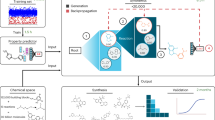
Abstract
Drug resistance caused by excessive and indiscriminate antibiotic usage has become a serious public health problem. The need of finding new antibacterial drugs is more urgent than ever before. Tyrosyl-tRNA synthase was proved to be a potent target in combating drug-resistant bacteria. In silico methodologies including molecular docking and 3D-QSAR were employed to investigate a series of newly reported tyrosyl-tRNA synthase inhibitors of furanone derivatives. Both internal and external cross-validation were conducted to obtain high predictive and satisfactory CoMFA model (q 2 = 0.611, r 2 pred = 0.933, r 2 m = 0.954) and CoMSIA model (q 2 = 0.546, r 2 pred = 0.959, r 2 m = 0.923). Docking results, which correspond with CoMFA/CoMSIA contour maps, gave the information for interactive mode exploration. Ten new molecules designed on the basis of QSAR and docking models have been predicted more potent than the most active compound 3-(4-hydroxyphenyl)-4-(2-morpholinoethoxy)furan-2(5H)-one (15) in the literatures. The results expand our understanding of furanones as inhibitors of tyrosyl-tRNA synthase and could be helpful in rationally designing of new analogs with more potent inhibitory activities.
Similar content being viewed by others
Introduction
Infectious diseases caused by bacteria are known as one of the most life-threatening health problem all over the world, whose chemotherapy using antimicrobial agents and antibiotics has been a critical public health tool for nearly a century, saving millions of lives around the world1. However, due to the indiscriminate usage of antibiotics in particular, surviving bacteria have evolved resistance against several antibiotics in recent decades2. Especially with the growth of multidrug resistance in bacteria, finding new antibacterial drugs becomes increasingly crucial in global researches3. Interruptions of protein synthesis have long been recognized as an attractive target of anti-bacterium, and its crucial enzyme aminoacyl-tRNA (aaRS) involved in protein synthesis catalyzed the bond between specific amino acid and its cognate tRNAs4. Hence, an increasing number of researches focused on aaRS inhibitors as a potent antibacterial agent. The inhibitors of leucyl-tRNA synthase of icofungipen and AN-2690 are both in clinical development for the treatment of onychomycosis5. Mupirocin, an inhibitor of isoleucyl-tRNA, shows good effect on infectious diseases as antibacterial agents6. Recently, a series of furan-2(5H)-one derivatives have been found remarkable inhibitory activities against tyrosyl-tRNA synthase5.
The three-dimensional quantitative structure-activity relationship (3D-QSAR) models, calculated via the most widely used comparative molecular field analysis (CoMFA)7 and comparative molecular similarity indices analysis (CoMSIA)8 incorporating the information of conformation or spatial orientation of molecules, are used for the rational design of most potent novel inhibitors. In this study, they were used to extract the structural features favored for tyrosyl-tRNA synthase inhibitors based on the skeleton of furan-2(5H)-one. The best model, which was developed from a dataset consisted of a 44 molecule training set and a 8 molecule test set, have been validated appropriately, and 10 novel compounds designed on the basis of the model have been predicted better activity than compound 15, the most active molecule in the literatures5, 9. Therefore, the established 3D-QSAR models of fifty-two molecules by CoMFA and CoMSIA could not only give the key structure requirements for the antimicrobial activity but also serve as a helpful guidance in design of novel antibiotics, especially for the control of drug-resistant superbugs.
Methodology
Methods and Data sets
Fifty-two furan-2(5H)-one tyrosyl-tRNA synthase inhibitors were collected from papers published by a certain research group5, 9. The biological data of the compounds and their structures were showed in Table 1. The pIC50(−logIC50) values converted from the origin IC50 was required as dependent variables in 3D-QSAR analysis. The pIC50 values covering 3 log units were considered as a homogenous and wide range dataset for 3D-QSAR studies10
. Eight molecules of structural variety and broad range of activity in the data were randomly chosen as test set to assess predictive ability of the resulting models, and therefore the remaining forty-four molecules were selected as a training set to generate the 3D-QSAR models.
Molecular modeling and alignment
The 3D structures of the furan-2(5H)-ones were build in SYBYL 8.1 (Tripos, Inc, St. Louis, MO, USA) molecular modeling package. The force field of standard Tripos molecular mechanics along with Gasteiger-Hűckel charge were employed to perform structure energy minimization11. The quality of molecular alignment was considered as a key factor for the robustness and predictive power of CoMFA and CoMSIA models4, 12. Here we applied molecular alignment to align all the molecules by using furan ring as the common skeleton and the most active compound 15 as the template molecule. To ensure the energy level of the conformations was reasonable, a further conjugate gradient method minimization of compound 15 was conducted and the convergence was reached. The aligned molecules were shown in Fig. 1.
CoMFA and CoMSIA analysis
The descriptor fields of both methods were calculated in a three-dimensional cubic with one angstrom grid spacing. The frontier of the box extended extra 4 angstrom units from the border of aligned structures in each direction.
For CoMFA method, incorporating steric and electrostatic fields13, the the probe atom of a charged sp3 hybridized carbon atom was applied to compute electrostatic and steric fields. The cutoff value was 30 kcal∙mol−1 14. As to CoMSIA approach, three extra fields including hydrogen bond acceptor, hydrogen bond donor and hydrophobic were considered15. A Gaussian function was also applied in calculating the similarity indices, making it accounts for all grid points16. The equation (1) for the similarity indices calculation is as below:
where A q means the similarity index of point q, and k represent the physicochemical properties of electrostatic and steric descriptors; W probe,k means the probe atom and the attenuation factor is a default value of 0.3; i means summation index of the molecule j, and W ik is the observed value k of a specific property of the atom i 17.
Partial least squares (PLS) analysis
In order to build a statistically significant model, PLS method18 was introduced to correlate the both fields to the pIC50 values linearly. Leave-one-out (LOO) method was performed first in the cross-validation in which each compound is deleted from the dataset and the activity of the “leave-one” molecule is predicted using the model build based on the remaining molecules in the dataset19. With the default value of column filtering at 2.0 kcal∙mol−1, the optimum number of components (ONC) was obtained on the lowest standard error of prediction (SEP), which is usually corresponds to the highest cross-validated squared coefficient (q 2). To avoid the model over-fit, higher numbers of component will not be accepted unless the q 2 could have risen by 10% or more20. Non-cross-validation was then conduct to build the final 3D-QSAR models. The correlation coefficient of LOO method (r 2 cv ) was defined by equation (2) as follows:
where Ymean, Ypre and Yobs, means average, calculated and actual pIC50, respectively21, 22.
Sensitivity of a PLS model
Most molecules of the data set may have “twins”, which make a near twin of each left-out molecule likely remain in the training data and lead to a good prediction, therefore, the q 2 statistic may give you a false sense of confidence. Progressive scrambling was often used to determine the sensitivity of a QSAR model to small systemic perturbations of the response variable for the model’s stability23, 24.
Predictive correlation coefficient (r 2 pred )
Normally q 2 is considered as a productive but not sufficient parameter in validating the model. Sometimes models with high r 2 cv and r 2 values may be improper in many cases. The external predictive correlation coefficient (r 2 pred )25 was calculated to estimate the predict ability. The predictive correlation coefficient (r 2 pred ) was calculated by the following equation (3):
where, SD is the sum of the squared deviations between the activity values of the test set and mean activities of the training molecules; PRESS stands for the sum of squared deviations between calculated and observed activity values for each structure of the test set26, 27.
Molecular docking and MD simulation
Molecular docking technique is an important method in discovering novel small-molecule drugs28,29,30,31,32. In our study, Surflex-Dock was used to perform the molecular docking. Tyrosyl-tRNA synthase’s crystal structure 3P0H which contains a ligand was obtained from the PDB database33. The hypothetical protomol was used to probe steric and electrostatic interactions of the active pocket. H-bond acceptor and donor substituent as well as hydrophobic fields were also investigated34, 35. Before docking, hydrogen atoms of the receptor were added first, and the Kollman-All force field was applied in the prepared structures36,37,38. The definition of active site definition was performed based on the original ligand in the crystal. We chose compound 15 as the subject to dock into the active pocket under the conditions previously optimized. As to the newly designed molecules, the docking study along with MD simulation was also conducted to validate the calculated activities and the interaction mechanisms. The docking method was similar as the docking process above and the binding conformations with highest score were subjected to MD simulations in AMBER molecular dynamics package39. Each MD time was 10 ns in explicit solvent. Antechamber tool was applied to generate the partial atomic charges of each molecule and the force field ff12SB was loaded to the receptor. Truncated octahedral water box by the TIP3P water model was chosen to encompass the complexes before minimization and MD. After the MD, analysis was performed with the ptraj analysis tool based on the last 2 ns trajectories of the simulation. The binding free energies of complexes were calculated using the MM-PBSA40.
Results and Discussion
CoMFA and CoMSIA results
Both models were obtained on the basis of a 44 molecules training set of whose pIC50 values ranging from 4 to 7. Table 2 listed the statistical parameters of CoMFA and CoMSIA models. And the results of the progressive scrambling demonstrated that the value for the slope in the five component model is admissible (Fig. 2), and with a minimum cSDEP and maximumQ 2 the optimum statistics are also seen for six components in Table 3. The cross-validated correlation coefficient (q 2) of CoMFA model based on both electrostatic and steric fields is 0.611 (>0.5). Other parameters such as ONC as 6, SEE (standard error estimate) of 0.179, non-cross-validated correlation coefficient (r 2) of 0.940, standard error estimate (S) of 0.179, F value of 96.577 and predictive correlation coefficient (r2 pred) of 0.759 was derived. The proportion of electrostatic and steric fields contributes 0.281 and 0.719. CoMSIA with an ONC of 8 gave a q 2 of 0.577 (>0.5), r 2 of 0.916, SEE of 0.212, F value of 67.037 and r 2 pred of 0.791. Contributions proportion of electrostaticsteric, steric electrostatic, hydrogen H-bond donor and hydrogen acceptor contributes were 0.152, 0.525, 0.221 and 0.101, respectively. The correlations between the calculated and actual pIC50 values of the whole data set were listed in Figs 3 and 4. These PLS statistics revealed that the our proposed CoMFA and CoMSIA models could adequately predict all the compounds in the test set.
CoMFA contour map analysis
CoMFA contour maps were drawn vividly to explore the areas in three-dimensional space around the compounds where modifications would alter activity. The contour maps are revealed in Fig. 5, with compound 15 as the template molecule. In Fig. 5a, the green blocks mean a bulky group favored area, while the yellow blocks indicate that minor substituent are preferable to enhance the activity. In Fig. 5b, the electron-donating group and electron-withdrawing group favored region are represented by blue and red contours, respectively.
CoMFA contour maps with compound 15 as template. (a) Steric: green contours indicates bulky groups favored, yellow contours means bulky groups disfavored. (b) Electrostatic fields: blue contours stand for electron-donating groups favored ragion, red contours located where electron-withdrawing groups favored.
As shown in Fig. 5a, a giant green block located near the hydroxyl group substituted to phenyl ring along with the large green contours around the non-aromatic hexatomic ring of compound 15 indicate that bulky groups here can increase the activity. These factors may explain why compounds 11, 12, 41, 42 and 47 with halogen substituents in this area are more potent than molecules without any substituents at this particular position. The yellow contour on the top or bottom of the non-aromatic hexatomic ring of compound 15 suggested the unfavorable influence of bulky groups. This might be the reason why compound 40 whose methoxyl substituent is not on the plane of the molecule showed significantly decreased activities.
As shown in Fig. 5b, red contours around substituent group R1 or R4, and para- and meta-positions of hexatomic ring of group R3 or R5 revealed that electron-withdrawing substituents were considered to be beneficial to the activity. The compounds 29, 41, 42 and 47, with electron-withdrawing groups at R1 and the meta-position of hexatomic ring of group R3 or R5, displayed the good bioactivity. On the contrary, the blue contour around meta-position of hexatomic ring of group R3 or R5 told us that electron-donating groups here would be expected. Therefore, compound 28 with the methyl group at the meta-position of benzene ring displayed good IC50 values.
CoMSIA contour map analysis
CoMSIA contour maps of steric, electrostatic, H-bond donor and acceptor field are revealed in Fig. 6. Generally the CoMSIA contour maps of electrostatic and steric field are similar to CoMFA models. However, the electrostatic field in CoMSIA model appended a blue contour around group R2, which means that electron-donating substituents were also preferred in this region.
CoMSIA contour maps with compound 15 as template. (a) Steric: Green contours favored bulky regions, and yellow contours bulkily disfavored regions. (b) Electrostatic: Red contours indicates electron-withdrawing groups favored, and blue contours means electron-donating groups favored. (c) H-bond acceptor: Magenta and red contours indicate h-bond acceptor favorable and unfavorable respectively. (d) H-bond donor: Cyan and purple contours stand for favorable and unfavorable respectively.
The hydrogen-bond donor field was presented in Fig. 6c. Cyan contour around hydroxyl substituent of compound 15 revealed that hydrogen bond donor was preferred in this region. In hydrogen bond acceptor field (Fig. 6d), the purple contour was around group R3, which revealed that hydrogen acceptor was preferred in this region.
Therefore, compounds 6 and 11 with hydrogen acceptor at group R3 showed good IC50 values. Furthermore, the hydrogen acceptor was also preferred in the side chain of furan ring.
Molecular Docking Analysis
The Figs 7 and 8 demostrated the binding modes of tyrosyl-tRNA synthase with compound 15. The hydroxyl groups at R1 position formed H-bonds with Tyr46 and Asp170 residue by acting as H-bond acceptor and donor simultaneously. The morpholine nitrogen in R3, which served as a H-bond acceptor, also formed a hydrogen bond with Glu40. These interactions summarized from Fig. 7 coincided satisfactorily with the contour maps derived from CoMSIA method above. Moreover, as can be seen in Fig. 8, a cavity located beneath the morpholine ring where steric contour map hints us a bulky group favored verified our results.
Summary of the structure-activity relationships
The SAR derived from the present work were illustrated in Fig. 9. To be specific, the bulky, electron-withdrawing and h-bond donor groups such as hydroxyl group at R1 or R4 position are essential to improve the antibacterial activity. The electron-withdrawing, H-bond acceptor or bulky groups like ether bond at para-position or meta-position of substituent group R3 or R5 may increase their activities. Introducing electron-donating groups at meta-position of substituent group R3 or R5 may also enhance the activity. Furthermore, the hydrogen acceptor was also preferred in the side chain at furan ring.
Design of Novel Derivatives
After gaining the SAR revealed by the foregoing study, ten new furan-2(5H)-ones (D1-D10) were designed and predicted. Molecular alignment was conduct on these new molecules and their activities were calculated to be better than compound 15 (pIC50 = 6.941). Such results indicated that these 3D-QSAR models with considerable predictive ability could be prospectively used in structure modification and optimization. The structures and calculated pIC50 of these newly designed molecules were listed in Table 4
.
Validation of Newly-designed Derivatives
These ten new furan-2(5H)-ones (D1-D10) were re-docked to the receptor 3P0H and based on which MD simulations were also performed to check the potencies by assess the binding affinity and free energy. The docking results was listed in the following Table 5. The ΔG calculated from the MD simulation results were also shown below. Compound D7 showinged significant affinity and the interactions between ligand and receptor were depicted in Fig. 10, where compound 15 was also exhibited as the reference. We can find that more hydrogen bonds with stronger bond energy formed based on the modified group, for instance the acylamino in R1 connecting Asp170 with a new hydrogen bond and interacting Tyr36 with a hydrogen bond of −3.3 K cal/mol is more favored than the original hydroxyl in this position whose hydrogen bond was −2.9 K cal/mol. However, comparing with the predicted pIC50 values, compounds with trichloromethyl or bromine demonstrated less potency, especially the compound D6 are of poor affinity. By analyzing the conformations optimized from the MD simulation, we found that although the substituent group of R3 are bulky favored, the group size is not unlimited, substituent group larger than furan ring with trifluoromethyl may cause crash of protein and ligand thus changed the equalized conformation after the MD simulation. Compound D1 suffered the same situation that showed dissatisfactory inhibitory activity in the docking and optimization. In Fig. 11, the conformation of compound D6 altered hugely to extend the trifluoromethyl out of the surface to reduce the tension. Additionally the amide group in R1 served as a H-bond donor by forming a stable hydrogen bond with His210 and verified the SAR obtained above. According to the docking and MD simulation study the compound D7 was also proven to be most promising in tyrosyl-tRNA synthase inhibition.
Conclusion
The tyrosyl-tRNA synthase inhibitors provide a promising approach in fighting against drug-resistant bacteria. In our present studies, we have established CoMFA model (q 2 = 0.611, r 2 = 0.940) and CoMSIA model (q 2 = 0.546, r 2 = 0.905) with satisfactory correlation and predictive abilities from fifty-two tyrosyl-tRNA synthase inhibitors. The CoMFA and CoMSIA contour maps provided information to summarize the SAR and characterized key features affecting the antibacterial activity. Moreover, the prediction ability of the model validated by the test set turned out to be satisfactory which means these models could be applied in predicting the activities of new compounds. Thus 10 new molecules were designed and predicted to be more potent than compound 15, these results were further validated by MD simulation study.All the work above confirmed that our models can provide a ponderable clue in designing novel antimicrobial agents.
References
Garciaalvarez, L., Dawson, S., Cookson, B. & Hawkey, P. Working across the veterinary and human health sectors. J Antimicrob Chemother 67, 37–49 (2012).
Estephane, J. et al. N-Acyl-3-amino-5H-furanone derivatives as new inhibitors of LuxR-dependent quorum sensing: Synthesis, biological evaluation and binding mode study. Bioorg Med Chem Lett 18, 4321–4324 (2008).
Chaudhary, A. S. A review of global initiatives to fight antibiotic resistance and recent antibiotics’ discovery. Acta Pharmaceutica Sinica B 6, 552–556 (2016).
Yanagisawa, T., Sumida, T., Ishii, R., Takemoto, C. & Yokoyama, S. A paralog of lysyl-tRNA synthetase aminoacylates a conserved lysine residue in translation elongation factor P. Nat Struct Mol Biol 17, 1136–1143 (2010).
Xiao, Z. P. et al. 4-Alkoxy-3-arylfuran-2(5H)-ones as inhibitors of tyrosyl-tRNA synthetase: Synthesis, molecular docking and antibacterial evaluation. Bioorg Med Chem Lett 19, 3884–3891 (2011).
Vijver, P. V. D. et al. Aminoacyl-tRNA Synthetase Inhibitors as Potent and Synergistic Immunosuppressants. J Med Chem 51, 3020–3029 (2008).
Cramer, R. D., Patterson, D. E. & Bunce, J. D. Comparative molecular field analysis (CoMFA): 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 110, 5959–5967 (1988).
Klebe, G., Abraham, U. & Mietzner, T. Molecular similarity indices in a comparative ananlysis (CoMSIA) of drug molecules to correlate and predict their biological activity. J Med Chem 37, 4130–4146 (1994).
Wang, X. D. et al. 3-Aryl-4-acyloxyethoxyfuran-2(5H)-ones as inhibitors of tyrosyl-tRNA synthetase: synthesis, molecular docking and antibacterial evaluation. Bioorg Med Chem 21, 4914–4922 (2013).
Cramer, R. D. 3rd, Patterson, D. E. & Bunce, J. D. Recent advances in comparative molecular field analysis (CoMFA). Prog Clin Biol Res 291, 161–165 (1989).
Ravichandran, V., Sankar, S. & Agrawal, R. K. Predicting anti-HIV activity of 1,1,3-trixox [1,2,4]-thiadiazine (TTD) derivatives: 3D QSAR approach. Med Chem Res 18, 511–522 (2009).
Zheng, J. et al. Exploring QSARs for 5-lipoxygenase (5-LO) inhibitory activity of 2-substituted 5-hydroxyindole-3-carboxylates by CoMFA and CoMSIA. Chem Biol Drug Des 78, 314–321 (2011).
Song, Q. L., Sun, P. H. & Chen, W. M. Exploring 3D-QSAR for ketolide derivatives as antibacterial agents using CoMFA and CoMSIA. Lett Drug Des Discov 7, 149–159 (2010).
Hu, R., Barbault, F., Delamar, M. & Zhang, R. Receptor- and ligand-based 3D-QSAR study for a series of non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorg Med Chem 17, 2400–2409 (2009).
Klebe, G. & Abraham, U. Comparative molecular similarity index analysis (CoMSIA) to study hydrogen-bonding properties and to score combinatorial libraries. J Comput Aided Mol Des 13, 1–10 (1999).
Zheng, J. et al. Insight into the interactions between novel isoquinolin-1,3-dione derivatives and cyclin-dependent kinase 4 combining QSAR and molecular docking. Plos One 9, e93704 (2014).
Politi, A. et al. Application of 3D QSAR CoMFA/CoMSIA and in silico docking studies on novel rennin inhibitors against cardiovascular diseases. Eur J Med Chem 44, 3703–3711 (2009).
Bush, B. L. & Jr, N. R. Sample-distance partial least squares: PLS optimized for many variables, with application to CoMFA. J Comput Aided Mol Des 7, 587–619 (1993).
Lu, X. Y. et al. Molecular docking-guided 3D-QSAR studies of substituted isoquinoline-1,3-(2H, 4H)-diones as cyclin-dependent kinase (CDK4) inhibitors. J Mol Model 16, 163–173 (2010).
Zhang, N. & Zhong, R. Docking and 3D-QSAR studies of 7-hydroxycoumarin derivatives as CK2 inhibitors. Eur J Med Chem 45, 292–297 (2010).
Sun, J., Cai, S., Yan, N. & Mei, H. Docking and 3D-QSAR studies of influenza neuraminidase inhibitors using three dimensional holographic vector of atomic interaction field analysis. Eur J Med Chem 45, 1008–1014 (2010).
Puntambekar, D. S., Giridhar, R. & Yadav, M. R. Understanding the antitumor activity of novel tricyclicpiperazinyl derivatives as farnesyltransferase inhibitors using CoMFA and CoMSIA. Eur. J Med Chem Res 41, 1279–1292 (2006).
Clark, R. D. & Fox, P. C. Statistical variation in progressive scrambling. J Comput Aided Mol Des 18, 563–576 (2004).
Luco, J. M. & Ferretti, F. H. QSAR based on multiple linear regression and PLS methods for the anti-HIV activity of a large group of HEPT derivatives. J Chem Inf Comput Sci 37, 392–401 (1997).
Golbraikh, A. & Tropsha, A. Beware of q2! J Mol Graph Model 20, 269–276 (2002).
Cichero, E., Cesarini, S., Mosti, L. & Fossa, P. CoMFA and CoMSIA analyses on 4-oxo-1,4-Dihydroq-uinoline and 4-oxo-1, 4-dihydro-1, 5-, -1, 6- and -1, 8-naphthyridine derivatives as selective CB2 receptor agonists. J Mol Model 16, 677–691 (2010).
Park, C. H. Y. et al. A comparative study of quantitative structure activity relationship methods based on antitumor diarylsulfonylureas. Eur J Med Chem 36, 829–836 (2011).
Wang, J., Kollman, P. A. & Kuntz, I. D. Flexible ligand docking: a multistep strategy approach. Proteins 36, 1–19 (1999).
Jain, A. N. Surflex: Fully automatic flexible molecular docking using a molecular similarity-based search engine. J Med Chem 46, 499–511 (2003).
Jain, A. N. Surflex-Dock 2.1: Robust performance from ligand energetic modeling, ring flexibility, and knowledge-based search. J Comput Aided Mol Des 21, 281–306 (2007).
Spitzer, G. M., Wellenzohn, B., Laggner, C., Langer, T. & Liedl, K. R. DNA minor groove pharmacophores describing sequence specific properties. J Chem Inf Model 47, 1580–1589 (2007).
Ambure, P. S., Gangwal, R. P. & Sangamwar, A. T. 3D-QSAR and molecular docking analysis of biphenyl amide derivatives as p38 alpha mitogen-activated protein kinase inhibitors. Mol Divers 16, 377–388 (2012).
Larson, E. T. et al. The double-length tyrosyl-tRNA synthetase from the eukaryote Leishmania major forms an intrinsically asymmetric pseudo-dimer. J Mol Biol 409, 159–176 (2011).
Ruppert, J., Welch, W. & Jain, A. N. Automatic identification and representation of protein binding sites for molecular docking. Protein Sci 6, 524–533 (1997).
Holt, P. A., Chaires, J. B. & Trent, J. O. Molecular docking of intercalators and groove-binders to nucleic acids using Autodock and Surflex. J Chem Inf Model 48, 1602–1615 (2008).
Li, Y. F. et al. Prediction and evaluation of thelipase inhibitory activities of tea polyphenols with 3D-QSAR models. Sci Rep 6, 34387 (2016).
Muthas, D., Sabnis, Y. A., Lundborg, M. & Karlen, A. Is it possible to increase hit rates in structure-based virtual screening by pharmacophore filtering? An investigation of the advantages and pitfalls of post-filtering. J Mol Graph Model 26, 1237–1251 (2008).
van Westen, G. J. & Overington, J. P. A ligand’s-eye view of protein similarity. Nat Methods 10, 116–117 (2013).
Case D. A., et al. AMBER 12, University of California, San Francisco (2012).
Peng, C. K. et al. Novel 4-(4-substituted amidobenzyl)furan-2(5H)-one derivatives as topoisomerase I inhibitors. Eur J Med Chem 127, 187–199 (2017).
Acknowledgements
This study was supported by the research grant from National Natural Science Foundation of China (No. 81573294, 81373285), Major Project for the Innovation of Industry and Research of Guangzhou City (No. 201508020128). Excellent Young Teachers Program of Guangdong Provincial colleges and universities(YQ 2015061), Pearl River S&T Nova Program of Guangzhou (201610010100).
Author information
Authors and Affiliations
Contributions
P.S. conceived and designed the experiments. S.L., J.H., M.H., and J.F. performed the experiments. S.L., C.P., B.W., L.L. and Y.C. wrote the main manuscript text. L.G. and G.X. provided editorial advices. The analysis and interpretation of data were supplied by J.F., J.Z., W.C., J.G. and G.L. reviewed the manuscript. P.S. revised the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, S., Fan, J., Peng, C. et al. New molecular insights into the tyrosyl-tRNA synthase inhibitors: CoMFA, CoMSIA analyses and molecular docking studies. Sci Rep 7, 11525 (2017). https://doi.org/10.1038/s41598-017-10618-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10618-1
This article is cited by
-
Insights into the c-Jun N-terminal kinase 3 (JNK3) inhibitors: CoMFA, CoMSIA analyses and molecular docking studies
Medicinal Chemistry Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.