Abstract
Mangroves forests of Avicennia marina occupy about 135 km2 in the Red Sea and represent one of the most important vegetated communities in this otherwise arid and oligotrophic region. We assessed the soil organic carbon (Corg) stocks, soil accretion rates (SAR; mm y−1) and soil Corg sequestration rates (g Corg m−2 yr−1) in 10 mangrove sites within four locations along the Saudi coast of the Central Red Sea. Soil Corg density and stock in Red Sea mangroves were among the lowest reported globally, with an average of 4 ± 0.3 mg Corg cm−3 and 43 ± 5 Mg Corg ha−1 (in 1 m-thick soils), respectively. Sequestration rates of Corg, estimated at 3 ± 1 and 15 ± 1 g Corg m−2 yr−1 for the long (millennia) and short (last century) temporal scales, respectively, were also relatively low compared to mangrove habitats from more humid bioregions. In contrast, the accretion rates of Central Red Sea mangroves soils were within the range reported for global mangrove forests. The relatively low Corg sink capacity of Red Sea mangroves could be due to the extreme environmental conditions such as low rainfall, nutrient limitation and high temperature, reducing the growth rates of the mangroves and increasing soil respiration rates.
Similar content being viewed by others
Introduction
Mangrove forests supply important ecosystem services that support the livelihoods of coastal societies in the tropics and subtropics1. However, these extend beyond the local communities to yield, through their role as a globally-relevant carbon sinks, climate change mitigation benefits to the global community2,3,4,5. Although mangroves occupy only 0.5% of the area of the global coastal ocean6 and represent only 0.7% of the tropical forests of the world7, they account for about 1% and 14% of the carbon sequestered annually by the world’s forests and the global ocean, respectively8.
The intense organic carbon (Corg) sequestration by mangrove forests is mainly due to their high net ecosystem production9, resulting in high loads of leaf litter and biomass to the soil, combined with their high capacity to trap and retain soil resulting in vertical accretion10 compared to other forests and coastal areas devoid of vegetation11. Further, high Corg preservation due to low oxygen availability12, 13 combined with lack of fires in the aquatic environment where mangroves thrive3, results in high carbon storage in mangroves14, 15.
Unfortunately, mangrove ecosystems have declined globally, with one third of the global mangrove area lost since World War II16. Mangrove deforestation leads to losses of their carbon sink capacity as well as greenhouse gas emissions from remineralization of the large Corg stocks that mangroves accrete3, 14. Annual CO2 emissions associated with mangrove deforestation have been estimated at 0.02 to 0.12 Pg C14, contributing to CO2 emissions through land-use change, the second largest anthropogenic source of CO2 to the atmosphere after fossil fuel combustion17.
The Red Sea has recently been identified as possibly the only mangrove province where mangroves are not only stable, but have expanded by 12% over the last four decades18. Lack of freshwater and soil inputs lead to acute nutrient limitation of Red Sea mangroves19, resulting in mangrove forests being dominated by dwarf trees20, similar to those in other arid areas (e.g. Gulf of California21 and areas lacking surface runoff such as the Indian River Lagoon in Florida22). Hence, we would expect mangrove forests in the Red Sea to have a relatively modest carbon sink capacity so that, despite the stability of the forests, their contribution to carbon sequestration would still be marginal. Yet, most estimates of carbon sequestration and stocks in mangrove forests have been, hitherto, derived from mangrove forests in the wet tropics6, 8, and whether those growing in the arid topics contribute significantly to carbon sequestration remains an open question. However, a recent assessment reports a Corg sequestration for dwarf mangrove forests in the arid shores of Baja California of, on average, 1000 Mg Corg ha−1 in 1.5 m-thick soils (adapted from ref. 23), similar to that found under some of the tallest tropical mangroves in the Mexican Pacific coast23, which has been linked to the stability of these forests. These observations question the assumption that dwarf mangroves in arid shores support low carbon sequestration rates and stocks.
Here we assess the Corg stock and sequestration rates and stocks supported by Avicennia marina mangrove forests in the Central Red Sea. We do so by combining measurements of soil Corg density down to 10 cm with soil chronologies derived from 210Pb and soil Corg density down to 1 m depth with soil chronologies derived from 14C, to estimate (a) the stock of Corg contained within the top meter of the soil, and (b) the burial rate of Corg over short-term (last 100 years) and long-term (last millennia) periods. Further, we use stable carbon and nitrogen isotopes of the organic matter in the soil and putative sources (mangroves, halophytes, seaweed, seagrass and seston) to estimate the potential contribution of different sources of Corg to mangrove soil carbon stocks.
Results
The distribution of soil grain size differed among mangrove forests, with soils sampled at Thuwal Island, Economic City and Khor Alkharar having a sandy texture, (∼85% of sand fraction >0.063 mm) compared to loamy sand in Petro Rabigh (32% of mud <0.063 mm) (Table 1), where the clay and silt contents in the soils was 2 to 3-fold higher (P < 0.001), (Tukey HSD multiple comparison post-hoc test, P < 0.05). Mangrove forests at Thuwal Island grow in the coarsest soil (80% particles between 0.125 and 1 mm) compared to the other study sites (47 to 65% particles between 0.125 mm and 1 mm, Table 1).
The Corg density and % Corg were generally low, ranging from 3 to 9 mg Corg cm−3 and 0.2% to 1.5% Corg (Table 2). The Corg density and % Corg significantly decreased with soil depth, while δ15N and δ13C significantly increased with depth, except for δ15N signatures at Thuwal Island which remained constant (Tukey HSD post hoc test, P < 0.05, Fig. 1 and Table 2). Mangrove soils in Khor Alkharar were characterized by relatively high soil Corg density (1 to 39 mg Corg cm−3 along the 170 cm soil profile), and high % Corg (0.2 to 17% Corg in the upper 25 cm), compared to the other locations (<14 mg Corg cm−3 and <1% Corg, Tukey HSD post hoc test, P < 0.05, Fig. 1). δ13C values did not differ among locations, whereas soil at Petro Rabigh mangroves had higher δ15N values (0.6 to 7.8‰ along the 170 cm soil profile) compared with those in other locations (<3.2‰ along the 170 cm soil profile) (Tukey HSD post hoc test, P < 0.05; Fig. 1 and Table 2).
Vertical profiles of (A) % organic carbon (Corg), (B) Corg density (g Corg cm−3), and (C,D) δ13C and δ15N (‰) in mangrove soils in central Red Sea. It was not possible to plot average values for the replicate cores against decompressed depths because replicate cores experienced different degrees of compaction during coring.
The seaweed species analyzed (i.e. Padina, Colpomenia, Turbinaria and Sargassum species, Table 3) were unusually enriched in δ13C and, therefore, quite similar in carbon isotopic values to seagrass. This was the case also for seston, suggesting that it is mostly comprised of seagrass and macroalgal detritus in the mangrove waters sampled. Hence, the discrimination between these three sources (seaweed, seston and seagrass) was poor and involved considerable uncertainty (Table 3). Likewise, halophytes and mangroves had comparable δ13C signatures, but much lighter than those derived from plants performing aquatic photosynthesis (Table 3). The analysis of δ15N and δ13C values of marine plants and soil indicated that the source of the Corg in mangroves soil from the Red Sea was composed, on average, of 2/3 of Corg derived from atmospheric photosynthesis (e.g. mangrove and halophytes) and 1/3 of Corg derived from aquatic photosynthesis (e.g. seaweed, seston and seagrass, Tables 2 and 3, Fig. 2).
The 210Pb concentration profiles of 6 out of 10 cores showed intense mixing of the upper layers or did not contain excess 210Pb, i.e. 210Pb concentrations were not significantly different than supported 210Pb and, thereby, it was not possible to apply a suitable 210Pb model to determine soil accretion rates (SAR). The 210Pb concentration profiles of 3 mangrove soil cores (KMC, KME and RMF) showed evidence of mixing in the upper 2 to 7 cm (Fig. 3a), and no apparent mixing in the top layers was observed for core TMF. Excess 210Pb concentrations at the surface were low, averaging 9 ± 2 Bq kg−1 in all cores, except for KME, which reached 90 Bq kg−1. Excess 210Pb concentrations decreased steadily below the surface mixed layer (when present) and down to 22, 12, 15 and 11 cm, in cores KMC, KME, RMF and TMF respectively. Supported 226Ra concentrations were similar among the 10 analyzed cores, averaging 10 ± 3 Bq kg−1, with KMC concentrations being significantly lower at 5.6 ± 0.6 Bq kg−1 (Fig. 3b, Table S5). The CF:CS model (Constant Flux: Constant Sedimentation rate24, 25) was applied to these cores (below the mixed layer when present), obtaining SAR ranging from 0.7 ± 0.1 to 3.7 ± 0.7 mm yr−1 over the last 100 years. Due to the presence of mixing, these rates must be considered as upper limits, especially for KMC and RMF. The resulting SAR averaged 2.2 ± 0.6 mm yr−1 over the past 100 years (Table 4). The range of estimates of SAR over longer time-scales derived from 14C age estimates were comparable (0.4 to 1.8 mm yr−1, Table 5) to those derived for the past century, while the average was 2.6 times lower. Long-term SAR did not differ among locations (Table 5, Tukey HSD post hoc test, P > 0.05).
The high Corg content at Khor Alkharar resulted in the highest Corg stock at this site, which was significantly higher compared to all other locations (Tables 4 and 5). Likewise, recent Corg sequestration rates averaged 15 ± 1 g Corg m−2 yr−1 (i.e. based on 210Pb dating) and varied three-fold, from the lowest rates at Petro Rabigh (9 ± 4 g Corg m−2 yr−1) to the highest rates at Khor Alkarar (23 ± 1 g Corg m−2 yr−1; Table 4, Tukey HSD post hoc test, P < 0.05). These differences were driven mostly by the differences in Corg density, as soil accretion rates were rather uniform and did not differ significantly among locations. The long-term Corg sequestration rates did not differ among locations, averaging 3.5 ± 1.1 g Corg m−2 yr−1 (Table 5, Tukey HSD post hoc test, P > 0.05).
Discussion
The sediment grain size we found was consistent with the findings of Gheith & Abou-ouf26 who reported sandy sediments in Khor Alkharar Sea becoming coarser, with a higher gravel component, towards the beach, and sediment at Rabigh to be mainly composed of medium grain size sand. Although, the clay and silt content of mangrove soils at Khor Alkharar were relatively low compared to the other locations, site KME had considerably high content of mud 24% (average top 20 cm; soil thickness where excess 210Pb is found), hence the high content of fine sediments at surface layers together with high concentrations of Corg can explain the higher inventory of excess 210Pb found at this site compared to other locations.
The average (±SE) Corg density in Central Red Sea mangrove soils (0.0044 ± 0.00028 g Corg cm−3) is remarkably low compared to values reported from a global compilation (e.g. average 0.055 g Corg cm−3 and minimum 0.023 g cm−3 in Kosrae Island 27, or 0.038 g Corg cm−3 and 0.061 g Corg cm−3 characteristic of estuarine and oceanic mangrove soils, respectively14). The Corg density in the mangrove soils of the Central Red Sea is 100 to 300-fold lower than in mangrove soils in wet temperate to subtropical climate at Rookery Bay, Florida (0.51 g cm−3; ref. 28) and dry tropical climate at Abu Dhabi, UAE (1.2 g cm−3; ref. 29). Soil Corg stocks are highly variable across hemispheres, latitudes, countries and plant community compositions30, thereby the relatively lower Corg density values found at Central Red Sea could be explained by particular habitat characteristics and geomorphological settings not favorable for Corg sequestration.
In contrast, the recent soil accretion rates of Central Red Sea mangroves soil (mean 2 mm yr−1) are close to the median global value of 2.8 mm yr−1 29 and half of the median value of 4.5 mm yr−1 reported for mangroves globally3, and within the range previously reported (0.1 to 21 mm yr−1 3, 8). Moreover, the values obtained for the Red Sea mangroves using 210Pb chronologies are higher than those recently reported using comparable methods for mangroves in Moreton Bay and southeastern Australia (1.2 and 1.7 mm yr−1, respectively31), and lower than the average rate reported in Florida (2.7 mm y−1)32.
The SAR obtained using the 210Pb method (for short-term periods, last 100 years) is up to 4-fold higher than those based on 14C (1 mm yr−1), which encompass long-term (millennia) accretion, in areas such as Economic City, Petro Rabigh and Khor Alkharar. These results suggest an increase in SAR during the last decades, which is consistent with coastal development in the Saudi coast of the Central Red Sea, that experienced industrial and urban developments over the past decades. This is supported by the high value of 15N, that suggests an increase of nutrients from land runoff and coastal development as sewage and fertilizers are released to the sea. Moreover, decreasing δ13C values and increasing mud content (silt and clay) towards recent periods also support this hypothesis. However, the presence of mixing in top layers, decomposition of organic matter with ageing, compaction of soils during diagenesis, and intrinsic differences in 210Pb and 14C methodologies could also explain the higher SAR found in recent times33,34,35.
Whereas SAR are not particularly low for Central Red Sea mangrove forests, their remarkably low soil Corg density resulted in low carbon sequestration rates. The average soil Corg sequestration rates of 15 g Corg m−2 yr−1 for Central Red Sea mangroves soils is 10-fold lower than the average value of 163 g Corg m−2 yr−1 reported for mangroves globally29, and are in the low range of values previously reported (10 to 920 g C m−2 yr−1)8. The long-term (millenary time scale) soil Corg sequestration rates in Central Red Sea mangroves (3.5 g Corg m−2 yr−1) was also well below the soil Corg sequestration rates derived from 14C chronologies reported for dwarf A. germinans forests in arid Baja California (256 g Corg m−2 yr−1)23 and Pohnpei Island, Micronesia dominated by Rhizophora apiculata, (93 g Corg m−2 yr−1)29, despite SARs at the Micronesia sites being comparable to those reported here for Red Sea mangroves, of 2 mm yr−1 36. Despite of the low long-term sequestration of carbon in Central Red Sea mangroves compared to other mangrove habitats, their capacity to sequester Corg is similar to that observed in tropical forest soils (2.3 to 2.5 g Corg m−2 yr−1)37.
The relatively low Corg stocks and Corg sequestration rates in Red Sea mangroves are most likely due to the oligotrophic nature and low allochthonous inputs to the Red Sea. The lack of rivers and the extremely arid conditions result in nutrient-limited mangrove growth19, reflected in low-biomass dwarfed trees, particularly within the study region in the Central Red Sea20. Moreover, the soils of Red Sea mangroves are mainly composed of biogenic coarse carbonates, which could also explain the relatively low Corg sequestration capacity of mangroves growing in unfavorable conditions for biomass production, soil accretion and preservation compared to mangrove habitats from temperate and sub-tropical habitats. The isotopic results showed that two-thirds of the soil Corg stocks originated from mangrove or tidal-marsh biomass, while one-third was derived from marine photosynthetic organisms. Previous studies demonstrated that terrigenous Corg inputs can contribute up to 30% of Corg stocks in mangrove soil associated with riverine ecosystems38, but the lack of rivers implies that there is no influx of riverine soil and organic matter in coastal areas and, therefore, Corg sequestration in Red Sea mangroves is limited to autochthonous production and fluxes from the ocean.
The lack of terrigenous inputs is also reflected in the heavy carbon isotopic signatures of suspended particulate matter (−11.3 ±1.6‰), indicative of sestonic organic matter of marine origin, as seston from river sources is characterized by lighter values ranging from −18.5 to −26.4‰39. Moreover, the seston carbon seems to be dominated by carbon derived from macrophytes as plankton-derived Corg is also relatively depleted in 13C (−24.7 to −26.0‰ in the open ocean, and −19.8 to −22.3‰ in semi-tropical regions)40.
We hypothesized that in contrast with other mangrove forests, CO2 capture by mangroves in the Red Sea would be extremely low due to the arid conditions of this region. Indeed, poor Corg preservation under coarse grained-soils, high hydraulic conductivity, and low moisture-holding capacity, could also result in low Corg storage, as previously demonstrated for mangroves from United Arab Emirates41. Considering the total mangrove area in the Red Sea (135 km2)18, the rates reported for Red Sea mangroves here represent about 2100 tons of carbon sequestered per year, which is a relatively low contribution. Whereas Red Sea mangroves remains a healthy and stable ecosystem in the Red Sea18, they offer a limited potential to support blue carbon strategies to mitigate CO2 emissions.
Methods
Study location, sampling and laboratory procedures
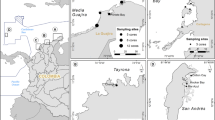
Sampling was conducted in mangrove forests of Avicennia marina at Thuwal Island, Economic City, Petro Rabigh and Khor Alkharar (Kingdom of Saudi Arabia, Fig. 4). The area encompassed by this study extends along 80 km of coastline, from Thuwal Island where mangroves grow on a shallow soil of weathered coral42, to Khor Alkharar, a coastal lagoon permanently connected to the Red Sea43. Petro Rabigh is a major industrial and petrochemical complex, whereas Economic City, about 40 km south of Petro Rabigh, is a newly developed city and harbor complex26, 44. Thuwal Island and Khor Alkharar lagoon are relatively away from direct sources of human disturbance whereas mangrove forests near Petro Rabigh and the Economic City are subjected to disturbances caused by industrial and coastal development, respectively.
Location of the Central Red Sea mangrove forests sampled. The map was produced with ArcMap Version 10.2. Background map credits: the World Administrative Divisions layer provided by Esri Data and Maps, and DeLorme Publishing Company. Redistribution rights are granted http://www.esri.com/~/media/Files/Pdfs/legal/pdfs/redist_rights_103.pdf?la=en.
In order to assess the Corg sink capacity of Red Sea mangroves, a total of 29 soil cores were sampled: 8 cores at Thuwal Island, 8 cores at Economic City, 6 cores at Petro Rabigh and 7 cores at Khor Alkharar (Fig. 4 and Table S1 in Supplementary materials). The soils were sampled using manual percussion and rotation of PVC pipes (170 cm long, high pressure class 12 with an inner diameter of 62.6 mm) that were gently hammered into the soil (<0.5 m water depth). The top core was sealed with PVC tape before retrieval to create vacuum and avoid losing the sample during recovery. The cores were sealed at both ends and transported vertically to the laboratory for further processing. Half of the cores collected at each location were kept intact and transported to the laboratory (hereafter referred to as ‘whole cores’). The other cores from each study location were sampled using a corer consisting of a PVC pipe with pre-drilled holes in the sidewall (3 cm wide and 3 cm apart; hereafter referred to as ‘port cores’), allowing sub-sampling of soil samples along the core in the field by inserting 60 ml syringes into the pre-drilled holes along the PVC pipes. The length of the core barrel inserted into the soil and the length of retrieved mangrove soil were recorded in order to correct the core lengths for compression effects and all variables studied here are referenced to the corrected, uncompressed depths. The volume of each subsample retrieved from the port cores was recorded in the field. The whole cores were sealed at both ends and transported vertically to the laboratory together with the subsamples obtained from the port cores.
PVC whole cores were cut lengthwise and cut into 1 cm-thick slices. Each slice from the whole cores and the subsamples from the port cores were weighed before and after oven drying at 60 °C until constant weight (dry weight; DW) to estimate dry bulk density (DBD in g DW cm−3). Then, every second slice of whole cores and all subsamples from port cores were divided into two subsamples by quartering. One subsample was ground and analyzed for organic carbon (Corg), and stable carbon and nitrogen isotopic composition (δ13C and δ15N), and the other subsamples were used for soil grain-size, 14C and 210Pb analyses.
Samples were acid-rinsed to ensure complete removal of inorganic carbon (i.e., carbonates) before Corg analysis, despite the fact that this procedure may lead to an underestimation of soil Corg stocks45, 46. For Corg, δ13C and δ15N analyses, 1 g of ground samples was acidified with 1 M HCl until bubbling stopped to remove inorganic carbon, centrifuged (3500 RPM; 5 min) and the supernatant with acid residues was removed using a pipette, then washed in deionized water once, the residues were centrifuged again and the supernatant removed. The residual samples were re-dried (70 °C) and encapsulated for analysis using a Thermo Delta V Conflo III coupled to a Costech 4010 at the UH Hilo Analytical Laboratory, USA (Table S2 in Supplementary materials). The content of Corg was calculated for the bulk (pre-acidified) samples. Organic carbon and Nitrogen isotope ratios are expressed as δ values in parts per thousand and relative to the Vienna Pee Dee Belemnite and atmospheric nitrogen using USGS 40 and USGS 41 standards, respectively. Analyses of replicates and reference materials were carried out to ensure reproducibility of the results.
The carbon and nitrogen elemental and isotopic composition was also analyzed in Avicennia marina macro-detritus (i.e. aerial roots, green and senescent leaves, stem, buds, flowers), halophytes (e.g. Salicornia spp. etc.), seaweeds (i.e. Padina, Colpomenia, Turbinaria and Sargassum spp.), seagrasses (i.e. rhizomes, roots and leaves from Halophila, Halodule, Thalassodendrum, Thalassia and Enhalus spp.) and seston particulate organic matter (fraction retained on a 0.7 μm pore diameter filter) collected at the four study locations. The samples from living materials were milled, and encapsulated for elemental and isotopic analyses as described above. Living material containing carbonates in their tissues were acidified with 1 M HCl before analyses (see Table S3 in Supplementary materials). The seston filters were pretreated with acid using the fumigation method47. In total 312 samples for carbon and nitrogen isotopic composition of putative carbon sources were analyzed.
For soil grain-size analysis, a Mastersizer 2000-Malvern was used following sieving (1 mm) digestion of <1 mm samples with 30% hydrogen peroxide at the Centro de Estudios Avanzados de Blanes, Spain. Grain size classification and texture were categorized following the Wentworth scale48.
The concentrations of 210Pb in the upper 20 to 30 cm of one to two cores per location were determined in the soil fraction <125 μm at the Universitat Autònoma de Barcelona (Spain) through the measurement of its granddaughter 210Po assuming radioactive equilibrium between both radionuclides49. Briefly, after addition of a known amount of 209Po as yield tracer, samples were acid digested in an analytical microwave, the polonium isotopes were auto-plated in silver discs and the concentration of 210Po quantified by alpha spectrometry. The concentrations of excess 210Pb used to obtain the age models were determined as the difference between total 210Pb and 226Ra (supported 210Pb). Concentrations of 226Ra were determined for selected samples along each core by low-background liquid scintillation counting method (Wallac 1220 Quantulus)50. These concentrations were found to be in agreement with the concentrations of total 210Pb at depth below the excess 210Pb horizons. Analyses of reagent blanks, replicates and a reference material (IAEA - 315, marine sediment) were carried out for both 210Pb and 226Ra to assess for any contamination and to ensure reproducibility of the results, (Table S4 in Supplementary materials).
A total of 94 radiocarbon analyses were conducted in 25 of the 29 cores sampled (2–5 cores per location) at two soil depths per core (cm 18–21 and the bottom cm towards the end for the ‘port core’, and cm 40 and the bottom cm towards the end for the ‘whole core’), at the AMS Direct Laboratory (USA). Samples consisted of pooled shells and bulk soil, (Table S5 in Supplementary materials).
The IsoSource software package51 was used, using δ13C and δ15N, to estimate the proportion of the Corg in the soil derived from different plants with atmospheric (e.g. A. marina and halophytes) and aquatic (e.g. seaweeds, seagrasses and suspended particulate organic matter ‘seston’) photosynthesis collected at the four study sites, using a 1% increment and 0.1 to 0.5‰ tolerance.
The soil Corg stocks per unit area (g Corg m−2) were estimated for 10 cm and 1 m soil thicknesses (i.e. cumulative mass). Where necessary (i.e. in 7 cores), we inferred Corg stocks below the limits of the reported data to 1 m, by extrapolating linearly the cumulative Corg stocks to 1 m.
Soil Corg sequestration rates (expressed in g DW m−2 y−1) for the last century and the last millennia were estimated using 210Pb (CF:CS model)24, and 14C age models, respectively. Mean soil accretion rates (SAR; mm y−1) over the last 200 to 5000 years (i.e. based on 14C) were determined by calibrating the raw radiocarbon dates reported by the Laboratory using the R routine “Bacon” (Marine13 curve) for Bayesian chronology building52 and corrected for the marine reservoir effect (i.e. subtracting Delta R value of 110 ± 38 for the Red Sea)53. From the Bacon routine output, the mean age was used to produce an age-depth weighted regression model forced through 0 (0 cm is cal. BP: 1950), using as weight the sum of the Euclidean distance of the minimum and maximum ages.
Sequestration rates of Corg were estimated by dividing the inventories in 100 cm-thick soil by the average soil accretion rate derived from 14C, whereas, the 210Pb-derived sequestration rate was calculated for 10 cm-thick soils by multiplying the soil accretion rate by the fraction of Corg accreted since 1900. For the cores that were not possibly dated, we assumed they supported the same accretion rates as the dated cores at the same location. The cores from Economic city could not be successfully dated with 210Pb (i. e. showed mixing) and we assumed that the soil accretion rate of that area is the average of the successfully dated cores in other locations.
Statistical analyses were carried out using JMP software, including descriptive statistics and ANOVA (for all 29 cores), and General Linear Mixed Models (GLMM) (for 25 cores, as cores less than 1 m long were excluded) to test for differences among forests, followed by Tukey HSD posthoc tests to assess pairwise differences among sites and soil depths.
Generalized Linear Mixed Models (GLMM) were used to take into account the potential non-independence of samples taken at different depths within the same core, since depth is a proxy for time in the cores. And given the spatial separation of cores within mangrove forests (hundreds of meters) we considered the cores themselves to be spatially independent. All response variables (bulk density, Corg, δ13C signatures and soil grain size fractions) were square-root transformed prior to analyses to homogenize their variances. Study sites (Thuwal Island, Economic City, Petro Rabigh and Khor Alkharar) and soil depth nested within cores were treated as fixed factors, whereas replicate cores within sites was treated as random factor.
References
Polidoro, B. A. et al. The loss of species: mangrove extinction risk and geographic areas of global concern. PLoS One 5, e10095, doi:10.1371/journal.pone.0010095 (2010).
Duarte, C. M., Middelburg, J. J. & Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2, 1–8 (2005).
Duarte, C. M., Losada, I. J., Hendriks, I. E., Mazarrasa, I. & Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nature Climate Change 3, 961–968 (2013).
Bouillon, S. et al. Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochemical Cycles 22 (2008).
Duarte, C. M. In EGU General Assembly Conference Abstracts. 1459.
Alongi, D. M. Carbon cycling and storage in mangrove forests. Annual review of marine science 6, 195–219 (2014).
Giri, C. et al. Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecology and Biogeography 20, 154–159, doi:10.1111/j.1466-8238.2010.00584.x (2011).
Alongi, D. M. Carbon sequestration in mangrove forests. Carbon management 3, 313–322 (2012).
Duarte, C. M. & Cebrian, J. The fate of marine autotrophic production. Limnology and Oceanography 41, 1758–1766 (1996).
Ewel, K., Twilley, R. & Ong, J. Different kinds of mangrove forests provide different goods and services. Global Ecology & Biogeography Letters 7, 83–94 (1998).
Kathiresan, K. How do mangrove forests induce sedimentation? Revista de biologia tropical 51, 355–360 (2003).
Komiyama, A., Ong, J. E. & Poungparn, S. Allometry, biomass, and productivity of mangrove forests: A review. Aquatic Botany 89, 128–137 (2008).
Lovelock, C. E. Soil respiration and belowground carbon allocation in mangrove forests. Ecosystems 11, 342–354 (2008).
Donato, D. C. et al. Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience 4, 293–297 (2011).
McLeod, E. et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment 9, 552–560, doi:10.1890/110004 (2011).
Valiela, I., Bowen, J. L. & York, J. K. Mangrove forests: One of the world’s threatened major tropical environments. Bioscience 51, 807–815, doi:10.1641/0006-3568(2001)051[0807:mfootw]2.0.co;2 (2001).
Van der Werf, G. R. et al. CO2 emissions from forest loss. Nature Geoscience 2, 737–738 (2009).
Almahasheer, H., Aljowair, A., Duarte, C. M. & Irigoien, X. Decadal stability of Red Sea mangroves. Estuarine, Coastal and Shelf Science 169, 164–172 (2016).
Almahasheer, H., Duarte, C. M. & Irigoien, X. Nutrient Limitation in Central Red Sea Mangroves. Frontiers in Marine Science 3, doi:10.3389/fmars.2016.00271 (2016).
Almahasheer, H., Duarte, C. M. & Irigoien, X. Phenology and Growth dynamics of Avicennia marina in the Central Red Sea. Scientific Reports 6, 37785 (2016).
Arreola-Lizárraga, J. A., Flores-Verdugo, F. J. & Ortega-Rubio, A. Structure and litterfall of an arid mangrove stand on the Gulf of California, Mexico. Aquatic botany 79, 137–143 (2004).
Feller, I. C., Whigham, D. F., McKee, K. L. & Lovelock, C. E. Nitrogen limitation of growth and nutrient dynamics in a disturbed mangrove forest, Indian River Lagoon, Florida. Oecologia 134, 405–414 (2003).
Ezcurra, P., Ezcurra, E., Garcillán, P. P., Costa, M. T. & Aburto-Oropeza, O. Coastal landforms and accumulation of mangrove peat increase carbon sequestration and storage. Proceedings of the National Academy of Sciences 113, 4404–4409 (2016).
Krishnaswamy, S., Lal, D., Martin, J. & Meybeck, M. Geochronology of lake sediments. Earth and Planetary Science Letters 11, 407–414 (1971).
Robbins, J. A., Edgington, D. N. & Kemp, A. L. W. Comparative 210Pb, 137Cs, and pollen geochronologies of sediments from Lakes Ontario and Erie. Quaternary Research 10, 256–278 (1978).
Gheith, A. M. & Abou-ouf, M. A. Textural characteristics, mineralogy and fauna in the shore zone sediments at Rabigh and Sharm Al-Kharrar, eastern Red Sea, Saudi Arabia. Marine Scienes-Ceased lssuerg 17, 1–2 (1996).
Chmura, G. L., Anisfeld, S. C., Cahoon, D. R. & Lynch, J. C. Global carbon sequestration in tidal, saline wetland soils. Global biogeochemical cycles 17 (2003).
Cahoon, D. R. & Lynch, J. C. Vertical accretion and shallow subsidence in a mangrove forest of southwestern Florida, USA. Mangroves and Salt Marshes 1, 173–186 (1997).
Breithaupt, J. L., Smoak, J. M., Smith, T. J., Sanders, C. J. & Hoare, A. Organic carbon burial rates in mangrove sediments: Strengthening the global budget. Global Biogeochemical Cycles 26 (2012).
Atwood, T. B. et al. Global patterns in mangrove soil carbon stocks and losses. Nature Climate Change (2017).
Lovelock, C. E. et al. The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature (2015).
Marchio, D. A., Savarese, M., Bovard, B. & Mitsch, W. J. Carbon sequestration and sedimentation in mangrove swamps influenced by hydrogeomorphic conditions and urbanization in Southwest Florida. Forests 7, 116 (2016).
Baskaran, M., Bianchi, T. & Filley, T. Inconsistencies between 14 C and short-lived radionuclides-based sediment accumulation rates: Effects of long-term remineralization. Journal of environmental radioactivity 174, 10–16 (2017).
Nittrouer, C., DeMaster, D., McKee, B. A., Cutshall, N. & Larsen, I. The effect of sediment mixing on Pb-210 accumulation rates for the Washington continental shelf. Marine Geology 54, 201–221 (1984).
Cochran, J. K. Particle mixing rates in sediments of the eastern equatorial Pacific: Evidence from 210 Pb, 239, 240 Pu and 137Cs distributions at MANOP sites. Geochimica et Cosmochimica Acta 49, 1195–1210 (1985).
Fujimoto, K. et al. Belowground carbon storage of Micronesian mangrove forests. Ecological Research 14, 409–413 (1999).
Laffoley, D. & Grimsditch, G. D. The management of natural coastal carbon sinks. (Iucn, 2009).
Smoak, J. M., Breithaupt, J. L., Smith, T. J. & Sanders, C. J. Sediment accretion and organic carbon burial relative to sea-level rise and storm events in two mangrove forests in Everglades National Park. Catena 104, 58–66 (2013).
Onstad, G. D., Canfield, D. E., Quay, P. D. & Hedges, J. I. Sources of particulate organic matter in rivers from the continental USA: lignin phenol and stable carbon isotope compositions. Geochimica et Cosmochimica Acta 64, 3539–3546 (2000).
Eadie, B. J. & Jeffrey, L. M. δ13C analyses of oceanic particulate organic matter. Marine Chemistry 1, 199–209 (1973).
Schile, L. M. et al. Limits on Carbon Sequestration in Arid Blue Carbon Ecosystems. Ecological Applications (2016).
Balk, M., Keuskamp, J. A. & Laanbroek, H. J. Potential Activity, Size, and Structure of Sulfate-Reducing Microbial Communities in an Exposed, Grazed and a Sheltered, Non-Grazed Mangrove Stand at the Red Sea Coast. Frontiers in microbiology 6 (2015).
Al-Washmi, H. Sedimentological aspects and environmental conditions recognized from the bottom sediments of Al-Kharrar Lagoon, eastern Red Sea coastal plain, Saudi Arabia. J. KAU Mar. Sci 10, 71–87 (1999).
Al-Farawati, R. Spatial and Seasonal Distribution of Total Dissolved Copper and Nickel in the Surface Coastal Waters of Rabigh, Eastern Red Sea, Saudi Arabia. Journal of King Abdulaziz University: Earth Sciences 22 (2011).
Phillips, S. C., Johnson, J. E., Miranda, E. & Disenhof, C. Improving CHN measurements in carbonate-rich marine sediments. Limnology and Oceanography: Methods 9, 194–203 (2011).
Brodie, C. R. et al. Evidence for bias in C and N concentrations and δ 13 C composition of terrestrial and aquatic organic materials due to pre-analysis acid preparation methods. Chemical Geology 282, 67–83 (2011).
Lorrain, A., Savoye, N., Chauvaud, L., Paulet, Y.-M. & Naulet, N. Decarbonation and preservation method for the analysis of organic C and N contents and stable isotope ratios of low-carbonated suspended particulate material. Analytica Chimica Acta 491, 125–133 (2003).
Wentworth, C. K. A scale of grade and class terms for clastic sediments. The Journal of Geology 30, 377–392 (1922).
Sánchez-Cabeza, J., Masqué, P. & Ani-Ragolta, I. 210Pb and210Po analysis in sediments and soils by microwave acid digestion. Journal of Radioanalytical and Nuclear Chemistry 227, 19–22 (1998).
Masqué, P., Sanchez-Cabeza, J., Bruach, J., Palacios, E. & Canals, M. Balance and residence times of 210 Pb and 210 Po in surface waters of the northwestern Mediterranean Sea. Continental Shelf Research 22, 2127–2146 (2002).
Phillips, D. L. & Gregg, J. W. Source partitioning using stable isotopes: coping with too many sources. Oecologia 136, 261–269 (2003).
Blaauw, M. & Christen, J. A. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian analysis 6, 457–474 (2011).
Southon, J., Kashgarian, M., Fontugne, M., Metivier, B. & Yim, W. W. Marine reservoir corrections for the Indian Ocean and Southeast Asia. Radiocarbon 44, 167–180 (2002).
Acknowledgements
The research reported in this paper was supported by King Abdullah University of Science and Technology through the baseline funding to C.M. Duarte and X. Irigoien. O.S. was supported by an ARC DECRA (DE170101524) and Edith Cowan University Collaboration Enhancement Scheme. PM and AAO acknowledge the support by the Generalitat de Catalunya (grant 2014 SGR-1356). This work is contributing to the ICTA ‘Unit of Excellence’ (MinECo, MDM2015-0552). AAO was supported by a PhD grant of Obra Social “la Caixa”. We thank CMOR staff for support and help during sampling the soil corers. And thank Vincent Saderne for his help with Bacon analysis.
Author information
Authors and Affiliations
Contributions
H.A., O.S., C.M.D. and X.I. designed the study. H.A. and O.S. carried out the field measurements. H.A., O.S., A.A.O. carried out the lab measurements. All authors contributed to the statistical analysis and writing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almahasheer, H., Serrano, O., Duarte, C.M. et al. Low Carbon sink capacity of Red Sea mangroves. Sci Rep 7, 9700 (2017). https://doi.org/10.1038/s41598-017-10424-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10424-9
This article is cited by
-
Spatial Variations in Element Concentrations in Saudi Arabian Red Sea Mangrove and Seagrass Ecosystems: A Comparative Analysis for Bioindicator Selection
Earth Systems and Environment (2024)
-
Assessment of heavy metals at mangrove ecosystem, applying multiple approaches using in-situ and remote sensing techniques, Red Sea, Egypt
Environmental Science and Pollution Research (2024)
-
Variability of blue carbon storage in arid evaporitic environment of two coastal Sabkhas or mudflats
Scientific Reports (2023)
-
Variability in soil organic carbon stock and isotopic signature in tropical island mangrove forests of India
Regional Environmental Change (2023)
-
Estimation of carbon pools in the biomass and soil of mangrove forests in Sirik Azini creek, Hormozgan province (Iran)
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.