Abstract
The inability to successfully adapt to stress produces pathological changes that can lead to depression. Molecular hydrogen has anti-oxidative and anti-inflammatory activities and neuroprotective effects. However, the potential role of molecular hydrogen in stress-related disorders is still poorly understood. The present study aims to investigate the effects of hydrogen gas on resilience to stress in mice. The results showed that repeated inhalation of hydrogen-oxygen mixed gas [67%:33% (V/V)] significantly decreased both the acute and chronic stress-induced depressive- and anxiety-like behaviors of mice, assessed by tail suspension test (TST), forced swimming test (FST), novelty suppressed feeding (NSF) test, and open field test (OFT). ELISA analyses showed that inhalation of hydrogen-oxygen mixed gas blocked CMS-induced increase in the serum levels of corticosterone, adrenocorticotropic hormone, interleukin-6, and tumor necrosis factor-α in mice exposed to chronic mild stress. Finally, inhalation of hydrogen gas in adolescence significantly increased the resilience to acute stress in early adulthood, which illustrates the long-lasting effects of hydrogen on stress resilience in mice. This was likely mediated by inhibiting the hypothalamic-pituitary-adrenal axis and inflammatory responses to stress. These results warrant further exploration for developing molecular hydrogen as a novel strategy to prevent the occurrence of stress-related disorders.
Similar content being viewed by others
Introduction
Stress-related disorders, such as depression and anxiety, are the most common and debilitating psychiatric diseases around the world1. Extensive evidence has shown that stress, especially chronic stress, is one of the most important factors responsible for depression2, 3. The individual’s coping style to psychosocial stress impacts the stress-induced pathological changes and the risk of psychological disorders such as depression. Resilience refers to the capacity of an individual to avoid negative social, psychological, and biological consequences of extreme stress that would otherwise compromise their psychological or physical well-being4. Recent reports indicated that resilience in humans represent an actively adaptive process, and not simply the absence of pathological responses that occur in more susceptible individuals5. Previous evidence showed that treatment with pharmacological and/or nonpharmacological strategies, such as environment enrichment and intermittent hypoxia, could increase the resilience of individuals to the subsequent stress6, 7. Our previous study demonstrated that predictable chronic mild stress in adolescence enhanced stress resilience in adulthood8. It is proposed that the stronger resilient ability an individual has, the lower the risk of psychological diseases2, 9, 10. Recently more attention has been focused on elucidating the mechanisms underlying resilience, and to construct new strategies for enhancing the resilience of individuals11.
The hypothalamic-pituitary-adrenal (HPA) axis is a highly adaptive neuroendocrine system strongly implicated in stress resilience and vulnerability5, 9. Modulation of HPA axis activity results in widespread hormonal, neurochemical, and physiological alterations5, 12. The interaction of stress and the immune system has become a major focus of psychiatric research since the introduction of the “cytokine hypothesis of depression” in 200813. A growing body of literature illustrates the connection between stress, proinflammatory cytokines, and depression in both humans and animals14,15,16,17. It has been found that circulating cytokines, such as tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), are increased in the patients with depression18. Children with higher circulating levels of IL-6 at age 9 are at a 10% greater risk for developing depression by age 18 than the general population or children with low levels of IL-619, 20. Raison et al. reported that infliximab, a monoclonal antibody against TNF-α, exhibited antidepressant efficacy in a subset of patients characterized by elevated plasma cytokines21. Recently, Hodes and colleagues reported that pre-existing differences in stress-responsive IL-6 release from bone marrow-derived leukocytes functionally contribute to stress-induced behavioral abnormalities and inhibition of peripheral IL-6 enhanced resilience to stress, suggesting that the peripheral immune system controlls behavioural susceptibility22. Altered regulation of this adaptive behavioural response to immune challenge by chronic illness or psychosocial stress contributes to depression. In both humans receiving immunotherapy and animal models of inflammation, administration of pro-inflammatory cytokines produces depression and anxiety-like behaviors23,24,25,26. Collectively, these studies suggest that aberrant periphery immune responses to stress can amplify the initial inflammatory signal that can directly or indirectly act on neuronal plasticity, which contributes to stress susceptibility and depression-like behavioral phenotypes22.
Molecular hydrogen has recently received significant attention by biomedical researchers due to its anti-oxidative, anti-apoptotic, and anti-inflammatory activities27. Molecular hydrogen has superior distribution properties due to it being small, electrically neutral, and nonpolar. Thus H2 can easily penetrate biomembranes such as the blood-brain barrier, placental and testis barrier, and reach target organs (e.g. brain) and organelles (e.g. mitochondria, nucleus, etc.). Inhalation of hydrogen gas in septic mice alleviated pathological damage, neuronal apoptosis, BBB disruption, and reversed cognitive decline, which was mediated by activation of the Nrf2 pathway and HO-1 induction28. Increasing evidence from animal and human studies indicate that molecular hydrogen offers significant neuroprotective effects in neuropathic pain, Parkinson’s disease, Alzheimer’s disease, and brain injury via alleviating excessive inflammatory response and oxidative stress27, 29,30,31. These neurological benefits may be mediated by second messenger systems such as H2-induced neuroprotective gastric ghrelin secretion32. Interestingly, elevating gastric ghrelin levels, via subcutaneous injections or caloric restriction, provided anxiolytic- and antidepressant-like response in the elevated plus maze and forced swim test33. Ad libitum consumption of hydrogen water attenuated lipopolysaccharide-induced neuroinflammation and promoted recovery of behavior sickness in mice31 (see comment for ref). Additionally, hydrogen water consumption ad libitum suppressed the increase in the oxidative stress markers malondialdehyde and 4-hydroxy-2-nonenal, prevented cognitive impairment and reversed neural proliferation in the dentate gyrus of the hippocampus induced by chronic physical restraint stress34. Most recently, Zhang and colleagues reported that hydrogen-rich water had antidepressant-like effects in CMS-treated mice by inhibiting oxidative stress, inflammation and apoptosis in the hippocampus and prefrontal cortex35. These studies strongly suggest that hydrogen, as a potential preventive and/or therapeutic molecule, has beneficial effects on resilience to stress and even to stress-related disorders including depression and anxiety.
In the present study, we examined the effects of repeated inhalation of high concentration of hydrogen gas on behavioral response to acute or chronic stress in mice, including depressive- and anxiety-like behaviors. We also assessed the HPA activity and immune response by measuring the serum levels of corticosterone (CORT), adrenocorticotropic hormone (ACTH), IL-6 and TNF-α to elucidate the potential mechanism(s) of hydrogen using a chronic mild stress (CMS) mouse model.
Materials and Methods
Animals
Two hundred male ICR mice (with a 5% attrition rate), weighing 22–24 g, were individually housed at a constant temperature (23 ± 2 °C) with 12 h/12 h light/dark cycles and free access to food and water. All mice were transferred to the experimental room 1 h before behavioural tests, and all drug administration and behavioural tests were performed in the dark phase. All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Local Animal Use Committee of Hebei Medical University.
Drugs and hydrogen inhalation
Fluoxetine hydrochloride [Sigma-Aldrich (Shanghai) Trading Co. Ltd.], acting as a positive control drug, was dissolved in saline and parallel injected (10 mg/kg, i.p.) for 14 consecutive days. As previously reported32, 33, 36, the 67% H2/33% O2 mixed gas (V/V) was produced using an AMS-H-3 hydrogen-oxygen nebulizer Machine (ASCLEPIUS MEDITEC, Shanghai, China), which decomposed water via electrolysis to produce H2 and O2 gas. A transparent closed box (20 × 18 × 15-cm, length × width × height) was used as the gas inhalation room for the mice. The water electrolysis-derived gas was directly leading in a closed room for inhalation. Before the experiment, we flushed the box with mix gas for no less than 15 min to replace the air in the box and NaHCO3 was also applied to the bottom of the closed box to keep the relatively lower concentration of CO2. During this inhalation, mice were awake and freely moving. Thermal trace GC ultra-gas chromatography (Thermo Fisher, MA, USA) was used to monitor the concentration of hydrogen gas in the closed box. The actual concentration of H2 gas in the closed box was kept in level of approximately 65%. The H2 concentration in the blood and hippocampus of mice exposed to H2/O2 mixture gas was detected by using a needle-type Hydrogen Sensor based on previous research37.
Tail suspension test
The tail suspension test was conducted according to our previous reports38, 39. Briefly, mice were suspended 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail for 6 minutes. Immobility was defined as the absence of limb or body movements, except for those caused by respiration when the mice hung passively and were completely motionless. During the test, mice were separated from each other to prevent possible visual and acoustical associations. The results were expressed as the time (in seconds) that animals spent immobile in the last 4 min of the 6 min session.
Forced swimming test
The forced swimming test was performed as previously described39, 40. Mice were placed into a 20-cm diameter ×35-cm high plastic cylinder filled to a depth of 20 cm with 23–25 °C water for 6 minutes. This session was videotaped, and the floating time was measured. Immobility was defined as the absence of movement, except for motion that was required to maintain the animal’s head above the water. The results were expressed as the time (in seconds) that animals spent immobile in the last 4 min of the 6 min session.
Open field test
The apparatus consisted of a (40 cm × 40 cm × 35 cm) square arena that was divided into 25 equal squares on the floor of the arena. Mice were individually placed in the centre of the cage, and the number that crossed to adjacent squares was counted as horizontal locomotor activity for 5 min39. The time in the central zone was recorded to reflect the anxiety-like behaviours of mice.
Novelty-suppressed feeding
The novelty-suppressed feeding test (NSF) was adapted from previous studies40, 41. The mice were deprived of food for 24 h before the test in their home cages. On the test day, mice were individually placed in an open field arena (40 cm × 40 cm × 35 cm) with small pellets of food placed in the centre. Each mouse was first placed in a corner of the cage. The latency to approach the food and begin eating was recorded (in seconds) as the main test parameter (maximum time, 5 min). Immediately after each mouse was taken back to its home cage, food consumption during the first 5 min was quantified to exclude the possibility that stress affected normal appetite and feeding. A more ‘anxious’ animal will take more time to begin eating in a novel environment.
Chronic Mild Stress
The chronic mild stress (CMS) protocol was based on previous reports40, 42. Briefly, mice were exposed to a variable sequence of mild, unpredictable stressors for 28 days. A total of 10 different stressors were used; two stressors were used per day. The stressors included restraint for 3 h, cold for 1 h at 4 °C, water deprivation for 24 h, vibration for 1 h, tilted cages (45°) for 24 h, forced cold swim for 5 min, crowding for 24 h, soiled bedding for 24 h, light/dark cycle reversal for 36 h, food deprivation for 24 h, and tail clamp for 1 min. Control mice were handled daily without any stress in the housing room.
Sucrose Preference Test
The sucrose preference test was performed as in previous studies40, 43. Mice were adapted to a 1% sucrose solution (w/v) for 48 h at the beginning of the experiment; two bottles of a 1% sucrose solution were placed in each cage. After adaptation, the mice were deprived of water for 24 h, which was followed by the sucrose preference test (SPT), during which mice were housed in individual cages for 24 h with exposure to two identical bottles; one was filled with a 1% sucrose solution, and the other was filled with water. Sucrose and water consumption (in g) were measured. Sucrose preference (%) = consumption × 100/(sucrose consumption + water consumption).
Enzyme-linked Immunosorbent Assay
The serum levels of CORT, ACTH, IL-6 and TNF-α were analysed by enzyme-linked immunosorbent assay (ELISA) according to our previous study44. Briefly, 1 ml of blood was collected from decapitation bleeding and kept at room temperature for 1 h, and then centrifuged at 3000 rpm for 10 min. The serum (supernatant fraction) was transferred into a new tube for subsequent assays. Serum CORT, ACTH, IL-6 and TNF-α levels were measured with commercially available ELISA kits (CORT, ml001959; ACTH, ml001895, IL-6, ml002293; TNF-α, ml002095, mlbio, China) according to the manufacturer’s instructions. To exclude the potential impact of diurnal rhythm on mouse hormone levels, blood samples were collected in the same time window of 4:00 to 6:00 pm.
Experimental design
Experiment 1: Effects of repeated inhalation of hydrogen gas on resilience to acute stress in adult mice
As shown in Fig. 1A, experiment 1 was aimed at determining the effects of molecular hydrogen on acute stress response of mice, assessed by the depressive- and anxiety-like behaviours. Mice were randomly divided into 6 groups (n = 8–11 per group): Saline group, 1h- O2/N2 group, 3 h O2/N2 group, 1h- H2/O2 group, 3h- H2/O2 group, and fluoxetine group. After a 5-day habituation, mice in H2/O2 group inhaled mixture of hydrogen and oxygen (67%:33%, V/V) for 1 h or 3 h daily for 14 consecutive days. Mice in O2/N2 group inhaled mixture of oxygen and nitrogen (33%:67%, V/V) for 1 h or 3 h daily to exclude the potential effects of high level of oxygen inhalation. Mice in fluoxetine group were injected with fluoxetine (10 mg/kg, i.p.) daily for 14 consecutive days, while mice in saline group were injected with saline (5 ml /kg, i.p.) daily for 14 consecutive days. Behavioural tests were conducted to assess response to acute stress 24 hours after the last hydrogen or fluoxetine treatment.
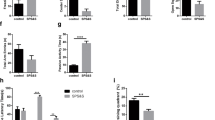
Repeated inhalation of hydrogen gas enhanced resilience to acute stress in mice. (A) Experimental procedure. After a 5-day adaptation period, the mice were given daily administration of saline, fluoxetine (10 mg/kg, i.p.), or inhaled mixture gas of H2/O2 [67%/33% (v/v)] or O2/N2 [33%/67% (v/v)] for 1, 3 h daily for 14 days. Beginning on day 15, behavioural tests were conducted to assess the depressive- and anxiety-like behaviours. Inhalation of hydrogen gas significantly decreased the immobility time of mice in the TST (B) and the floating time in the FST (C), increased the time spent in the central zone (D) without affecting the crossing activities (E) in the OFT, and decreased the latency to feeding (F) without affecting the total feeding in homecages (G) during the NSF test. *P < 0.05, **P < 0.01 versus the saline-treated control group. n = 8–11 per group. TST, tail suspension test; FST, forced swimming test; OFT, open field test; NSF, novelty suppressed feeding test.
Experiment 2: Effects of repeated inhalation of hydrogen gas on resilience to chronic stress in mice
To further assess the effects of molecular hydrogen on stress resilience in mice response to chronic stress, CMS procedure was used in this experiment as shown in Fig. 2A. Mice were divided into 5 groups (n = 8–10 per group): Control, CMS + saline, CMS + 3h-N2/O2, CMS + 3h-H2/O2, and CMS + fluoxetine. After a 5-day habituation, mice in CMS groups were treated with a consecutive 28-day chronic stress procedure. Since the 14th day during CMS procedure, CMS-treated mice were randomly divided into 4 subgroups and were injected with saline (10 ml/kg, i.p.), fluoxetine (10 mg/kg, i.p.) or 3 h H2/O2 and N2/O2 inhalation daily for 14 days. Mice in control group were left in their homecages with saline injections daily for 14 days. Behavioural tests, including SPT, NSF, OFT, were conducted 24 hours after the last hydrogen or drug treatment.
Repeated inhalation of hydrogen gas blocked the depressive- and anxiety-like behaviours in chronically stressed mice. (A) Experimental procedure. After a 5-day adaptation, mice were treated by chronic stress for 28 days. Beginning on day 14, mice inhaled mixture gas of H2/O2 [67%/33% (v/v)] or O2/N2 [33%/67% (v/v)] for 3 h daily or were injected with fluoxetine (10 mg/kg i.p.) daily 0.5 h before stress for 14 days. During day 28–30, behavioural tests were conducted to assess the depressive- and anxiety-like behaviours. Repeated inhalation of hydrogen gas significantly blocked the chronic stress-induced decrease in sucrose preference (B) without affecting the total intake (C) in the SPT, the increased latency to feeding (D) without affecting the total feeding in home cage (E) in the NSF test, and the decreased time spent in the central zone (F) without affecting the crossing activities (G) in the OFT. #P < 0.05 and ##P < 0.01 versus the control group; *P < 0.05 and **P < 0.01 versus the saline-treated CMS group. n = 8–10 per group. CMS, chronic mild stress; SPT, sucrose preference test; NSF, novelty suppressed feeding test; OFT, open field test.
Experiment 3: Effects of repeated inhalation of hydrogen gas on HPA axis activity and inflammatory response to chronic stress in mice
Experiment 3 was aimed at investigating whether the modulatory effects of hydrogen gas on stress response of mice are associated with the regulation of HPA axis activity and/or immune system. Mice were divided into 5 groups (n = 4–6 per groups) with CMS and hydrogen treatments similar to those in the experiment 2. Mice were decapitated 24 hours after the last hydrogen or drug treatment without any behavioural tests, and the blood was collected to detect the serum concentration of CORT, ACTH, IL-6 and TNF-α by ELISA analysis.
Experiment 4: Effects of repeated inhalation of hydrogen gas in adolescence on resilience to acute stress in adulthood
To further assess the long-lasting effects of molecular hydrogen on stress resilience in mice; the mice inhaled hydrogen gas within the adolescent period (i.e. postnatal day 28–42), which continued for 14 days. On postnatal day 28, the mice were randomly assigned to 5 groups (n = 9–10 per group). Four groups of mice inhaled N2/O2 or H2/O2 gas for 1 h or 3 h daily for 14 days, and the last group of mice were assigned to the control group and underwent similar handling every day for 14 days, without any treatment. Behavioural tests began on PND 52, and each mouse was subjected to NSF, FST and TST to assess depressive- and anxiety-like behaviours.
Data analysis
Data are expressed as the mean ± SEM. Statistical analysis of the data from acute and chronically stressed mice was performed by one-way analysis of variance (ANOVA), respectively, which was followed by a post hoc Dunnett’s test. (For details, see the Results section). p < 0.05 was considered statistically significant.
Results
Repeated inhalation of hydrogen gas enhanced resilience to acute stress
The results showed that H2 concentration in both hippocampus and blood was significantly increased in mice exposed to H2/O2 mixture gas and kept at relative higher concentration at even 30 min after H2 treatment (see Supplementary Fig. 1), indicating that H2 inhalation could be a effective method for H2 treatment.
Before the conducted The effects of repeated inhalation of molecular hydrogen on behavioural response to acute stress were assessed. Mice were randomly divided into 6 groups (n = 8–11 per group) and were treated (i.p.) with saline, fluoxetine (10 mg/kg), 1 h or 3 h H2 (67% H2 + 33% O2, V/V) inhalation, 1 h or 3 h O2 (67% N2 + 33% O2, V/V) inhalation daily for 14 consecutive days. Behavioural tests were conducted 24 hours after the last hydrogen inhalation.
One-way ANOVA of the TST data revealed a significant group effect [F5, 57 = 2.782, p = 0.027]. Post hoc analyses showed that repeated hydrogen gas inhalation 3 h daily for 14 days significantly reduced immobility time (p = 0.039), but the 1 h inhalation treatment had no effects on the immobility time compared with saline-treated mice. The positive control fluoxetine (10 mg/kg) significantly reduced immobility time in the TST (p = 0.012) compared with saline-treated mice (Fig. 1B).
One-way ANOVA of the FST data showed a significant group effect [F5, 57 = 4.023, p = 0.004]. Post hoc analyses showed that repeated hydrogen gas inhalation 3 h daily for 14 days significantly reduced floating time (p = 0.007), but the 1 h inhalation treatment had no effects on the floating time compared with saline-treated mice. The positive control fluoxetine (10 mg/kg) significantly reduced floating time in the FST (p = 0.003) compared with control mice (Fig. 1C). The results suggest that repeated inhalation of hydrogen gas enhanced resilience to acute stress.
Next, the potential anxiolytic effects of molecular hydrogen were evaluated using OFT. One-way ANOVA of the data revealed a significant group effect in the OFT [F5, 57 = 5.322, p = 0.001] and in the NSF [F5, 57 = 6.601, p = 0.001]. Post hoc analyses showed that repeated hydrogen gas inhalation for 1 h (p = 0.041), 3 h (p = 0.015) and fluoxetine administration (p = 0.001) significantly increased the time spent in the central zone in the OFT (Fig. 1D). Repeated fluoxetine and hydrogen gas inhalation for 1 h and 3 h had no significant effects on the crossing activities in the OFT (Fig. 1E). Repeated hydrogen gas inhalation for 1 h (p = 0.011), 3 h (p = 0.002) and fluoxetine administration (p = 0.003) significantly decreased the latency to feeding in the NSF (Fig. 1F), compared with saline-treated mice. Repeated fluoxetine and hydrogen gas inhalation for 1 h and 3 h did no change total food intake in homecages (Fig. 1G). Taken together, these results indicated that repeated hydrogen gas inhalation enhanced resilience to acute stress.
Repeated inhalation of hydrogen gas increased resilience against chronic stress
We further assessed the effects of molecular hydrogen on behavioural changes of mice response to chronic stress (Fig. 2A). Mice subjected to CMS exhibited key depressive-like phenotypes (e.g. ahedonia), reflected by a decrease in sucrose preference in SPT (F1, 17 = 15.905, p = 0.001, Fig. 2B). Both repeated hydrogen gas inhalation and fluoxetine treatment significantly increased sucrose preference (F3, 34 = 15.905, p = 0.001; F3, 34 = 91.291, p = 0.001 respectively, Fig. 2B) of CMS-treated mice. Hydrogen inhalation or fluoxetine treatment had no significant effects on the total water intake in the SPT (Fig. 2C).
The OFT and NSF were conducted for further investigation. One-way ANOVA analysis showed that mice subjected to CMS exerted a prolonged latency to feeding in the NSF (F1,17 = 8.755, p = 0.009, Fig. 2D) and decreased time spent in the central zone in the OFT (F1,17 = 9.24, p = 0.001, Fig. 2F), compared with control mice. Similar to the positive control fluoxetine treatment, repeated hydrogen gas inhalation inhibited those behavioural changes of mice induced by CMS, such as decreasing the prolonged latency to feeding in the NSF (F3,34 = 7.906, p = 0.003, p = 0.043, respectively, Fig. 2D), and increasing the time spent in the central zone in the OFT (F3,34 = 9.983, p = 0.001, Fig. 2F). Repeated hydrogen gas inhalation had no effects on total feeding in homecages (Fig. 2E) or the locomotion activity (Fig. 2G). Taken together, these results indicate that repeated hydrogen gas inhalation prevented CMS-induced depressive- and anxiety-like behaviours.
Repeated inhalation of hydrogen gas blocked changes of serum levels of CORT, ACTH, IL-6 and TNF-α in chronic stress treated mice
The effects of hydrogen gas inhalation on HPA activity were determined by analysing the serum CORT and ACTH levels (Fig. 3A). Mice were decapitated 24 hours after the last hydrogen or drug treatment, without any behavioural tests, and the blood was collected to detect the serum concentration of CORT, ACTH, IL-6 and TNF-α by ELISA analysis. One-way ANOVA analysis showed that the mice subjected to CMS had significant higher serum levels of CORT (F1,11 = 14.945, p = 0.003, Fig. 3B) and ACTH (F1,11 = 12.828, p = 0.005, Fig. 3C), compared with the control group. Hydrogen gas inhalation and fluoxetine treatment during the CMS process significantly prevented the CMS-induced increase in serum CORT (F3,22 = 5.736, p = 0.016 and p = 0.003 respectively, Fig. 2B) and ACTH (F3,22 = 15.653, p = 0.01, and p = 0.001 respectively, Fig. 3C) levels.
Repeated inhalation of hydrogen gas inhibited the chronic stress-induced increase in serum levels of CORT, ACTH, IL-6, and TNF-α. (A) Experimental procedure. After a 5-day adaptation, mice were treated with chronic stress procedure for 28 days. Beginning on day 14, mice were inhaled with mixture gas of H2/O2 [67%/33% (v/v)] or O2/N2 [33%/67% (v/v)] for 3 h daily 0.5 h before stress for 14 days. On day 29, mice were decapitated and serum samples were collected for ELISA analysis. Inhalation of hydrogen gas significantly blocked the increased serum levels of CORT (B), ACTH (C), IL-6 (D), and TNF-α (E) in chronically stressed mice. #P < 0.05 and ##P < 0.01 versus the control group; *P < 0.05 and **P < 0.01 versus the saline-treated CMS group. n = 4–6 per group. CMS, chronic mild stress; CORT, corticosterone; ACTH, adrenocorticotropic hormone; IL-6, interlukin-6; TNF-α, tumour necrosis factor-α.
Moreover, one-way ANOVA analysis showed that four weeks of CMS significantly increased the serum IL-6 (F1,11 = 5.786, p = 0.031, Fig. 3D) and TNF-α (F1,11 = 21.383, p = 0.007, Fig. 3E) levels, compared with control group. Similar to the effects of fluoxetine administration, repeated hydrogen gas inhalation significantly prevented the CMS-induced increase in serum IL-6 (F3, 22 = 11.017, p = 0.014, Fig. 3D) and TNF-α (F3, 22 = 7.747, p = 0.011, Fig. 3E) levels.
Repeated inhalation of hydrogen gas in adolescence increased stress resilience in adulthood
We further assessed the long-lasting effects of hydrogen gas inhalation in adolescence on the behavioural changes of mice response to acute stress in early adulthood (Fig. 4A).
Repeated inhalation of hydrogen gas in adolescence increased resilience to acute stress in adulthood. (A) Experimental procedure. On the 28th postnatal day (PND), the mice were inhaled mixture gas of H2/O2 [67%/33% (v/v)] or O2/N2 [33%/67% (v/v)] for 1, 3 h daily for 14 days. Behavioural tests were conducted to assess the acute stress-induced depressive- and anxiety-like behaviours. Inhalation of hydrogen gas significantly decreased the floating time in the FST (B) and the immobility time of mice in the TST (C), decreased the latency to feeding (D), but without affecting the total feeding in homecages (E) during the NSF test. *P < 0.05, **P < 0.01 versus the control group. n = 9–10 per group. TST, tail suspension test; FST, forced swimming test. NSF, novelty suppressed feeding test.
One-way ANOVA of the FST data showed a significant group effect [F4,45 = 3.229, p = 0.019]. Post hoc analyses showed that repeated hydrogen gas inhalation 1 h or 3 h daily for 14 days significantly reduced floating time (p = 0.044, p = 0.004 respectively, Fig. 4B).
One-way ANOVA of the TST data revealed a significant group effect [F4, 46 = 4.104, p = 0.007]. Post hoc analyses showed that both 1-h and 3-h repeated hydrogen gas inhalation 3 h daily for 14 days significantly reduced the immobility time (p = 0.018, p = 0.003 respectively) compared with the mice in control group. Mice with oxygen inhalation for 1 h or 3 h daily had no significant effects on the immobility time (p > 0.05) compared with the mice in control group (Fig. 4C).
The potential anxiolytic effects of molecular hydrogen were evaluated. One-way ANOVA of the data showed a significant group effect in the NSF [F4,46 = 4.806, p = 0.003]. Post hoc analyses showed that repeated hydrogen gas inhalation for 1 h (p = 0.003), 3 h (p = 0.001) significantly decreased the latency to feeding in the NSF test (Fig. 4D). Repeated hydrogen gas inhalation for 1 h and 3 h did not change total food intake in homecages (Fig. 4E). Taken together, these results indicate that repeated hydrogen gas inhalation enhanced resilience to acute stress.
Discussion
In the current study, using acute and chronic stress mice model, we explored the potential effects of molecular hydrogen on behavioural changes in response to stress in adult mice. We found that repeated inhalation of hydrogen gas enhanced resilience of mice subjected to acute or chronic stress by blocking the normal stress-induced depressive-and anxiety-like behaviours. In addition, we found that repeated inhalation of hydrogen gas inhibited hyperactivity of the HPA axis and the inflammatory response induced by chronic mild stress. This indicates that the enhanced resilience to stress by repeated hydrogen inhalation may be associated with modulation of the HPA axis activity and the immune system. Interestingly, the enhanced resilience effects of hydrogen can provide resilience against depression and anxiety caused by acute stress in early adulthood of mice treated with hydrogen inhalation in adolescence.
Biological effects of molecular hydrogen had been investigated for over 4 decades27. In 1975, Dole and colleagues reported in Science that hyperbaric hydrogen therapy (2.5% oxygen and 97.5% hydrogen under 8-atmospheric pressure) reduced the size of the squamous cell carcinoma tumors in hairless mice45. In 2007, Ohsawa and colleagues published a report in Nature Medicine that showed that inhalation of 1–4% hydrogen gas markedly reduced the size of cerebral infarction in rats, which ignited interest in the biomedical effect of molecular hydrogen in various diseases46, 47. A large number of studies have reported that molecular hydrogen has significant protective effects on many neurological diseases, including neuropathic pain, cerebral injury, Alzheimer’s Disease, Parkinson’s Disease and so on48,49,50. Research from Nagata and colleagues showed that continuous consumption of hydrogen water reduced oxidative stress in the brain, and prevented the stress-induced decline in learning and memory caused by chronic physical restraint in mice, highlighting the potential application of hydrogen in cognitive-impairment and stress-related disorders34.
Most recently, high concentration of H2 inhalation was also reported to exert anti-inflammatory and ant-apoptotic effects in vivo and in vitro. Our present study aimed to investigate the potential neuroprotective effects of high concentration of H2 inhalation32. Our present results showed that the concentration of hydrogen in the hippocampus and blood of mice exposed to H2/O2 mixture gas was significantly increased. Surprisingly, the highest H2 concentration (not more than 45 μM) in the blood collected immediately after 3-h of H2/O2 gas mixture inhalation is much lower than what is predicted. these low H2 concentrations may be explained in the following ways. H2 does not clearly follow its predicted equilibrium concentration based on Henry’s Law. First, Previous studies showed significant differences in H2 concentration depending on the organ or the location from which the measurement was obtained. Secondly, because H2 has very high diffusivity, some of the gas may have leaked out of the system, which reduces the total percentage46, 51, 52. Finally, perhaps the breathing system was not delivering 66.7% H2 for all the air inhaled by the mice. More research needs to be done on the exact pharmacokinetics of H2, the method of H2 inhalation to verify the total percentage of H2 administered, and the analytical techniques and methodology to confirm accuracy and precision.
Depression is increasingly considered to be a “whole body” illness involving the dysregulation of multiple systems, including the nervous system, the endocrine system and the immune system53, 54. Stress is well known to be one of the most important factors responsible for depression3. The forced swim test (FST), tail suspension test (TST), and chronic unpredictable stress are widely used animal models for examining stress vulnerability and resilience in rodents38, 55. In CMS paradigms, animals are exposed to varying mild stressors sequentially for a period of about 4 weeks. CMS produces a range of significant depressive- and anxiety-like phenotypes in rodents, including ahedonia, despair, and anxiety behaviours, which can be reversed by antidepressants, or blocked by pre-treatment of nonpharmacological strategies8. Sucrose preference test is the most widely used parameter to assess the ahedonia of depressed animals, the lower sucrose preference, the higher level of depression of animals8, 40. In the present study, we found that repeated inhalation of hydrogen gas in mice significantly decreased the immobility time in the FST and TST in response to acute stress, and blocked the decrease of sucrose preference induced by CMS. Potential anxiolytic effects of hydrogen were also observed in the OFT and NSF models in response to both acute and chronic stress. Importantly, repeated inhalation of hydrogen gas did not affect the locomotor activity, excluding the possibility that the effects of molecular hydrogen resulted from alteration of the locomotion activity of mice. Our behavioural results indicate that repeated inhalation of hydrogen gas enhanced the resilience of mice to acute stress without any side effects.
Resilience likely results from successful allosteric mechanisms in the hypothalamus-pituitary-adrenal (HPA) axis, immune system and the central nervous system1, 3. The causative relationship between inflammation and depression is gradually becoming more consistent18. Increasing evidence shows that molecular hydrogen exerts anti-oxidative, anti-inflammatory, and anti-apoptotic activities in both animal and human diseases44, 48, 50. The antioxidant effects of hydrogen was reported to be due to direct elimination of hydroxyl radical and peroxynitrite46, and the nuclear factor E2-related factor 2 (Nrf2)-Keap1 system was also reported to be associated with the antioxidant effects of molecular hydrogen56. To exert multiple functions in addition to anti-oxidative roles, H2 regulates various signal transduction pathways and the expression of many genes. For examples, The down-regulatory effects of hydrogen on TNF-α, IL-1β, and IL-6 levels are hypothesized as the reason for its protective role against ultraviolet light radiation or lipopolysaccharide. Hydrogen could attenuate TNF-α-induced activation of the NF-κB pathway36, 50, 57. H2 protects neural cells and stimulates energy metabolism by stimulating the hormonal expression of ghrelin and fibroblast growth factor 21 (FGF21) respectively. In contrast, H2 relieves inflammation by decreasing pro-inflammatory cytokines. Most recently, Luchi K and colleagues reported that exposure of cultured cells to the H2-dependently autoxidized phospholipid species reduced Ca2+ signal transduction and mediated the expression of various genes as revealed by comprehensive microarray analysis. H2 suppressed free radical chain reaction-dependent peroxidation and regulated the concentration of cellular Ca2+ and the Ca2+-dependent gene expression. indicating that H2 might regulate gene expression via the Ca2+ signal transduction pathway28. To investigate the potential mechanism underlying the effects of molecular hydrogen, we measured the serum CORT, ACTH and inflammatory mediators’ levels in chronically stressed mice. Our present results revealed that repeated inhalation of hydrogen significantly blocked the increase of serum CORT, ACTH, IL-6, and TNF-α in mice subjected to CMS, indicating the neuroprotective effect of molecular hydrogen is associated with the regulation of the HPA axis homeostasis and inflammatory response to stressful conditions, which is consistent with the previous studies.
In summary, this study demonstrated that repeated inhalation of hydrogen gas enhanced resilience of mice to acute and chronic stress without having apparent adverse effects. The long-lasting effect of repeated hydrogen gas inhalation in adolescence on resilience to acute stress in adulthood was also confirmed. The enhanced resilience effects by repeated hydrogen inhalation are associated with a normalization of the stress-induced HPA axis dysfunction and the inhibitory effects on the inflammatory response to stress. Thus neuroprotective effects of molecular hydrogen may be mediated via neuroimmune mechanisms. Further investigation should be carried out to explore the underlying molecular mechanisms for the neuroprotective effects of hydrogen, and whether the effects of molecular hydrogen on stress resilience in mice can be transferred to clinical application. Considering the comparable efficiency to prescription medication with no reported side effects, the high safety profile and ease of administration, molecular hydrogen should be further developed as a new strategy for preventing stress-related disorders, including depression and anxiety in the future.
References
Hodes, G. E., Kana, V., Menard, C., Merad, M. & Russo, S. J. Neuroimmune mechanisms of depression. Nature neuroscience 18, 1386–1393, doi:10.1038/nn.4113 (2015).
Russo, S. J., Murrough, J. W., Han, M. H., Charney, D. S. & Nestler, E. J. Neurobiology of resilience. Nature neuroscience 15, 1475–1484, doi:10.1038/nn.3234 (2012).
Franklin, T. B., Saab, B. J. & Mansuy, I. M. Neural mechanisms of stress resilience and vulnerability. Neuron 75, 747–761, doi:10.1016/j.neuron.2012.08.016 (2012).
Zemishlany, Z. Resilience and vulnerability in coping with stress and terrorism. The Israel Medical Association journal: IMAJ 14, 307–309 (2012).
Cramer, T., Kisliouk, T., Yeshurun, S. & Meiri, N. The balance between stress resilience and vulnerability is regulated by corticotropin-releasing hormone during the critical postnatal period for sensory development. Developmental neurobiology 75, 842–853, doi:10.1002/dneu.22252 (2015).
Kim, E. H. & Sufka, K. J. The effects of environmental enrichment in the chick anxiety-depression model. Behavioural brain research 221, 276–281, doi:10.1016/j.bbr.2011.03.013 (2011).
Zhu, X. H. et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. The Journal of neuroscience: the official journal of the Society for Neuroscience 30, 12653–12663, doi:10.1523/JNEUROSCI.6414-09.2010 (2010).
Suo, L. et al. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 38, 1387–1400, doi:10.1038/npp.2013.67 (2013).
Gillespie, C. F., Phifer, J., Bradley, B. & Ressler, K. J. Risk and resilience: genetic and environmental influences on development of the stress response. Depression and anxiety 26, 984–992, doi:10.1002/da.20605 (2009).
Daskalakis, N. P. & Yehuda, R. Early maternal influences on stress circuitry: implications for resilience and susceptibility to physical and mental disorders. Frontiers in endocrinology 5, 244, doi:10.3389/fendo.2014.00244 (2014).
Zannas, A. S. & West, A. E. Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience 264, 157–170, doi:10.1016/j.neuroscience.2013.12.003 (2014).
ter Heegde, F., De Rijk, R. H. & Vinkers, C. H. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology 52, 92–110, doi:10.1016/j.psyneuen.2014.10.022 (2015).
Maes, M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro endocrinology letters 29, 287–291 (2008).
Goldsmith, D. R., Rapaport, M. H. and Miller, B. J. A. meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Molecular psychiatry, doi:10.1038/mp.2016.3 (2016).
Lotrich, F. E. Inflammatory cytokine-associated depression. Brain research 1617, 113–125, doi:10.1016/j.brainres.2014.06.032 (2015).
Udina, M. et al. Cytokine-induced depression: current status and novel targets for depression therapy. CNS & neurological disorders drug targets 13, 1066–1074 (2014).
Bai, Y. M., Chiou, W. F., Su, T. P., Li, C. T. & Chen, M. H. Pro-inflammatory cytokine associated with somatic and pain symptoms in depression. Journal of affective disorders 155, 28–34, doi:10.1016/j.jad.2013.10.019 (2014).
Loftis, J. M., Huckans, M. & Morasco, B. J. Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiology of disease 37, 519–533, doi:10.1016/j.nbd.2009.11.015 (2010).
Masten, A. S. & O’Connor, M. J. Vulnerability, stress, and resilience in the early development of a high risk child. Journal of the American Academy of Child and Adolescent Psychiatry 28, 274–278, doi:10.1097/00004583-198903000-00021 (1989).
Wells, R. D. & Schwebel, A. I. Chronically ill children and their mothers: predictors of resilience and vulnerability to hospitalization and surgical stress. Journal of developmental and behavioral pediatrics: JDBP 8, 83–89 (1987).
Raison, C. L. et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry 70, 31–41, doi:10.1001/2013.jamapsychiatry.4 (2013).
Hodes, G. E. et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences of the United States of America 111, 16136–16141, doi:10.1073/pnas.1415191111 (2014).
Haroon, E., Raison, C. L. & Miller, A. H. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 37, 137–162, doi:10.1038/npp.2011.205 (2012).
Kim, Y. K., Na, K. S., Myint, A. M. & Leonard, B. E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Progress in neuro-psychopharmacology & biological psychiatry 64, 277–284, doi:10.1016/j.pnpbp.2015.06.008 (2016).
Du, Y. J. et al. Association of pro-inflammatory cytokines, cortisol and depression in patients with chronic obstructive pulmonary disease. Psychoneuroendocrinology 46, 141–152, doi:10.1016/j.psyneuen.2014.04.020 (2014).
Al-Maskari, M., Al-Shukaili, A. & Al-Mammari, A. Pro-inflammatory cytokines in Omani type 2 diabetic patients presenting anxiety and depression. Iranian journal of immunology: IJI 7, 124–129, doi:IJIv7i2A8 (2010).
Ichihara, M. et al. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Medical gas research 5, 12, doi:10.1186/s13618-015-0035-1 (2015).
Iuchi, K. et al. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Scientific reports 6, 18971, doi:10.1038/srep18971 (2016).
Kawaguchi, M., Satoh, Y., Otsubo, Y. & Kazama, T. Molecular hydrogen attenuates neuropathic pain in mice. PloS one 9, e100352, doi:10.1371/journal.pone.0100352 (2014).
Liu, G. D. et al. Molecular hydrogen regulates the expression of miR-9, miR-21 and miR-199 in LPS-activated retinal microglia cells. International journal of ophthalmology 6, 280–285, doi:10.3980/j.issn.2222-3959.2013.03.05 (2013).
Spulber, S. et al. Molecular hydrogen reduces LPS-induced neuroinflammation and promotes recovery from sickness behaviour in mice. PloS one 7, e42078, doi:10.1371/journal.pone.0042078 (2012).
Cui, J. et al. Inhalation of water electrolysis-derived hydrogen ameliorates cerebral ischemia-reperfusion injury in rats - A possible new hydrogen resource for clinical use. Neuroscience 335, 232–241, doi:10.1016/j.neuroscience.2016.08.021 (2016).
He, Y. et al. Effects of Hydrogen Gas Inhalation on Endometriosis in Rats. Reproductive sciences, doi:10.1177/1933719116655622 (2016).
Nagata, K., Nakashima-Kamimura, N., Mikami, T., Ohsawa, I. & Ohta, S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 34, 501–508, doi:10.1038/npp.2008.95 (2009).
Zhang, Y. et al. Effects of hydrogen-rich water on depressive-like behavior in mice. Scientific reports 6, 23742, doi:10.1038/srep23742 (2016).
Wang, R. et al. Postconditioning with inhaled hydrogen promotes survival of retinal ganglion cells in a rat model of retinal ischemia/reperfusion injury. Brain research 1632, 82–90, doi:10.1016/j.brainres.2015.12.015 (2016).
Hayashida, K. et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochemical and biophysical research communications 373, 30–35, doi:10.1016/j.bbrc.2008.05.165 (2008).
Zhu, W. L. et al. Green tea polyphenols produce antidepressant-like effects in adult mice. Pharmacological research 65, 74–80, doi:10.1016/j.phrs.2011.09.007 (2012).
Shi, H. S. et al. PI3K/Akt signaling pathway in the basolateral amygdala mediates the rapid antidepressant-like effects of trefoil factor 3. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 37, 2671–2683, doi:10.1038/npp.2012.131 (2012).
Wu, S. et al. Sulforaphane produces antidepressant- and anxiolytic-like effects in adult mice. Behavioural brain research 301, 55–62, doi:10.1016/j.bbr.2015.12.030 (2016).
Jung, Y. H. et al. Strain differences in the chronic mild stress animal model of depression and anxiety in mice. Biomolecules & therapeutics 22, 453–459, doi:10.4062/biomolther.2014.058 (2014).
Liu, X. L. et al. Fluoxetine regulates mTOR signalling in a region-dependent manner in depression-like mice. Scientific reports 5, 16024, doi:10.1038/srep16024 (2015).
Zhang, Y. et al. NLRP3 Inflammasome Mediates Chronic Mild Stress-Induced Depression in Mice via Neuroinflammation. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 18, doi:10.1093/ijnp/pyv006 (2015).
Wei, R., Zhang, R., Xie, Y., Shen, L. & Chen, F. Hydrogen Suppresses Hypoxia/Reoxygenation-Induced Cell Death in Hippocampal Neurons Through Reducing Oxidative Stress. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 36, 585–598, doi:10.1159/000430122 (2015).
Dole, M., Wilson, F. R. & Fife, W. P. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science 190, 152–154 (1975).
Ohsawa, I. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature medicine 13, 688–694, doi:10.1038/nm1577 (2007).
Zhang, C. B., Tang, Y. C., Xu, X. J., Guo, S. X. & Wang, H. Z. Hydrogen gas inhalation protects against liver ischemia/reperfusion injury by activating the NF-kappaB signaling pathway. Experimental and therapeutic medicine 9, 2114–2120, doi:10.3892/etm.2015.2385 (2015).
Shao, A. et al. Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-kappaB Pathway and NLRP3 Inflammasome. Molecular neurobiology, doi:10.1007/s12035-015-9242-y (2015).
Chen, Q. et al. Hydrogen-rich saline attenuated neuropathic pain by reducing oxidative stress. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques 40, 857–863 (2013).
Wang, C. et al. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-kappaB activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neuroscience letters 491, 127–132, doi:10.1016/j.neulet.2011.01.022 (2011).
Liu, C. et al. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Scientific reports 4, 5485, doi:10.1038/srep05485 (2014).
Ono, H. et al. A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patients for safety check with physiological parameters and measurement of blood H2 level. Medical gas research 2, 21, doi:10.1186/2045-9912-2-21 (2012).
Van den Hove, D. L. et al. Vulnerability versus resilience to prenatal stress in male and female rats; implications from gene expression profiles in the hippocampus and frontal cortex. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology 23, 1226–1246, doi:10.1016/j.euroneuro.2012.09.011 (2013).
Stiller, A. L., Drugan, R. C., Hazi, A. & Kent, S. P. Stress resilience and vulnerability: the association with rearing conditions, endocrine function, immunology, and anxious behavior. Psychoneuroendocrinology 36, 1383–1395, doi:10.1016/j.psyneuen.2011.03.012 (2011).
Vialou, V. et al. Serum response factor promotes resilience to chronic social stress through the induction of DeltaFosB. The Journal of neuroscience: the official journal of the Society for Neuroscience 30, 14585–14592, doi:10.1523/JNEUROSCI.2496-10.2010 (2010).
Meng, X., Chen, H., Wang, G., Yu, Y. & Xie, K. Hydrogen-rich saline attenuates chemotherapy-induced ovarian injury via regulation of oxidative stress. Experimental and therapeutic medicine 10, 2277–2282, doi:10.3892/etm.2015.2787 (2015).
Hattori, Y. et al. Maternal molecular hydrogen treatment attenuates lipopolysaccharide-induced rat fetal lung injury. Free radical research 49, 1026–1037, doi:10.3109/10715762.2015.1038257 (2015).
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (31371140), Special Foundation for Excellent Youth Investigator from Hebei Province of China (To H.-s. S.), Foundation for Heath Science from HFPC of Hebei Province (20170470, 20170471), Special Foundation for Excellent Undergraduate Students of Hebei Province (USIP201562A, USIPA201519A). We thank Prof. Zhi-min Kang and Prof. Da-gui Chen for their technical help.
Author information
Authors and Affiliations
Contributions
H.-s.S., S.-c.L. and Y.-x.M. conceived and designed the experiments. Q.G., H.S., X.-t.W., Y.L., Y.-j.X. and Y.-x. L. performed the behavioural Tests. Y.G., H.S., Q.-j.G. and S.-c.L. analysed the behavioural data and prepared the figures. H.S. and X.Y. conducted the measurement of H2 concentration assay. Q.G., X.Y. and H.-s.S. conducted the ELISA assays and analysed the data. H.-s.S., S.-c.L. and T.L. wrote and/or revised the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, Q., Song, H., Wang, Xt. et al. Molecular hydrogen increases resilience to stress in mice. Sci Rep 7, 9625 (2017). https://doi.org/10.1038/s41598-017-10362-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10362-6
This article is cited by
-
Molecular Hydrogen: an Emerging Therapeutic Medical Gas for Brain Disorders
Molecular Neurobiology (2023)
-
Microbial hydrogen “manufactory” for enhanced gas therapy and self-activated immunotherapy via reduced immune escape
Journal of Nanobiotechnology (2022)
-
Drinking hydrogen water improves photoreceptor structure and function in retinal degeneration 6 mice
Scientific Reports (2022)
-
Neuroprotective Effects of Molecular Hydrogen: A Critical Review
Neuroscience Bulletin (2021)
-
Local generation of hydrogen for enhanced photothermal therapy
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.