Abstract
The cotton aphid, Aphis gossypii (Hemiptera: Aphididae), is a serious pest of cotton across the globe, particularly in the cotton agroecosystems of northern China. Parasitic wasps are deemed to be important natural enemies of A. gossypii, but limited information exists about their species composition, richness and seasonal dynamics in northern China. In this study, we combine sampling over a broad geographical area with intensive field trials over the course of three cropping seasons to describe parasitoid-hyperparasitoid communities in cotton crops. We delineate a speciose complex of primary parasitoids and hyperparasitoids associated with A. gossypii. Over 90% of the primary parasitoids were Binodoxys communis. Syrphophagus sp. and Pachyneuron aphidis made up most of the hyperparasitoids. Parasitism rates changed in a similar way following the fluctuation of the aphid population. Early in the growing period, there were more hyperparasitoids, while later, the primary parasitoids provided control of A. gossypii. The first systematic report of this cotton aphid parasitoid complex and their population dynamics in association with their hosts presented a comprehensive assessment of cotton parasitoid species and provided important information for the establishment and promotion of their biological control of cotton aphids.
Similar content being viewed by others
Introduction
Aphids cause economic losses in many crops worldwide. In many cropping systems, parasitoids play a central role in the control of aphid pests. Diverse parasitoid communities, composed of both primary and secondary parasitoids, establish intricate trophic relationships with their aphid hosts and associated host plants. For example, 13 species of Aphidiinae (Hymenoptera: Braconidae) parasitoids from seven genera are associated with aphids on pome and stone fruit trees, establishing as many as 69 different tritrophic associations1. On alfalfa crops in Spain, up to 13 species of aphidiine wasps and four species of aphelinids have been found as well as eight hyperparasitoids2. Lastly, cereal aphids are subject to parasitism rates of 30–80% on Danish and New Zealand winter wheat, mostly from Aphidius ervi Haliday and Aphidius rhopalosiphi De Stefani-Perez3.
Hyperparasitoids that oviposit eggs directly into the eggs or larvae of primary parasitoids inside a premummified aphid are called true hyperparasitoids, such as Alloxysta (Cynipidae)4, while species of Asaphes and Pachyneuron (Pteromalidae) and Dendrocerus (Megaspilidae), which feed externally on the primary or secondary larval parasitoids inside the mummies, are mummy parasitoids5. Hyperparasitoids may disrupt primary parasitoids’ limited biological control of aphid populations6. The percentage of hyperparasitism of Lysiphlebus hirticornis Mackauer in colonies of Metopeurum fuscoviride (Stroyan) aphids can reach over 50% and causes significant mortality among primary parasitoids7. However, successful biological control has been reported in the presence of significant hyperparasitism8,9,10. The overall impact on aphid and parasitoid populations might be explained by low average fecundity7. Overall, the function and influence of hyperparasitoids, the top consumers in this system, on aphids and parasitoids is complicated.
The cotton aphid, Aphis gossypii Glover, is a major pest of cotton worldwide11. In northern China, this pest primarily affects cotton crops in the early growth stages12,13,14. Its climatic adaptability, reproductive ability, and capacity to rapidly attain high field populations contribute significantly to its pest status. Although A. gossypii directly impacts cotton yields through sap feeding and honeydew secretion, it also acts as an efficient vector of multiple plant viruses, such as cucumber mosaic virus (CMV) and others15, 16. Although parasitoids are important natural enemies of A. gossypii, parasitism rates tend to remain below 30%17,18,19,20,21. The dynamics of parasitoid wasp populations were only presented with the abundance of aphid mummies22, 23, mummification rates20 or Aphididae and hyperparasitoid proportions18. Cotton aphid parasitoids mainly belong to Aphidiinae (Braconidae) and Aphelinidae, with the former subfamily including Aphidius, Binodoxys, Lipolexis, Lysiphlebia, Lysiphlebus and Trioxys species. Among these, Lysiphlebia japonica (Ashmead) has received most of the scientific attention18, 21, 24,25,26,27,28,29. Aphelinidae are represented by Aphelinus asychis Walker30 and A. basilicus Fatima & Hayat31. In addition to the above groups of primary parasitoids, hyperparasitoids can play a prominent role in shaping aphid-parasitoid trophic interactions in cotton agroecosystems. However, little or no attention has been paid to hyperparasitoids in cotton crops around the world or within China. In eastern China, a total of four hyperparasitoid species have been reported in cotton fields, including Lygocerus koebelei Ashmead (Hym., Ceraphronidae) and Syrphophagus sp. (Hym., Encyrtidae)18. However, up to now, no comprehensive study has been conducted regarding the primary and secondary parasitoid species associated with A. gossypii in northern China.
In this study, we describe the parasitoid communities associated with A. gossypii in northern China. More specifically, we record 1) the species diversity and relative abundance of both primary and secondary parasitoids of A. gossypii, and 2) the seasonal aphid × parasitoid dynamics. Our work helps determine the importance of parasitoid-mediated biological control of A. gossypii in northern China and lays the necessary groundwork for the subsequent development of biological control tactics for conservation.
Results
Study #1: Species composition
In 2014, a total of 1,448 parasitoid specimens were collected, which included two primary parasitoid species (72.2%), Binodoxys communis (Gahan) and Aphidius gifuensis (Ashmead), and ten different hyperparasitoid species (27.8%), Alloxysta brevis (Thomson), A. pusilla (Kieffer), Asaphes suspensus (Nees), Dendrocerus laticeps (Hedicke), Pachyneuron aphidis (Bouché), Phaenoglyphis villosa (Hartig), Syrphophagus aphidivorus (Mayr), S. eliavae Japoshvili, S. taeniatus (Förster) and Syrphophagus sp. In 2015, a total of 2,901 parasitoid specimens were collected, including the same ten hyperparasitoid species (62.7%) as 2014, B. communis and a different primary parasitoid species, Aphelinus albipodus Hayat and Fatima (37.3%).
Of the primary parasitoids sampled in 2014 and 2015, 99.7 and 93.5%, respectively, were B. communis. In 2015, A. albipodus made up 6.5% (n = 40) of the primary parasitoids. For the hyperparasitoids, the same species were found in similar relative proportions with little differences across years, like A. brevis (0.2% for 2014, 0.4% for 2015), Syrphophagus sp. (33.8% for 2014, 28.6% for 2015), S. taeniatus (32.3% for 2014, 37.7% for 2015) etc. In 2014, Syrphophagus sp. was the most abundant hyperparasitoid, at 33.8%, followed by S. taeniatus (32.3%), S. aphidivorus (11.7%), and P. aphidis (10.0%). The remaining species collectively accounted for < 10% of all hyperparasitoids collected. In 2015, the most abundant hyperparasitoids were S. taeniatus (37.7%), Syrphophagus sp. (28.6%) and P. aphidis (19.5%) (Fig. 1, 2). Photographs of the main parasitoids and their key morphological features can be found in the Supplemental information (S1).
Study #2: Aphid-parasitoid seasonal dynamics
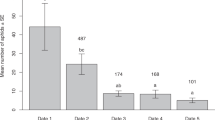
In 2015, A. gossypii populations increased from late May and reached an initial peak on June 14th, with 7,553 aphids per 100 plants, and a second peak on July 14th (Fig. 3). In 2016, the A. gossypii populations reached an initial peak on June 9th and then a maximum on July 19th, with a density of 31,029 individuals per 100 plants. On July 24th, the density suddenly dropped to 2,615 and then underwent only small fluctuations thereafter (Fig. 4).
Cotton aphid, Aphis gossypii, density and parasitism dynamics (A), the proportion of cotton aphid primary parasitoid and hyperparasitoid diversity and composition at the seedling stage (5/30-6/19) (B) and at the bud stage (6/20-6/29) (C), the flowering stage (6/30-7/14) (D) and the boll stage (7/15-8/23) (E) in 2015 in cotton fields in Langfang, Hebei, China.
Cotton aphid, Aphis gossypii, density and parasitism dynamics (A), the proportion of cotton aphid primary parasitoid and hyperparasitoid diversity and composition at the seedling stage (6/4-7/4) (B) and at the bud stage (7/5-7/14) (C), the flowering stage (7/15-7/24) (D) and the boll stage (7/29-9/12) (E) in 2016 in cotton fields in Langfang, Hebei, China.
Parasitoid populations lagged behind the A. gossypii build-up patterns. In 2015, parasitism rate peaked at 12.3% on June 24th (Fig. 3). In 2016, peak parasitism levels were recorded on June 20th, August 8th and September 2nd, with parasitism rates above 10% from August 19th to September 2nd (Fig. 4).
In Langfang, B. communis and A. albipodus were the two dominant primary parasitoids in both 2015 and 2016. Eight hyperparasitoid species were found in 2015 and ten in 2016. Compared to study #1, one additional hyperparasitoid species was found, i.e., Dendrocerus carpenteri (Curtis).
For the percentages of primary parasitoids and hyperparasitoids, there were significant differences throughout the whole growing period in both years (2015: χ2 = 16.49, df = 3, P = 0.0009; 2016: χ2 = 330.35, df = 3, P < 0.0001). In 2015, the hyperparasitoids dominated the parasitoid complex, with the highest percentage of 98.0% at the seedling stage and the lowest percentage of 66.7% at the bud stage, while the percentage of hyperparasitoids gradually decreased from 93.3% to 13.8% in 2016. A. albipodus only appeared at the flowering stage, with a percentage of 11.1%, and no significance difference was found throughout the growing period in 2015 (χ2 = 0.71, df = 3, P = 0.8698). However, the percentages of the two primary species were significantly different in 2016 (χ2 = 103.97, df = 3, P < 0.0001), and B. communis increased from 0.0% at the seedling stage to 99.7% at the boll stage. In 2015 and 2016, the proportions of the various hyperparasitoids were significantly different (2015: χ2 = 137.01, df = 21, P < 0.0001; 2016: χ2 = 82.81, df = 27, P < 0.0001). The hyperparasitoid species with the highest percentage varied among the different stages, with S. taeniatus (45.0%) highest at the seedling stage, S. taeniatus/P. aphidis (50.0%) highest at the bud stage, P. aphidis (58.8%) highest at the flowering stage and A. suspensus (56.0%) highest in 2015. In addition, P. aphidis led all the hyperparasitoids before the boll stage, with percentages over 45.0%, but S. taeniatus and Syrphophagus sp. made up 41.3% and 39.1%, respectively, ranking first instead of P. aphidis at boll stage in 2016 (Figs 3, 4, Table S1).
Discussion
The cotton aphid has long been considered as an important pest in cotton fields13. As the parasitic natural enemies of aphids, aphid parasitoids, such as Aphidius spp., have been successfully used in the suppression of aphid populations32. Our study constitutes the first systematic description of A. gossypii parasitoid communities in the cotton agroecosystems of northern China, reporting a total of three primary parasitoid species and 12 hyperparasitoids. Parasitoids are important natural enemies of cotton aphids in northern China14, 33, with Lysiphlebia japonica widely assumed to be the dominant parasitoid species18, 21, 23,24,25,26,27,28,29. This parasitic wasp has been broadly studied in China, and applied research has quantified its impact on aphid population dynamics33, 34, its oviposition behaviour and preferences in terms of host instar35 and host plants36, and the effect of environmental conditions on its biological control efficacy37. Despite the wealth of laboratory trials and manipulative assays using L. japonica, it is rather surprising to note a complete absence of comprehensive, field-level records on A. gossypii parasitoid communities and aphid-parasitoid dynamics from cotton agroecosystems in northern China.
Our study reports a complete absence of L. japonica from some of China’s principal cotton-growing areas in Hebei, Beijing and Tianjin. Instead, Binodoxys communis, Aphidius gifuensis and Aphelinus albipodus were the three primary parasitoid species attacking cotton aphids at our research sites. The dominant species B. communis is indeed an effective parasitoid of A. gossypii 38, 39 but was earlier only recorded at low population levels in Shaanxi Province and the Shanghai region27, 40. Additionally, previous studies reported A. gifuensis as the dominant aphid parasitoid or natural enemy in cotton fields in northern China14, 41 and the Yellow River region8. Furthermore, our manuscript constitutes the first record of A. albipodus in Chinese cotton systems (except for Luo and Gan, 1986)40.
Hyperparasitoids represent an important trophic level in the aphid-parasitoid-hyperparasitoid community that should not be overlooked18, 42, 43. Nevertheless, few cotton aphid parasitoid studies have examined hyperparasitoids. In an investigation of the Aphidiidae in cotton fields in Jiangsu Province in southeast China, four hyperparasitoids were recorded. Only Lygocerus koebelei was identified to the species level, while others were indicated only by family (Pteromalidae and Figitidae) or genus (Aphidencyrtus [Syrphophagus]), and the corresponding host aphids were not recorded10. Such limited knowledge underrepresents the hyperparasitoids associated with cotton aphids in cotton fields in northern China. Our study found 12 hyperparasitoid species belonging to four families and six genera. Of these, Pachyneuron aphidis and species of Syrphophagus were the dominant hyperparasitoids. These results are very similar to those from our earlier hyperparasitoid surveys in wheat fields in northern China (unpublished data), except for those concerning the Alloxysta species, with A. pusilla being found in both wheat and cotton, while a new species, A. brevis, was found in this study in cotton.
Increases in parasitism levels were delayed relative to increases in aphids in this study. The highest parasitism rates were 12.3% in 2015 and 14.4% in 2016. The parasitism rate varies with the investigation time and site, ranging from 5 to 30%21. We found more hyperparasitoids at the seedling and bud stages, while there were more primary parasitoids in the late-season flowering and boll stages. This is the opposite result from that in the wheat fields in the earlier hyperparasitoid study, where hyperparasitoids occurred later in the growing season (unpublished data). Since cotton fields start growing after the wheat harvest in northern China, it would appear that hyperparasitoids in wheat fields might migrate to cotton to find new aphid hosts. Parasitoids often move through different habitats or crops in the agricultural landscape and over a range of distances44. By spraying rubidium chloride on wheat plants, Dolichogenidea tasmanica (Cameron) parasitoids could be tracked by that biological marker, and the appropriate flowering buckwheat, Fagopyrum esculentum Moench, could be deployed for biological control45. However, the uncultivated margins providing a resource for Aphidiinae parasitoids seemed to have a much more limited contribution than expected based on analysis with a molecular technique46. In the study of how the presence of maize (non-host plant) influences the movement of the parasitoid Pediobius foveolatus (Crawford) in the absence of hosts, the density of parasitoid wasps might primarily be determined by emigration rates47. The intercrops would be more effective in pest suppression if they are colonized by natural enemies before the pest-susceptible time48. Wheat-cotton intercropping preserved and augmented more parasitoids than cotton monoculture, especially when intercropping with an aphid-susceptible wheat variety, which was consistent with previous research results14. This migration phenomenon could explain the early occurrence of hyperparasitoids in cotton and the high similarity in the hyperparasitoid communities between wheat and cotton. Intercropping wheat and cotton fields may thus enhance the population size, survival, fecundity, longevity and behaviour of parasitoids and improve their control of cotton aphids14, 49,50,51.
Recently, emerging molecular techniques have made it easier to carry out in-depth studies of aphid-parasitoid interactions42 and have allowed us to better understand food web relationships52. To facilitate the establishment of a molecular detection system for cotton aphid parasitoids, knowledge of the parasitoid species involved is needed42, 53. This comprehensive survey of cotton aphid parasitoid species and composition allows for the establishment of such molecular detection techniques and the determination of the relative biological control effects of different parasitoids.
Methods
Study #1: Parasitoid species composition
Study area
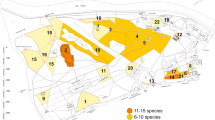
This study was conducted in three provinces or cities (Hebei, Tianjin, and Beijing) of northern China. Twenty-three cotton fields were randomly selected in 2014 and 16 in 2015, and each field was visited and sampled three times. The location of each site was recorded using a handheld mobile GPS set (MG768W, Unistrong, Beijing, China). Cotton fields of >1 ha in size were randomly chosen, and individual fields were spaced at a minimum distance of 3.5 km (max. 75.4 km). Fields were planted in late April and managed by individual growers using crop management practices common in the region. Field sampling lasted from July 15th to August 7th in 2014 and from July 11th to August 14th in 2015 with intervals of 6–7 days between each sampling date in both years.
Sampling method
On each collection date, at least 150 aphid mummies were collected from five random locations within each field. Mummies were individualized in 1.5 mL centrifuge tubes and kept at 25 ± 1 °C, 65–75% RH and a 16:8 h L:D photoperiod until parasitoid emergence. Each tube was closed with an absorbent cotton ball. Parasitoid emergence was recorded on a daily basis, and wasps were stored in 75% ethanol at 4 °C for subsequent identification.
Species identification
Field-collected parasitoids were morphologically identified using Doğanlar (1986), Huang (1994), Shi and Shen (1995), Gibson and Vikberg (1998), Xiao and Huang (2000), Alekseev and Radchenko (2001), Chen and Shi (2001), Gibson (2001), Japoshvili (2007), Xiao et al. (2009), Rakhshani et al. (2012), and Ferrer-Suay et al. (2013a,b)54,55,56,57,58,59,60,61,62,63,64,65,66. For each parasitoid, key morphological features were observed and photographed using a polarizing microscope (DM2500, Leica, Germany) and a digital camera (EOS 505D, Canon, Japan).
Study #2: Aphid-parasitoid seasonal dynamics
Study area. In late April 2015 and 2016, three replicate cotton field plots (var. SGK321) were planted at the Langfang Experimental Station (GPS coordinates 116.6°E, 39.5°N), Institute of Plant Protection (IPP) of the Chinese Academy of Agricultural Sciences (CAAS) in Langfang, Hebei Province. Each cotton field plot was 15 × 15 m and separated from the others by at least 5 m. All the field plots were located in the middle of large-area cotton fields (>1 ha) and at least 20 m away from other surrounding crops. No insecticides or herbicides were used, but all other common agronomic practices of northern China were applied, including regular inter-tillage, weeding and pruning. Prior to planting, all plots were fertilized with 375 kg/ha urea, 225 kg/ha phosphorus diamine, and 150 kg/ha potassium sulfate. One week after seedling emergence, 150 g/ha mepiquat chloride was applied.
Sampling method
Sampling was carried out every five days from May 30th to September 7th in 2015 and from June 4th to September 12th in 2016. During each sampling event, the number of cotton aphids and mummies were recorded on 20 cotton plants at each of five randomly selected sites per plot. Mummies were collected from the field and transported to the laboratory. Using the protocols described above, parasitoids were reared from each field-collected mummy and kept until further morphology-based identification.
Data analysis
The species composition and the seasonal dynamics of the wheat aphids and parasitoids were investigated. The standard errors are presented in the figures as the error bars along with the mean number of aphids in the population. For study #2, a chi-squared test (proc freq) was performed to assess differences in the proportions of different parasitoid taxa, which included the two primary parasitoids and various hyperparasitoids, collected from cotton during different growing stages in 2015 and 2016. All analyses were performed using SAS 9.3 software (SAS Institute Inc., Cary, USA).
References
Rakhshani, E. Aphid parasitoids (Hymenoptera: Braconidae, Aphidiinae) associated with pome and stone fruit trees in Iran. J. Crop. Prot. 1, 81–95 (2012).
Pons, X., Lumbierres, B., Antoni, R. & Starý, P. Parasitoid complex of alfalfa aphids in an IPM intensive crop system in northern Catalonia. J. Pest Sci. 84, 437–445 (2011).
Sigsgaard, L. A survey of aphids and aphid parasitoids in cereal fields in Denmark, and the parasitoids’ role in biological control. J. Appl. Ent. 126, 101–107 (2002).
Matejko, I. & Sullivan, D. J. Bionomics and behavior of Alloxysta megourae, an aphid hyperparasitoids (Hymenoptera: Cynipidae). J. N. Y. Entomol. Soc. 87, 275–282 (1979).
Sullivan, D. J. Hyperparasites. In Aphids, their biology, natural enemies and control, vol. B (eds A. K. Minks & P. Harrewijn), pp. 189–203. Amsterdam, The Netherlands: Elsevier (1988).
Rosenheim, J. A. Higher-order predators and the regulation of insect herbivore populations. A. Rev. Entomol. 43, 421–447 (1998).
Mackauer, M. & Völkl, W. Regulation of aphid populations by aphidiid wasps: does parasitoid foraging behavior or hyperparasitism limit impact? Oecologia 94, 339–350 (1993).
van den Bosch, R. et al. Biological control of the walnut aphid in California: impact of the parasite. Trioxys pallidus. Hilgardia 47, 1–13 (1979).
Hughes, R. D., Woolcock, L. T., Roberts, J. A. & Hughes, M. A. Biological control of the spotted alfalfa aphid, Therioaphis trifolii F. Maculata, on lucerne crops in Australia by the introduced parasitic hymenopteran Trioxys complanatus. J. Appl. Ecol. 24, 515–537 (1987).
Farrell, J. A. & Stufkens, M. W. The impact of Aphidius rhopalosiphi (Hymenoptera: Aphidiidae) on populations of the rose grain aphid, Metopolophium dirhodum (Hemiptera: Aphididae) on cereals in Canterbury, New Zealand. Bull. Entomol. Res. 80, 377–383 (1990).
Slosser, J. E., Pinchak, W. E. & Rummel, D. R. A review of known and potential factors affecting the population dynamics of the cotton aphid. Southwest. Entomol. 14, 302–313 (1989).
Zhang, Z. Q. The natural enemies of Aphis gossypii Glover (Hom., Aphididae) in China. J. Appl. Ent. 114, 251–262 (1992).
Wu, K. M. & Guo, Y. Y. The evolution of cotton pest management practices in China. Annu. Rev. Entomol. 50, 31–52 (2005).
Ma, X. M. et al. Assessment of cotton aphids, Aphis gossypii, and their natural enemies on aphid‐resistant and aphid‐susceptible wheat varieties in a wheat–cotton relay intercropping system. Entomol. Exp. App. 121, 235–241 (2006).
Wang, B., Chen, J. Q., Zhang, P. F., Ma, L. & Wang, Y. M. Effects of post-acquisition fast on cucumber mosaic virus transmission by the cotton aphid, Aphis gossypii. Acta Ent. Sin. 46, 259–266 (In Chinese) (2003).
Zhang, S. et al. Study on cotton aphid-borne viruses via high-throughput sequencing. Cotton Sci. 26, 539–545 (In Chinese) (2014).
Shi, D. S. Studies on the hymenopterous parasite complex and its fluctuation on the cotton aphid (Aphis gossypii Glover) in Shanghai. Contr. Shanghai Inst. Ent. 1, 215–219 (In Chinese) (1980).
Tian, L. X., Yang, L. F. & Gao, S. G. A preliminary study on aphid parasites in cotton fields (Hymenoptera: Aphidiidae). Chin. J. App. Entomol. 18, 158–160 (In Chinese) (1981).
Xi, X. & Zhu, Z. L. Preliminary studies on aphid parasites in Jiangsu Province. J. Enviro. Entomol. 6, 49–52 (In Chinese) (1984).
Luo, Z. Y. & Gan, G. P. Population dynamics of cotton aphids on cotton during square-boll stage and the relation between population age structure and parasitization. Acta Ent. Sin. 29, 56–61 (In Chinese) (1986).
Xie X. Y. & Sterling, W. L. Computer simulation of cotton aphid population dynamics. Acta Phytophy. Sin. 14, 151–156 (In Chinese) (1987).
Ali, A., Desneux, N., Lu, Y. H., Liu, B. & Wu, K. M. Characterization of the natural enemy community attacking cotton aphid in the Bt cotton ecosystem in Northern China. Sci. Rep. 6, 24273 (2016).
Yao, Y. S. et al. Transgenic Bt cotton does not disrupt the top-down forces regulating the cotton aphid in central China. PLoS One. 11, e0166771 (2016).
Starý, P. & Schlinger, E. A revision of Far East Asian Aphidiidae (Hymenoptera) Series Entomologica 3. Dr. W. Junk, the Hague (1967).
Takada, H. Aphidiidae of Japan (Hymenoptera). Ins. Matsum. 30, 67–124 (1968).
Institute of Plant Protection, Hubei Academy of Agricultural Sciences. Photo album of cotton pests and their natural enemies. Hubei People’s Press, Wuhan, China (In Chinese) (1980).
Zhao, J. Z. Natural enemies of cotton pest in China. Wuhan Publishing House Press, Wuhan, China (1995).
Deng, Y. X. & Tsai, J. H. Development of Lysiphlebia japonica (Hymenoptera: Aphidiidae), a parasitoid of Toxoptera citricida (homoptera: aphididae) at five temperatures. Flo. Entomol. 81, 415–423 (1998).
Gao, X. K. et al. Preliminary study of the prevention and control of Lysiphlebia japonica Ashmead on Aphis gossypii Glover. China Cotton 43, 8–10 (In Chinese) (2016).
Schirmer, S., Sengonca, C. & Blaeser, P. Influence of abiotic factors on some biological and ecological characteristics of the aphid parasitoid Aphelinus asychis (Hymenoptera: Aphelinidae) parasitizing Aphis gossypii (Sternorrhyncha: Aphididae). Eur. J. Entomol. 105, 121–129 (2008).
Lokeshwari, D., Hayat, M., Krishna Kumar, N. K., Manjunatha, H. & Venugopalan, R. First occurrence of the aphid parasitoid, Aphelinus basilicus (Hymenoptera: Aphelinidae), on Aphis gossypii (Hemiptera: Aphididae) color forms in India. Fla. Entomol. 97, 809–813 (2014).
Van Lenteren, J. C. The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl 57, 1–20 (2012).
Li, J. The indirect effect of stress from Propylaea japonica Thunberg and Lysiphlebia japonica Ashmead on development, fecundity and fitness of Aphis gossypii Glover. Master thesis. Hunan Agricultural University. 2008.
Li, J., Long, D. B., Xiao, T. G. & Ouyang, F. The impacts of stress from Lysiphlebia japonica Ashmead on the development and fecundity of the three successive generations of aphids. Chinese J. App. Entomol. 50, 951–958 (In Chinese) (2013).
Lan, J. L. & He, F. D. A study on parasitization preferences of Lysiphlebia japonica (Ashmead) to different instars of Aphis gossypii. Plant Protection, 30, 37–39 (In Chinese) (2004).
Hu, D. W. et al. Preference of Lysiphlebia japonica for Aphis gossypii Glover reared on different host plants. Chinese J. Biol. Control, 32, 581–586 (In Chinese) (2016).
Sun, Y. C., Feng, L., Gao, F. & Ge, F. Effects of elevated CO2 and plant genotype on interactions among cotton, aphids and parasitoids. Insect Sci. 18, 451–451 (2011).
Desneux, N. et al. Cryptic species of parasitoids attacking the soybean aphid, Aphis glycines Matsumura (Hemiptera: Aphididae), in Asia: Binodoxys communis Gahan and Binodoxys koreanus Stary sp. n. (Hymenoptera: Braconidae: Aphidiinae). Ann. Entomol. Soc. Am. 102, 925–936 (2009a).
Desneux, N., Barta, R. J., Hoelmer, K. A., Hopper, K. R. & Heimpel, G. E. Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 60, 387–398 (2009b).
Luo, Z. Y. & Gan, G. P. Population dynamics of cotton aphids on cotton during square-boll stage and the relation between population age structure and parasitization. Acta Ent. Sin. 29, 56–61 (In Chinese) (1986).
Zheng, Y.S., Zhang, X. Y. & Wang, Z. H. Classification of aphid parasitoid in cotton. Chin. J. App. Entomol. 22, 175-180 (In Chinese) (1985).
Traugott, M. et al. Endoparasitism in cereal aphids: molecular analysis of a whole parasitoid community. Mol. Ecol. 17, 3928–3938 (2008).
Tylianakis, J. M. & Binzer, A. Effects of global environmental changes on parasitoid–host food webs and biological control. Biol. Control 75, 77–86 (2014).
Lavandero, B., Wratten, S., Hagler, J. & Jervis, M. The need for effective marking and tracking techniques for monitoring the movements of insect predators and parasitoids. Int. J. Pest. Manage. 50, 147–151 (2004).
Scarratt, S. L., Wratten, S. D. & Shishehbor, P. Measuring parasitoid movement from floral resources in a vineyard. Biol. Control 46, 107–113 (2008).
Derocles, S. A. P. et al. Molecular analysis reveals high compartmentalization in aphid–primary parasitoid networks and low parasitoid sharing between crop and noncrop habitats. Mol. Ecol. 23, 3900–3911 (2014).
Coll, M. & Bottrell, D. G. Movement of an insect parasitoid in simple and diverse plant assemblages. Ecol. Entomol. 21, 141–149 (1996).
Corbett, A. & Plant, R. E. Role of movement in the response of natural enemies to agroecosystem diversification: a theoretical evaluation. Environ. Entomol. 22, 519–531 (1993).
Parajulee, M. N., Montandon, R. & Slosser, J. E. Relay intercropping to enhance abundance of insect predators of cotton aphid (Aphids gossypii Glover) in Texas cotton. Int. J. Pest. Manage. 43, 227–232 (1997).
Landis, D. A., Wratten, S. D. & Gurr, G. M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 45, 175–201 (2000).
Velders, R. M., Cui, J. J., Xia, J. Y. & Van der Werf, W. Influence of transgenic cotton on the cotton aphid (Aphis gossypii) and its two major enemies in north China. Cotton Sci. 14, 175–179 (2002).
Van Veen, F. J. F., Müller, C. B., Pell, J. K. & Godfray, H. C. J. Food web structure of three guilds of natural enemies: predators, parasitoids and pathogens of aphids. J. Anim. Ecol. 77, 191–200 (2008).
Chen, Y., Pike, K. S., Greenstone, M. H. & Shufran, K. A. Molecular markers for identification of the hyperparasitoids Dendrocerus carpenteri and Alloxysta xanthopsis in Lysiphlebus testaceipes parasitizing cereal aphids. BioControl 51, 183–194 (2006).
Doğanlar, M. Morphological studies of the hypopygium and its importance to the taxonomy of the genera Pachyneuron and Euneura (Hymenoptera: Pteromalidae), with description of a new species of Pachyneuron from Turkey. Fen. Bil. Derg. 4, 23–32 (1986).
Huang, J. The classification of Aphelinidae (Hymenoptera: Chalcidoidea). Chongqing Publishing Group (In Chinese) (1994).
Shi, Z. Y. & Shen, X. C. Parasitoid identification. Chinese Agricultural Science and Technology Press (In Chinese) (1995).
Gibson, G. A. P. & Vikberg, V. The species of Asaphes Walker from America north of Mexico, with remarks on extralimital distributions of taxa (Hymenoptera: Chalcidoidea, Pteromalidae). J. Hymenopt. Res. 7, 209–256 (1998).
Xiao, H. & Huang, D. W. A taxonomic study on Asaphes (Hymenoptera: Pteromalidae) from China, with descriptions of four new species. Entomologia Sinica 7, 193–202 (2000).
Alekseev, V. N. & Radchenko, T. D. Ceraphronoid wasps (Hymenoptera, Ceraphronoidea) of the fauna of the Ukraine-Communication 1[J]. Vestnik zoologii 35, 3–16 (2001).
Chen, J. H. & Shi, Q. X. Systematic studies on aphidiidae of China (Hymenoptera: Aphidiidae). Fujian Science & Technology Publishing House (In Chinese) (2001).
Gibson, G. A. P. The Australian species of Pachyneuron Walker (Hymenoptera: Chalcidoidea: Pteromalidae). J. Hymenopt. Res. 10, 29–54 (2001).
Japoshvili, G. New data on species of Syrphophagus (Hymenoptera: Encyrtidae) from Transcaucasia and Turkey. Ann. Entomol. Soc. Am. 100, 683–687 (2007).
Xiao, H., Jiao, T. Y. & Huang, D. W. Pachyneuron (Hymenoptera: Pteromalidae) from China. Orient. Insects 43, 341–359 (2009).
Rakhshani, E. et al. Parasitoids (Hymenoptera: Braconidae: Aphidiinae) of northeastern Iran: Aphidiine-aphid-plant associations, key and description of a new species. J. Insect Sci. 12, 43 (2012).
Ferrer-Suay, M., Selfa, J. & Pujade-Villar, J. Charipinae fauna (Hymenoptera: Figitidae) from Asia with a description of 11 new species. Zool. Stu. 52, 41 (2013a).
Ferrer-Suay, M. et al. A contribution to the knowledge of Charipinae (Hymenoptera: Cynipoidea: Figitidae) associated with aphids (Hemiptera: Aphididae) from Iran, including new records. North-West J. Zool. 9, 30–44 (2013b).
Acknowledgements
We greatly thank Dr. Guoyan Zhang (Institute of Zoology, Chinese Academy of Sciences) for technical assistance for species identification. We also thank the graduate trainees at Langfang Experimental Station, IPP-CAAS, during the period of 2014–2016 for their assistance with the field work. This study was supported by National Natural Science Foundation of China (31572019, 31621064), and a grant from the Ministry of Education, Science and Technological Development of the Republic of Serbia (III43001).
Author information
Authors and Affiliations
Contributions
Y.L., and Y.G. conceived the original idea. Y.L. designed research. F.Y., Y.W., L.X., Q.W., Z.Y., V.Ž., Ž.T., M.F., J.S., J.P., and Y.L. performed research, analyzed the data and interpreted the results. All authors wrote, reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, F., Wu, YK., Xu, L. et al. Species composition and richness of aphid parasitoid wasps in cotton fields in northern China. Sci Rep 7, 9799 (2017). https://doi.org/10.1038/s41598-017-10345-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10345-7
This article is cited by
-
Effects of sublethal fipronil exposure on cross-generational functional responses and gene expression in Binodoxys communis
Environmental Science and Pollution Research (2024)
-
A molecular detection approach for a cotton aphid-parasitoid complex in northern China
Scientific Reports (2019)
-
Efficacy of using DNA barcoding to identify parasitoid wasps of the melon-cotton aphid (Aphis gossypii) in watermelon cropping system
BioControl (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.