations or more. The feature stability is defined as the proportion of runs that a lncRNA is identified to be differentially expressed and thus selected as a feature for the multivariate predictive models. More stable features are less sensitive to data partitioning. Log fold change and p-values of lncRNAs in validation sets were estimated and averaged across 100 iterations. Dashed line on p-value bar chart is the indicator of the 0.05 cutoff.
Differentially expressed lncRNAs are subject to change across iterations due to the change in the composition of the discovery set. We chose stable lncRNAs as those identified to be differentially expressed in more than 50% of iterations. Accordingly, 34 lncRNAs were selected, including all lncRNAs listed in Table 1 except for FLJ45340, LOC100272216, LOC100506013, LOC284454, LOC339988, MALAT1, and MEG3 which identified as differentially expressed in 38, 29, 38, 3, 26, 20, and 26 iterations, respectively. For each of these 34 lncRNAs, Fig. 2C plots the feature “stability” as the proportion of runs where the corresponding lncRNA was differentially expressed and thus selected as a feature of the predictive models. Figure 2C also shows the fold change values and p-values of these lncRNAs comparing CAFs versus NOFs in the validation sets averaged across iterations. The contribution of each lncRNA in the prediction performance of the RF model was estimated across iterations and visualised in Supplementary Fig. S2, which shows the overall positive contribution of lncRNAs in the model accuracy. Additionally, the predictive power of each of 38 lncRNAs was individually assessed using a univariate LR model whose sole predictor is the lncRNA expression across discovery samples (Table S2). The results highlight the advantage of using multivariate predictive models and confirm the previous findings that compared to single molecules, molecular signatures are more robust biomarkers and more powerful predictors of disease phenotypes31.
Functional roles of the identified lncRNAs in metastasis
The functional roles of deregulated lncRNAs in ovarian CAFs were predicted by first constructing the context-specific regulatory network of transcription factor (TF)-lncRNA and TF-target gene (TG) interactions. The network comprises 42 enriched TFs targeting 31 lncRNAs and 646 TGs differentially expressed in CAFs versus NOFs, totaling 1,266 interactions (375 TF-lncRNA and 891 TF-TG interactions). The network flat file is available in Supplementary Data and visualised in Supplementary Fig. S1. For each TF, targets with a similar expression pattern across all samples clustered together (using hierarchical clustering), forming coregulatory modules (clusters with p-value < 0.05). All targets within the same module are regulated by the same TF and expressed consistently across samples and thus assumed to function correspondingly. The focus of this study is on the metastatic role of lncRNAs. Pathways expressed in ovarian cancer metastases have been identified previously32; all metastasis-associated pathways considered in this study have been provided in Supplementary Table S3. An enrichment analysis was performed to identify metastasis-related pathways overrepresented by TGs within each module. Figure 3 shows TFs whose predominant coregulatory module (i.e., the largest cluster) enriches multiple (>5) metastatic pathways and comprises at least one lncRNA yet the number of lncRNAs is less than the number of coregulated TGs. Figure 3 demonstrates the association of 7 lncRNAs upregulated in CAFs (FLJ39739, GAS5, H19, LOC100499466, MALAT1, NEAT1, and TUG1) with multiple pathways in ovarian cancer metastasis including pathways involved in the interaction of cells with the microenvironment, namely focal adhesion, and ECM receptor interaction. We also identified co-regulatory modules containing lncRNAs whose downregulation was associated with enrichment of metastasis-associated pathways. These included lncRNAs CASC2, DLEU2, HCG18 and LOC100133669. Complete results including clusters formed by all TFs’ targets along with the corresponding enriched pathways are available in Supplementary Table S4.
Functional prediction of CAF-associated lncRNAs. (A) Hierarchical clustering dendrograms showing the lncRNAs in the predominant co-regulatory module for each TF. (B) Metastatic pathways enriched by TFs’ predominant clusters; links between a TF and a pathway represents the enrichment of the pathway by targets of the TF in the prevalent cluster; histogram bars show the corresponding p-value log10 scaled and negated to improve readability.
Discussion and Conclusion
The tumour microenvironment and more specifically CAFs are now accepted to play an important role in several aspects of tumour development and progression in many cancer types, including ovarian. The relative genetic stability of CAFs also makes them an attractive therapeutic target, but given this genetic stability, the factors that regulate gene expression in ovarian CAFs are not well elucidated. We sought to identify whether lncRNAs are involved in the CAF phenotype, firstly by comparing lncRNA expression profiles of ovarian CAFs to NOFs in order to identify those lncRNAs that could be used to confidently differentiate CAFs from NOFs. Then, given the known role of CAFs in promoting metastasis, we hypothesised that some of the differentially expressed lncRNAs may play a role in the pro-metastatic phenotype of CAFs. As the function of many lncRNAs is still not well known, we took an integrative computational approach to identify those lncRNAs which may be involved in the pro-metastatic function of CAFs.
To date, studies analysing lncRNA expression in ovarian cancer have identified a number that are aberrantly expressed33,34,35. However, these studies used ovarian cancer samples from bulk tumour; therefore the involvement of lncRNAs specifically in tumour stroma or CAFs could not be determined. By using gene expression data from laser-capture microdissected samples, we were able to identify 39 lncRNAs aberrantly expressed in ovarian CAFs compared to NOFs. This is likely an underestimate of the total number of lncRNAs that are differentially expressed between CAFs and NOFs due to the limited number of probes corresponding to lncRNAs on the array used. Of the lncRNAs identified, there were several that have previously been implicated in ovarian cancer. These include NEAT1 36, 37, TUG1 38, MALAT1 39, H19 40,41,42, XIST 43, 44, GAS5 45, 46 and MEG3 47. Also included were lncRNAs reported to play roles in other cancer types but that have not previously been associated with ovarian cancer such as FLJ39739 48, DLEU2 49, CASC2 50, 51, LINC00152 52, MIR100HG 53, 54 and MIR22HG 55. Our data suggest that these lncRNAs could play a role in ovarian CAFs rather than, or as well as, the tumour cells themselves. This is supported by the fact that 28 out of the 39 differentially expressed lncRNAs were not differentially expressed in tumour epithelium compared to ovarian surface epithelium. This is the first report in ovarian cancer to show lncRNAs to be aberrantly expressed in the tumour stroma. This is also likely to be the case in other tumour types; however, to date this has not been widely investigated. A recent study by Zhang et al. used chromogenic in situ hybridisation to examine a panel of 6 lncRNAs previously implicated in breast cancer in order to identify the cellular components expressing each lncRNA56. Interestingly, the lncRNAs H19 and MEG3 were shown to be overexpressed in the breast tumour stroma rather than the tumour cells, while MALAT1 was found to be expressed in both tumour and stromal tissue. While limited to a panel of 6 lncRNAs, these data strongly support our findings in ovarian CAFs and suggest that ovarian CAF-associated lncRNAs may also play a role in CAFs from other tumour types. Furthermore, CAFs are thought to most strongly resemble activated myofibroblasts, which are also increased in other pathologies such as liver fibrosis. Two lncRNAs identified in our study as differentially expressed in CAFs have previously been shown to be increased in activated hepatic stellate cells: MALAT1 57, 58 and GAS5 59.
The identification of several lncRNAs differentially expressed in ovarian CAFs supports our hypothesis that lncRNAs play a role in regulating gene expression in CAFs. We then wished to determine whether any of these lncRNAs may play a role in the pro-tumorigenic function of CAFs. CAFs have been shown to influence the behavior of tumour cells in several ways, a major role of which is to promote metastasis. We therefore constructed a context specific interaction network to identify those lncRNAs that play a role in the pro-metastatic phenotype of CAFs. This led to the identification of several metastasis-associated lncRNAs, several of which have been implicated in cancer metastasis but have not previously been associated with the metastatic role of CAFs. In addition, these lncRNAs clustered with several genes that have been shown to be overexpressed in CAFs and contribute to their ability to promote metastasis such as MMP2 60 and IL8 61. In ovarian cancer, overexpression of NEAT1 36, 37, TUG1 38 and MALAT1 39 have previously been associated with increased tumour grade, FIGO stage and an increase in distant metastasis. However, these studies used whole tumour specimens for analysis; therefore, the role of these lncRNAs in CAFs was not determined. Interestingly, these three lncRNAs have all been shown to regulate epithelial to mesenchymal transition in multiple cancer types in vitro 62,63,64,65, which is a process necessary for cancer cells to metastasise but is also known to be regulated by CAFs. It is possible that the ability for CAFs to promote epithelial to mesenchymal transition in tumour cells could in part be mediated by these lncRNAs and warrants further investigation.
CAFs are well known to play important roles in cancer and metastasis and as such, represent an attractive target for novel therapies in multiple cancer types. However, a better understanding of the molecular factors that differentiate CAFs from normal fibroblasts is essential for the development of therapies that specifically target CAFs. While gene expression profiling studies have determined that CAFs differ from normal fibroblasts, the lack of genetic mutations suggests that other complex mechanisms of gene regulation are at play in the tumour microenvironment. For the first time in any cancer, we have shown that lncRNAs represent one possible mechanism of gene expression regulation in CAFs that can be used to differentiate them from NOFs. Furthermore, several of these CAFs are also predicted play a role in the metastasis-promoting phenotype of CAFs. These data provide a greater understanding of the complexities involved in the CAF phenotype, despite their genetic stability and may lead to the design of targeted CAF therapies.
Materials and Methods
Tissue Specimens
Primary tumour specimens from 67 women diagnosed with HGSOC were obtained as previously described18, 66. All specimens were from HGSOC patients prior to treatment hospitalised at the Brigham and Women’s Hospital between 1990 and 2000. All specimens and their corresponding clinical information were collected by written consent under protocols approved by the review board of the Brigham and Women’s Hospital Ethics Committee. All procedures were performed in accordance with the approved guidelines and regulations. Classification was determined according to the International Federation of Gynecology and Obstetrics (FIGO) standards. Normal ovaries were obtained from 10 patients who underwent surgery for benign gynaecologic conditions. The characteristics of tumour samples included in this study are shown in Table 2. There was no significant difference between the ages of the oophorectomy patients (mean age ± Std = 62.3 ± 5.272) and the HGSOC patients at time of surgery (P = 0.674, Mann Whitney U Test).
Microdissection, RNA isolation, amplification and hybridisation
Microdissection, RNA isolation, amplification and hybridisation to GeneChip Human Genome U133 Plus 2.0 Oligonucleotide arrays (Affymetrix) were performed as described previously18. Gene expression of endothelial cell markers (TIE-2 and VEGFR1) and T cell markers (CD8 and CD45) were below the level of detection in the samples, indicating a lack of immune or endothelial components of the stroma and enrichment for fibroblasts18. All gene array data are available through Gene Expression Omnibus (GEO) accession number GSE40595.
Data pre-processing and differential expression analysis
Data pre-processing was performed using R Bioconducter,’affy’ package. Data were normalised and background corrected using the Robust Multi-Array Average method67 and expression values Log2 transformed. Differentially expressed probes (both lncRNAs and protein-coding genes) between CAFs versus NOFs were identified using the moderated t-test as defined in the Bioconductor limma package68; p-values were adjusted for multiple hypothesis testing using the False Discovery Rate (FDR) correction. The 2,448 probes corresponding to 1,970 lncRNAs were identified previously by Zhang et al.69. The gene symbols and titles corresponding to these probes were matched by Affymetrix U133 Plus 2.0 Array annotation file.
Analysis of the predictive power of the identified lncRNAs
A well-balanced dataset is critical for creating powerful prediction models as most existing classifiers tends to optimise the overall prediction accuracy and thus perform poorly on the minority class examples when the dataset is very imbalanced. We adjusted for the imbalanced class distribution between CAFs and NOFs using an oversampling/undersampling machine learning approach typically used to construct classifiers from unbalanced datasets70. Over/undersampling techniques have shown improved prediction performance when used to handle imbalanced clinical datasets71, 72. We used SMOTE: Synthetic Minority Oversampling Technique 30, a well-known and powerful technique which synthetically generates new examples of the minority class using the nearest neighbours of the cases and randomly samples from the majority class examples in order to produce a more balanced dataset. SMOTE was implemented using R package ‘DMwR’.
Once the original data was over/undersampled to produce a more balanced dataset, to assess the predictive power of differentially expressed lncRNAs, samples were first randomly partitioned into two disjoint sets of discovery (50% of samples) and validation (50% of samples). LncRNAs differentially expressed in the discovery set (i.e., |log2(fold-change)| > 1 and p-value < 0.05) were then selected as features/predictors of three widely-used multivariate predictive models namely logistic regression (LR)73, Random Forest (RF)74, and Support Vector Machine (SVM)75 with linear kernel function. All models were trained on the discovery set. The resultant models were then used to predict CAFs versus NOFs based on the expression values of identified lncRNAs in validation samples. The quality of the models was assessed based on the standard statistical performance measures of classification tests, i.e., accuracy, sensitivity and specificity. The accuracy is the proportion of true predictions (both CAFs and NOFs) among the total number of cases in the validation set. Sensitivity measures the proportion of CAFs in the validation set that are correctly identified as such. Specificity measures the proportion of NOFs in the validation set that are correctly identified as such. For statistical rigour, to account for random partitioning of the samples into discovery and validation sets, the whole process was repeated 100 times and the averaged measures of performance were reported. The importance or relative contribution of each feature (differentially expressed lncRNA) in the RF performance was estimated based on the ‘mean decrease accuracy’ measure74 as described in Supplementary Fig. S2. All analyses were performed using R ‘caret’, ‘RandomForest, and ‘e1071’packages.
Network-based functional analysis of the identified lncRNAs
Construction of a context-specific network of transcription factor (TF)-lncRNA and TF-gene regulations
TF-lncRNA regulatory relationships were extracted from ChIPBase database, which provides a comprehensive annotation of TF-lncRNA interaction map using ChIP-Seq data generated from multiple organisms76. We searched ChIPBase using default parameters (i.e., regulatory region of 5 kb upstream and 1 kb downstream) and filtered for mammalian organisms to obtain the list of TF interacting with the identified lncRNAs. The names of the interacting TFs were disambiguated using NCBI to obtain their official symbols provided by the HGNC (HUGO Gene Nomenclature Committee). These TFs were used to search ORTI77, a recently compiled repository of mammalian transcriptional interactions, to obtain their experimentally-validated (Rank 1) target genes (TGs). The compiled TF-TG interactions are sourced from a range of experimental conditions. In order to identify TFs modulated under the specific context of the study, we used ORTI application 177, which performs TF enrichment analysis on a list of genes differentially expressed in CAFs versus NOFs (p-value < 0.05 and |log2(fold-change)| > 1, using a similar moderated t-test employed to derive differentially expressed lncRNAs). TFs with enrichment p-values < 0.05 were then considered as ‘active’ in the context of the study. Subsequently, a context-specific regulatory network consisting of interactions between enriched TFs and differentially expressed TGs and lncRNAs was constructed for the subsequent analysis.
Prediction of lncRNA functions
The functional roles of lncRNAs deregulated in ovarian CAFs were characterised based on the functions of the co-expressed TGs using the constructed context-specific regulatory network. Accordingly, for each TF, targets with highly correlated expression patterns were clustered together and assumed to conduct similar functions (in the context of study) given that they were targeted by an identical ‘active’ TF and followed similar expression pattern across different samples. We performed hierarchical clustering on expression profiles of targets of each TF using R ‘pvclust’ package which conducts multiscale bootstrap resampling to calculate p-values for each cluster. Sample correlation was chosen as the distance measure and the ‘average’ agglomerative method was used in the hierarchical clustering. Clusters whose estimated p-value was < 0.05 were identified as co-regulatory modules of the TF.
The functional roles of lncRNAs were then predicted using the functions of the co-regulating TGs. In this study, we were focused on the metastatic role of lncRNAs in ovarian CAFs. Pathways expressed in metastatic ovarian tumours were identified previously32. We chose KEGG pathways whose enrichment p-value was <0.05 and performed pathway enrichment analysis on genes involved in each co-regulatory module using the right-sided Fisher’s exact test where the p-value for the null hypothesis is computed based on the hypergeometric distribution. Consequently, lncRNAs in each module were predicted to be involved in the metastatic pathways enriched (adjusted p-value < 0.1) by the co-regulating TGs in the module. The entire workflow for the network and subsequent functional analysis is depicted in Fig. 4.
References
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J Clin 63, 11–30 (2013).
Bovicelli, A., D’Andrilli, G. & Giordano, A. New players in ovarian cancer. J Cell Physiol 226, 2500–4 (2011).
Cannistra, S. A. Cancer of the ovary. N Engl J Med 351, 2519–29 (2004).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–74 (2011).
Duda, D. G. et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA 107, 21677–82 (2010).
Grum-Schwensen, B. et al. Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res 65, 3772–80 (2005).
Hwang, R. F. et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 68, 918–26 (2008).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–61 (2009).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–48 (2005).
Lau, T. S. et al. Cancer cell-derived lymphotoxin mediates reciprocal tumour-stromal interactions in human ovarian cancer by inducing CXCL11 in fibroblasts. J Pathol 232, 43–56 (2014).
Lawrenson, K. et al. NPPB is a novel candidate biomarker expressed by cancer-associated fibroblasts in epithelial ovarian cancer. Int J Cancer 136, 1390–401 (2015).
Leung, C. S. et al. Calcium-dependent FAK/CREB/TNNC1 signalling mediates the effect of stromal MFAP5 on ovarian cancer metastatic potential. Nat Commun 5, 5092 (2014).
Yeung, T. L., Leung, C. S. & Mok, S. C. CAF reprogramming inhibits ovarian cancer progression. Cell Cycle 13, 3783–4 (2014).
Sadlonova, A. et al. Identification of molecular distinctions between normal breast-associated fibroblasts and breast cancer-associated fibroblasts. Cancer Microenviron 2, 9–21 (2009).
Navab, R. et al. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc Natl Acad Sci USA 108, 7160–5 (2011).
Ghosh, S. et al. Up-regulation of stromal versican expression in advanced stage serous ovarian cancer. Gynecol Oncol 119, 114–20 (2010).
Kataoka, F. et al. EGRI and FOSB gene expressions in cancer stroma are independent prognostic indicators for epithelial ovarian cancer receiving standard therapy. Genes Chromosomes Cancer 51, 300–12 (2012).
Moran-Jones, K. et al. Connective tissue growth factor as a novel therapeutic target in high grade serous ovarian cancer. Oncotarget 6, 44551–62 (2015).
Allinen, M. et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 6, 17–32 (2004).
Qiu, J. J. et al. Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol Oncol 134, 121–8 (2014).
Walter, K., Omura, N., Hong, S. M., Griffith, M. & Goggins, M. Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer Biol Ther 7, 882–8 (2008).
Fiegl, H. et al. Breast cancer DNA methylation profiles in cancer cells and tumor stroma: association with HER-2/neu status in primary breast cancer. Cancer Res 66, 29–33 (2006).
Hanson, J. A. et al. Gene promoter methylation in prostate tumor-associated stromal cells. J Natl Cancer Inst 98, 255–61 (2006).
Hu, M. et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet 37, 899–905 (2005).
Jiang, L. et al. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res 68, 9900–8 (2008).
Mitra, A. K. et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov 2, 1100–8 (2012).
Geisler, S. & Coller, J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol 14, 699–712 (2013).
Wilusz, J. E., Sunwoo, H. & Spector, D. L. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 23, 1494–504 (2009).
Signal, B., Gloss, B. S. & Dinger, M. E. Computational Approaches for Functional Prediction and Characterisation of Long Noncoding RNAs. Trends in Genetics 32, 620–637 (2016).
Chawla, N. V., Bowyer, K. W., Hall, L. O. & Kegelmeyer, W. P. SMOTE: Synthetic minority over-sampling technique. Journal of Artificial Intelligence Research 16, 321–357 (2002).
Backes, C., Meese, E. & Keller, A. Specific miRNA Disease Biomarkers in Blood, Serum and Plasma: Challenges and Prospects. Mol Diagn Ther 20, 509–518 (2016).
Brodsky, A. S. et al. Expression profiling of primary and metastatic ovarian tumors reveals differences indicative of aggressive disease. PLoS One 9, e94476 (2014).
Akrami, R. et al. Comprehensive analysis of long non-coding RNAs in ovarian cancer reveals global patterns and targeted DNA amplification. PLoS One 8, e80306 (2013).
Du, Z. et al. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat Struct Mol Biol 20, 908–13 (2013).
Guo, Q. et al. Comprehensive analysis of lncRNA-mRNA co-expression patterns identifies immune-associated lncRNA biomarkers in ovarian cancer malignant progression. Sci Rep 5, 17683 (2015).
Chai, Y., Liu, J., Zhang, Z. & Liu, L. HuR-regulated lncRNA NEAT1 stability in tumorigenesis and progression of ovarian cancer. Cancer Med 5, 1588–98 (2016).
Chen, Z. J., Zhang, Z., Xie, B. B. & Zhang, H. Y. Clinical significance of up-regulated lncRNA NEAT1 in prognosis of ovarian cancer. Eur Rev Med Pharmacol Sci 20, 3373–7 (2016).
Kuang, D., Zhang, X., Hua, S., Dong, W. & Li, Z. Long non-coding RNA TUG1 regulates ovarian cancer proliferation and metastasis via affecting epithelial-mesenchymal transition. Exp Mol Pathol 101, 267–273 (2016).
Zhou, Y. et al. The Long Noncoding RNA MALAT-1 Is Highly Expressed in Ovarian Cancer and Induces Cell Growth and Migration. PLoS One 11, e0155250 (2016).
Matouk, I. J. et al. Oncofetal H19 RNA promotes tumor metastasis. Biochim Biophys Acta 1843, 1414–26 (2014).
Tanos, V. et al. Expression of the imprinted H19 oncofetal RNA in epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol 85, 7–11 (1999).
Yan, L. et al. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene 34, 3076–84 (2015).
Huang, K. C. et al. Relationship of XIST expression and responses of ovarian cancer to chemotherapy. Mol Cancer Ther 1, 769–76 (2002).
Kawakami, T. et al. Characterization of loss-of-inactive X in Klinefelter syndrome and female-derived cancer cells. Oncogene 23, 6163–9 (2004).
Gao, J. et al. Long non-coding RNA growth arrest-specific transcript 5 is involved in ovarian cancer cell apoptosis through the mitochondria-mediated apoptosis pathway. Oncol Rep 34, 3212–21 (2015).
Li, J. et al. Decreased expression of long non-coding RNA GAS5 promotes cell proliferation, migration and invasion, and indicates a poor prognosis in ovarian cancer. Oncol Rep (2016).
Sheng, X. et al. Promoter hypermethylation influences the suppressive role of maternally expressed 3, a long non-coding RNA, in the development of epithelial ovarian cancer. Oncol Rep 32, 277–85 (2014).
Wan, X. et al. Identification of androgen-responsive lncRNAs as diagnostic and prognostic markers for prostate cancer. Oncotarget (2016).
Xue, G. et al. c-Myc-mediated repression of miR-15-16 in hypoxia is induced by increased HIF-2alpha and promotes tumor angiogenesis and metastasis by upregulating FGF2. Oncogene 34, 1393–406 (2015).
Cao, Y. et al. Downregulation of lncRNA CASC2 by microRNA-21 increases the proliferation and migration of renal cell carcinoma cells. Mol Med Rep 14, 1019–25 (2016).
Huang, G. et al. The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep 6, 26524 (2016).
Zhao, J. et al. Long non-coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle 14, 3112–23 (2015).
Emmrich, S. et al. LincRNAs MONC and MIR100HG act as oncogenes in acute megakaryoblastic leukemia. Mol Cancer 13, 171 (2014).
Shang, C. et al. Characterization of long non-coding RNA expression profiles in lymph node metastasis of early-stage cervical cancer. Oncol Rep 35, 3185–97 (2016).
Li, D. S., Ainiwaer, J. L., Sheyhiding, I., Zhang, Z. & Zhang, L. W. Identification of key long non-coding RNAs as competing endogenous RNAs for miRNA-mRNA in lung adenocarcinoma. Eur Rev Med Pharmacol Sci 20, 2285–95 (2016).
Zhang, Z. et al. Long non-coding RNA chromogenic in situ hybridisation signal pattern correlation with breast tumour pathology. J Clin Pathol 69, 76–81 (2016).
Wu, Y. et al. Silent information regulator 1 (SIRT1) ameliorates liver fibrosis via promoting activated stellate cell apoptosis and reversion. Toxicol Appl Pharmacol 289, 163–76 (2015).
Yu, F. et al. MALAT1 functions as a competing endogenous RNA to mediate Rac1 expression by sequestering miR-101b in liver fibrosis. Cell Cycle 14, 3885–96 (2015).
Yu, F. et al. Long Non-coding RNA Growth Arrest-specific Transcript 5 (GAS5) Inhibits Liver Fibrogenesis through a Mechanism of Competing Endogenous RNA. J Biol Chem 290, 28286–98 (2015).
Bates, A. L. et al. Stromal matrix metalloproteinase 2 regulates collagen expression and promotes the outgrowth of experimental metastases. J Pathol 235, 773–83 (2015).
Wang, T. et al. Senescent Carcinoma-associated Fibroblasts Upregulate IL8 to Enhance Pro-metastatic Phenotypes. Mol Cancer Res (2016).
Lu, Y. et al. The long non-coding RNA NEAT1 regulates epithelial to mesenchymal transition and radioresistance in through miR-204/ZEB1 axis in nasopharyngeal carcinoma. Tumour Biol 37, 11733–11741 (2016).
Sun, J. et al. The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial-mesenchymal transition. J Transl Med 14, 42 (2016).
Wang, L. et al. Long non-coding RNA TUG1 promotes colorectal cancer metastasis via EMT pathway. Oncotarget (2016).
Ying, L. et al. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst 8, 2289–94 (2012).
Bonome, T. et al. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res 65, 10602–12 (2005).
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3, Article3 (2004).
Zhang, X. et al. Long non-coding RNA expression profiles predict clinical phenotypes in glioma. Neurobiol Dis 48, 1–8 (2012).
Chawla, N.V. Data mining for imbalanced datasets: an overview. in Data mining and knowledge discovery handbook 875-886 (Springer US, 2010).
Akalin, A. et al. Classification of malignant and benign tumors of the lung by infrared spectral histopathology (SHP) (vol 95, pg 697, 2015). Laboratory Investigation 95, 697–697 (2015).
Mostafizur Rahman, M. & Davis, D. N. Addressing the Class Imbalance Problem in Medical Datasets. International Journal of Machine Learning and Computing 3, 224–228 (2013).
Hosmer, D.W., Lemeshow, S. & Sturdivant, R.X. Applied Logistic Regression, (John Wiley & Sons, 2013).
Breiman, l. Random Forests. Machine Learning 45, 5-32 (2001).
Cristianini, N. & Shawe-Taylor, J. An introduction to support vector machines: and other kernel-based learning methods, (Cambridge University Press, 2000).
Yang, J. H., Li, J. H., Jiang, S., Zhou, H. & Qu, L. H. ChIPBase: a database for decoding the transcriptional regulation of long non-coding RNA and microRNA genes from ChIP-Seq data. Nucleic Acids Res 41, D177–87 (2013).
Vafaee, F. et al. ORTI: An Open-Access Repository of Transcriptional Interactions for Interrogating Mammalian Gene Expression Data. PloS one 11, e0164535 (2016).
Acknowledgements
This work was supported in part by a Cancer Institute NSW Fellowship (12ECF204 to E.K.C.); Cure Cancer Australia Foundation and Cancer Australia (PdCCRS 1050101 to E.K.C.); National Institutes of Health (R01CA169200, R01CA142832 to S.C.M.); The University of Texas MD Anderson Cancer Center Ovarian Cancer Specialized Program of Research Excellence grant (P50CA083639 to S.C.M.); MD Anderson Support Grant from the National Institutes of Health; the U.S. Department of Health and Human Services (P30CA016672 to S.C.M.).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: F.V., E.K.C., V.M.H. and G.S. Generation and analysis of data: F.V., E.K.C., S.C.M. and G.S. Prepared manuscript: F.V. and E.K.C. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vafaee, F., Colvin, E.K., Mok, S.C. et al. Functional prediction of long non-coding RNAs in ovarian cancer-associated fibroblasts indicate a potential role in metastasis. Sci Rep 7, 10374 (2017). https://doi.org/10.1038/s41598-017-10869-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10869-y
This article is cited by
-
MacroH2A restricts inflammatory gene expression in melanoma cancer-associated fibroblasts by coordinating chromatin looping
Nature Cell Biology (2023)
-
MicroRNAs as the critical regulators of Cisplatin resistance in ovarian cancer cells
Journal of Ovarian Research (2021)
-
The impact of ultraviolet- and infrared-based laser microdissection technology on phosphoprotein detection in the laser microdissection-reverse phase protein array workflow
Clinical Proteomics (2020)
-
The promising role of noncoding RNAs in cancer-associated fibroblasts: an overview of current status and future perspectives
Journal of Hematology & Oncology (2020)
-
Proteogenomic analysis of Inhibitor of Differentiation 4 (ID4) in basal-like breast cancer
Breast Cancer Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.