Abstract
Gray matter (GM) anomalies may represent a critical pathology underlying obstructive sleep apnea (OSA). However, the evidence regarding their clinical relevance is inconsistent. We conducted a meta-analysis of voxel-based morphometry (VBM) studies of patients with OSA to identify their brain abnormalities. A systematic search was conducted based on PRISMA guidelines, and a meta-analysis was performed using the anisotropic effect-size-based algorithms (ASE-SDM) to quantitatively estimate regional GM changes in patients with OSA. Fifteen studies with 16 datasets comprising 353 untreated patients with OSA and 444 healthy controls were included. Our results revealed GM reductions in the bilateral anterior cingulate/paracingulate gyri (ACG/ApCG), left cerebellum (lobules IV/V and VIII), bilateral superior frontal gyrus (SFG, medial rostral part), right middle temporal gyrus (MTG), and right premotor cortex. Moreover, GM reductions in the bilateral ACG/ApCG were positively associated with body mass index (BMI) and age among patients with OSA, and GM reductions in the SFG (medial rostral part) were negatively associated with Epworth sleepiness scale (ESS) scores and sex (male). These abnormalities may represent structural brain underpinnings of neurocognitive abnormalities and respiratory-related abnormalities in OSA. In particular, this study adds to Psychoradiology, which is a promising subspecialty of clinical radiology mainly for psychiatric disorders.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) involves sleep-induced apnea with complete or partial obstruction of the upper airway during sleep, leading to reduced brain oxygenation, transient arousal from sleep and sleep fragmentation. OSA affects approximately 9% of middle-aged men and 4% of middle-aged women1; estimates for the middle-aged to elderly population range from 49.7% to 79.2% in men and 23.4% to 54.3% in women2. OSA is a growing health concern because it has many unfavorable consequences, including daytime drowsiness and associated cardiovascular diseases, cognitive impairment (involving memory, attention and vigilance3, 4), and emotional problems, including depression5. OSA may be related to increased risk of neurodegenerative conditions, such as Alzheimer’s disease6.
In most epidemiological studies, the definition of OSA is based on the apnea-hypopnea index score (AHI) (the number of obstructive respiratory events per hour during sleep), which reflects the degree of departure from the normal physiology of breathing during sleep. OSA is defined as AHI ≥ 5 (mild = 5–15; moderate = 15–30; severe > 30) with associated symptoms (daytime sleepiness, cognitive impairments)7, although some studies have based OSA diagnoses on the respiratory disturbance index (RDI). AHI is defined by the American Academy of Sleep Medicine (AASM) as a ≥ 50% reduction in airflow for 10 seconds or longer or a clear reduction (but < 50%) with either an arousal or ≥ 3% (or ≥ 4%) oxygen desaturation, and RDI is defined as AHI with or without respiratory effort-related arousals (RERAs)8. In addition to AHI and RDI, body mass index (BMI), the Epworth sleepiness scale (ESS) and the oxyhemoglobin desaturation index (ODI) are frequently used to characterize patients with OSA. BMI is an indicator of body mass, and obesity (BMI ≥ 30) increases the risk of OSA9. The ESS is a self-administered questionnaire used to subjectively measure daytime sleepiness10. A score ≥ 10 is considered to represent excessive daytime sleepiness (EDS)11, 12. The ODI is calculated as the number of times per hour that the blood’s oxygen desaturation is ≥ 3% or ≥ 4% from baseline during sleep8. Significant correlations between ODI and BMI13 and between AHI and ODI14 have been reported, but the correlation between AHI and ODI is diminished among obese patients, indicating that oximetry may be more important in obese than in normal-weight patients15.
During the past three decades, developments in neuroimaging using MRI have come to provide highly sensitive, noninvasive approaches for investigating the neural mechanisms of brain alterations associated with various medical disorders, such as OSA. Although the exact pathology of OSA remains unclear, both hypoxemia and sleep fragmentation contribute to the neural deficits related to this disease16. To date, structural neuroimaging studies using voxel-based morphometry (VBM) have demonstrated widespread gray matter (GM) deficits in patients with OSA in multiple neocortical regions (frontal, prefrontal, ventral medial prefrontal, occipital, lateral temporal and parietal cortices), limbic regions (anterior/mid-cingulate gyrus, insula, amygdala, hippocampus/parahippocampal gyrus), basal ganglia, and cerebellum17,18,19. Researchers have also explored the relationships between structural brain abnormalities and clinical signs and symptoms of OSA. Celle et al.20 reported that AHI and ODI scores were inversely correlated with the GM volume in the bulbopontine area and cerebellum in patients with OSA. However, the localization of deficits varies considerably across studies, and some results are conflicting. For example, the insula has been reported to show both significant GM atrophy18, 21 and hypertrophy22, 23. The reasons accounting for these discrepancies include demographic (e.g., age and sex) and methodological factors (e.g., statistical thresholds and smaller patient cohorts with variability in the chronic and acute severity of OSA).
Reviews and meta-analyses have been conducted to evaluate these results and identify robust conclusions across studies. Regarding review articles, Zimmerman & Aloia24 found that the hippocampus may be a common atrophic region, and Huynh et al.19 suggested that there were few robust differences between OSA patients and healthy controls. Celle et al.25 stated that measuring GM volume changes in patients with OSA would not yield valid results unless large cohorts were studied to permit statistical control for various clinical and demographic confounding influences. In addition to these qualitative observations, coordinate-based meta-analyses have been used to facilitate quantitative analyses combining data from different studies. The first VBM meta-analysis, conducted by Weng et al.26 utilizing ALE software, evaluated 8 VBM studies16, 21, 27,28,29,30,31,32 containing 213 patients and 195 controls. They found that patients with OSA exhibited significant GM reductions in the bilateral parahippocampus and frontotemporal regions (although the evidence of the latter was less convincing). Recently, Tahmasian et al.33 published another coordinate-based meta-analysis using ALE software. In their study, VBM and functional magnetic resonance imaging (fMRI) studies were combined (4 VBM studies21, 30,31,32, 9 fMRI studies34,35,36,37,38,39,40,41,42, and 2 VBM & fMRI studies22, 43), which contained 290 OSA patients and 290 controls. The authors reported significant structural atrophy and functional disturbances in the right amygdala/hippocampus and right insula.
The previous two meta-analyses have provided useful information concerning OSA. However, there were important limitations. First, the VBM literature on OSA has grown substantially since the first VBM meta-analysis was published. Thus, a new meta-analysis including more VBM studies should provide more precise results. Although it included updated literature, the meta-analysis conducted by Tahmasian et al.33 explored only convergent brain changes on VBM and resting-state fMRI studies. Such a combination might fail to identify all regions with anatomic alterations. Second, the meta-analytic tool used by previous studies did not consider the effects of important demographic and clinical factors. One newer coordinate-based meta-analytic tool, the anisotropic effect-size-based algorithm (AES-SDM), enables such exploration and allows subgroup and sensitivity analyses to establish the robustness of the results. Third, although both software platforms are coordinate-based, there are some inherent differences between them, e.g., SDM takes the p value of each coordinate into account, while ALE does not.
Therefore, we conducted a meta-analysis using AES-SDM software to identify the most prominent and replicable GM changes among patients with OSA. We performed an updated literature search, and a sensitivity analysis was applied to evaluate the robustness of the results. Second, we examined clinical and demographic characteristics of the patient groups in the studies included in our meta-analysis, and we performed a meta-regression analysis to explore the associations between these features and identified brain alterations.
Methods
Inclusion of studies
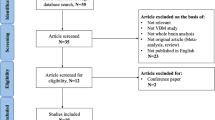
We searched the PubMed, Science Direct, Medline, Embase and Web of Science databases for literature from January 2000 to December 2016 using the keywords (“obstructive sleep apnea” OR “sleep apnea” OR “sleep disordered breathing” OR “sleep related breathing disorders” OR “OSA”) and (“voxel” OR “morphometry” OR “voxel-based morphometry” OR “VBM”). Furthermore, we checked the reference list of each study to identify additional studies for inclusion. The following were criteria for inclusion: (1) voxel-based morphometry was applied to analyze whole-brain gray matter volume (GMV) or gray matter concentration (GMC) or gray matter density (GMD) in patients with OSA (since both GMC and GMD reflect GM volumetric changes, we uniformly use GMD in the following text); (2) untreated patients with OSA were compared with healthy control subjects; and (3) articles reported whole-brain GM alterations in Montreal Neurological Institute (MNI) or Talairach stereotactic space coordinates (x, y, z). We followed the PRISMA guidelines to identify studies44 as illustrated in the PRISMA flowchart in Fig. 1.
VBM meta-analysis
Prior to conducting the meta-analysis, the reported peak coordinates of regional alterations were extracted to construct an analysis file. We also recorded the following variables from each study: sample size, percentage of male subjects, mean age of subjects, clinical characteristics of the patient group (BMI, AHI, ODI, and ESS), and method utilized to correct whole-brain results for multiple comparisons.
The analytical processes used in the meta-analysis are described in the AES-SDM tutorial (http://sdmproject.com/software/Tutorial.pdf) and related publications. As an intrinsic whole-brain analysis technique, AES-SDM does not restrict analyses to a priori regions of interest, thereby providing a regionally unbiased assessment of neuroanatomical differences on a point-by-point basis across the entire brain45. Because it can make use of the reported peak coordinates to recreate maps of the effect size of group differences, this method has been widely applied in many meta-analytic studies of neuropsychiatric disorders46, 47. Because this method has been described in detail elsewhere48, we describe below the primary analysis steps in brief. First, we ensured that the extracted coordinates from each study utilized the same threshold throughout the whole brain to avoid biases toward liberally thresholded brain regions. In cases in which the results were reported as both uncorrected and corrected for multiple comparisons, we analyzed the corrected results. Second, an effect-size signed map of the patient-control differences in GM was recreated for each study independently. Third, the mean map was generated by voxel-based calculation of the mean of all study maps and weighted by the inverse of each study variance to account for inter-study heterogeneity. We used the standard threshold (uncorrected p < 0.005, extent threshold of clusters > 10 voxels) recommended by the AES-SDM software developer, which was proposed to optimally balance the sensitivity and specificity of results. Other parameters included the following: anisotropy = 1.0; isotropic full-width at half-maximum (FWHM) = 20 mm; and peak height threshold = 1. MRIcron software (http://www.cabiatl.com/mricro/mricron/) was used to visualize AES-SDM maps by overlaying them onto a high-resolution brain template generated by the International Consortium for Brain Mapping.
Sensitivity analysis
A whole-brain jack-knife sensitivity analysis was used to test the replicability of the results using the same threshold as the pooled meta-analysis. We repeated the main analysis n times (n = the number of total datasets), each time discarding a different study, to determine whether the results of the pooled meta-analysis remained significant.
Subgroup analysis
We initially planned to conduct three subgroup analyses: male-only patient group vs. female-included patient group; middle-aged group (mean age = 40–60 years) vs. old-age group (mean age ≥ 60 years); and EDS group vs. non-EDS group. However, since the numbers of studies including females and elderly/non-EDS studies were insufficient to obtain reliable results, analyses of these subgroups were precluded.
Meta-regression analysis
Several relevant demographic and clinical factors, such as the percentage of male patients and the mean age, BMI, AHI, ODI, and ESS of patients, were applied in the regression analysis to explore their association with GM changes. To minimize the detection of spurious relationships, we set the probability threshold to p < 0.0005 in these analyses, as recommended by the software developer. The regression needed to be detected both in the slope and in one of the extremes of the regressors, and analyses were restricted to brain regions identified in the primary meta-analysis comparing patients and controls48.
Results
The search strategy initially identified 1,668 records; after screening, 17 studies16, 18,19,20,21,22,23, 27,28,29,30,31,32, 43, 49,50,51 met our inclusion criteria. Two additional studies were excluded because their coordinates were unavailable27, 51. One of the included studies, which was conducted by Huynh et al.19, separated the OSA patients into two groups: patients treated with a sham continuous positive airway pressure (CPAP) device and those treated with an active CPAP device. The authors separately compared the two groups of patients at baseline (i.e., before treatment) with the same healthy control group; thus, the respective resultant coordinates were included as separate datasets in our study. Therefore, 15 studies with 16 datasets containing 353 patients with OSA (321 males and 32 females; mean age, 49.3 years) and 444 healthy controls (330 males and 114 females; mean age, 52.0 years) were included in our meta-analysis. Each study was reviewed by at least 2 authors to obtain agreement on its clinical and demographic aspects, as summarized in Table 1. Between the OSA group and healthy controls, we detected no significant difference in mean age, and there was a sex ratio difference between patients with OSA and healthy controls in only one study50.
VBM meta-analysis
The meta-analysis revealed six brain regions with GM reductions in patients with OSA relative to healthy controls: (1) bilateral anterior cingulate/paracingulate gyri (ACG/ApCG, one cluster involving both brain sides); (2) bilateral superior frontal gyrus (SFG, medial rostral part) (one cluster involving both brain sides); (3) left cerebellum, hemispheric lobules IV/V; (4) left cerebellum, hemispheric lobule VIII; (5) right middle temporal gyrus (MTG); and (6) right premotor cortex (Table 2 and Fig. 2).
The meta-analysis revealed six brain regions showing reduced gray matter in patients with OSA compared with that in healthy controls. (a) Bilateral ACG/ApCG; (b) Bilateral SFG (medial rostral part); (c) Left cerebellum, hemispheric lobules IV/V; (d) Left cerebellum, hemispheric lobule VIII; (e) Right MTG; (f) right premotor cortex. Abbreviations: ACG/ApCG, anterior cingulate/paracingulate gyri; MTG, middle temporal gyrus; SFG, superior frontal gyrus.
Sensitivity analysis
The whole-brain jack-knife sensitivity analysis revealed that GM reductions in the bilateral ACG/ApCG and bilateral SFG (medial rostral part) were highly reliable and preserved throughout all 16 dataset combinations. The results in the left cerebellum, hemispheric lobule VIII were preserved in 15 dataset combinations. The other clusters failed to be repeated in two or three combinations, especially when the studies by Joo et al.21, Kim et al.18 and Morrell et al.31 were excluded (Table 3).
Subgroup analysis
In the male-only subgroup analysis of 9 datasets, the results remained largely unchanged. Compared with the pooled analysis of males and females, all clusters remained except two regions: the left cerebellum (lobule VIII) and right MTG (Table 3).
The results of the middle-aged subgroup (containing 15 datasets) and EDS subgroup (containing 9 datasets) analyses were both identical to the results of the pooled analysis (Table 3).
Meta-regression analysis
The GM in the cluster of bilateral ACG/ApCG was positively associated with age (r = 0.40, permutation-derived p < 0.0001, Fig. 3a) and BMI among patients with OSA (r = 0.60, permutation-derived p < 0.0001, Fig. 3b), and both relationships were primarily observed in four studies18, 21, 23, 43. The GM in the bilateral SFG (medial rostral part) was negatively associated with ESS scores (r = −0.39, permutation-derived p < 0.0005, Fig. 3c) and the percentage of male patients among patients with OSA (r = −0.28, permutation-derived p < 0.0001, Fig. 3d); these results were also primarily observed in four studies18, 21, 30, 43. No significant linear relationships were observed between neuroanatomic measures and the AHI or ODI scores.
Meta-regression results showed that the mean age (a) and BMI (b) of patients with OSA were positively correlated with gray matter in the bilateral ACG/ApCG and that the ESS scores of patients with OSA (c) and the percentages of male patients with OSA (d) were negatively correlated with gray matter in the bilateral SFG (medial rostral part). The effect sizes in this graph were extracted from the peak of maximum slope significance, and the dot size reflects the sample size of each study. Abbreviations: ACG/ApCG, anterior cingulate/paracingulate gyri; SFG, superior frontal gyrus.
Discussion
Our study identified consistent and robust regional GM alterations in patients with OSA compared with healthy controls. Additionally, to the best of our knowledge, this meta-analysis is the first to explore the potential effects of age, sex and important clinical characteristics on GM alterations due to OSA. Our main finding was that patients with OSA exhibited reduced GM in bilateral ACG/ApCG, bilateral SFG (medial rostral part), left cerebellum (hemispheric lobules IV/V and VIII), right MTG and right premotor cortex. These results partially overlapped with those of two previous meta-analyses, particularly the meta-analysis by Wang et al.47 (regions of the frontal and temporal lobes). Differences mainly existed regarding the cerebellum and some subcortical structures, which might have been detected given the larger number of studies included in our analysis. Our results remained similar when the analyses were repeated with the subgroups of male subjects, middle-aged subjects and subjects who scored equal or greater than 10 on the ESS scale, which supports the robustness of our results. Furthermore, the GM values from brain regions with GM alterations were significantly correlated with mean age, BMI, ESS score and percentage of male patients in the OSA group, suggesting that these GM abnormalities may be associated with these clinical factors.
Findings in the anterior cingulate/paracingulate gyri
The anterior cingulate cortex (ACC) is part of the limbic system, which is a crucial structure in humans that participates in the control of various autonomic, cognitive and emotional functions52. Although recent accounts of ACC function have placed more emphasis on its role in cognitive and emotional processes, other research has clearly established that the ACC is involved in central cardiovascular and respiratory control53. Respiratory arousal has been positively correlated with ACC thinning54, and response signals were altered in the ACC during Valsalva maneuvers and expiratory loading tasks in patients with OSA40, 41. GM reductions in the cingulate cortex have been consistently reported in patients with OSA18, 21, 23, 27. In one of our included VBM studies, GMV within the ACC in OSA patients was reduced before and after multi-level surgical treatment compared with that in controls, suggesting that the reduced GMV of this structure might be an irreversible deficit following OSA23. The ACC participates in attention and executive processes55, and an atrophic ACC may partially explain the cognitive deficits in patients with OSA. From a network perspective, the dorsal part of the ACC is a key node in the salience network (SN), which is involved in detecting, integrating and filtering relevant interoceptive, autonomic and emotional information56. The insula, which has been reported to be abnormal in OSA, is another key node in the SN. Several structural studies have demonstrated GM alternations in the insula in patients with OSA. However, the results have not been concordant across studies. For example, the insula has been reported to exhibit both significant GM atrophy18, 21 and enlargement22, 23. This evidence indicates a potential role of the SN in OSA. The paracingulate gyrus is adjacent to the cingulate gyrus, located at the border of Brodmann areas (BAs) 32 and 10. The exact function of this structure is less well understood, but the paracingulate gyrus has been related to the understanding of intentions during social interactions57. Further work is needed to explore this structure in OSA.
Although the current meta-analysis did not reveal alterations in other components of the limbic system, it is noteworthy that the hippocampus, parahippocampus and amygdala are three limbic structures commonly found to be abnormal in OSA studies, as reported in some of our included VBM studies16, 18, 21, 29, 30, 32, 49. The previous meta-analysis conducted by Tahmasian et al.33 revealed structural atrophy and functional disturbances in the right basolateral amygdala/hippocampus and the right central insula, suggesting an impact of OSA on these structures that might contribute to the cognitive and affective alterations seen in patients with the disorder. The “non-significance” of these three structures in the current meta-analysis was in part due to contradictory findings, e.g., the report by Fatouleh et al.22 of increased GMV in the left hippocampus.
Findings in the frontal lobe
In the current study, GM reductions were observed in two parts of the frontal lobe, including the medial rostral part of the SFG bilaterally and the right premotor cortex. Alteration in the SFG’s rostral and ventral extension to the orbital frontal cortex (OFC) suggests an important contribution of this region to the neurobehavioral aspects of OSA. The OFC is the ventromedial part of the frontal lobe and shares extensive connections with other parts of the prefrontal cortex, with the limbic, motor, premotor, sensory and association cortices, and with multiple subcortical regions58. This region of the ventromedial prefrontal cortex (VMPFC) appears vulnerable to sleep loss. For example, total overnight sleep deprivation was associated with reductions in cerebral glucose metabolism in the prefrontal cortex, which appeared to be particularly prominent in the VMPFC59. The OFC is a critical structure in the nervous system that facilitates cognitive functions and the integration of cognitive and emotional processes guiding decision-making60. According to behavioral data, sleep loss leads to impaired decision-making, increased risk-taking behaviors61 and emotional difficulties62. It is of note that the medial OFC is a component of the default mode network (DMN), which is typically more active during rest than during cognitive activity. Within this network, altered functional connectivity and regional brain activity were observed in the resting state63, and abnormal deactivation was observed during working memory tasks in patients with OSA36. Thus, the GM atrophy of the OFC identified in the current study may represent a brain change resulting from low sleep quality and hypoxia during sleep, leading to the cognitive deficits and emotional problems seen in patients with OSA.
Another region of GM alteration in the frontal lobe was located in right premotor cortex. In healthy humans, the right premotor cortex is important in voluntary behavior, including volitional inspiration64 and voluntary breathing65. However, in the apnea state, the functional activation of the premotor cortex is altered bilaterally66. The function of the premotor cortex is not limited to the motor domain; it is also active during executive cognitive processes67, 68. Sleep loss has also been shown to affect the functional activation in the premotor cortex during finger motor tasks69. Among patients with OSA, structural studies have demonstrated GM atrophy bilaterally21, and one study reported increased GMV in the left premotor cortex22.
Findings in the middle temporal lobe
The MTG is located on the lateral surface of the temporal lobe and is involved in multiple cognitive processes70. One tractography-based parcellation study suggested that the anterior subregion of the MTG is primarily involved in the DMN, sound recognition, and semantic retrieval; that the middle subregion is predominantly involved in semantic memory and semantic control networks; and that the posterior subregion is important for language. The sulcus defining the posterior MTG is associated with coding gaze and perception of motion in the visual field71. Structural and functional deficits in this region have been reported in epilepsy72, schizophrenia73 and major depression74. Many structural studies have reported GM reductions in the temporal lobe in patients with OSA18, 21, 27, 30, 31. According to functional studies, altered cerebral activation in the temporal cortex is significantly related to nocturnal hypoxemia duration (hemoglobin oxygen saturation levels < 90% during sleep) in OSA patients36, 75, and nocturnal hypoxemia is thought to be important in mediating cardiovascular damage76. In our study, the anterior part of the MTG, which is adjacent to the right temporal pole, was the region most consistently altered in OSA across different VBM studies. This finding overlaps with the results of the meta-analysis of studies on major depression conducted by Zhang et al.74 and is consistent with the view that alterations in this region may also contribute to emotional and cognitive changes in OSA. At the network level, the anterior MTG is traversed by abundant white matter tracts77, including the uncinate fasciculus, arcuate fasciculus, fornix, anterior commissure and inferior longitudinal fasciculus, which participate in different brain networks. For example, a resting-state fMRI study found that the anterior MTG exhibited positive connectivity with other components of the DMN, including the hippocampus, medial superior frontal cortex, and posterior cingulate cortex, as well as negative connectivity with frontotemporal brain areas71. Thus, deficits in this region may cause undesirable impacts on both intrinsic and integrated communications from the DMN, which would represent a significant alteration in resting brain neurophysiology.
Findings in the cerebellum
Findings in the left cerebellum also indicate the involvement of a motor-related network along with a cluster in the premotor cortex. Although the cerebellum is a crucial module in motor control systems, it is also connected to widespread brain regions, including cortical association areas and limbic structures78. Via these connections, the cerebellum participates in respiratory modulation79, sensorimotor activities, motor learning, emotional functions80, and even social functions81. The cerebellum is also important for maintaining sleep82, and sleep loss may disturb cerebellar function. Sleep deprivation disrupts fMRI signals from many brain regions, including the cerebellum during sensorimotor tasks83. Most neuroimaging studies have reported decreased GMV18, 20, 21, 30, 31 and elevated neural activation of the cerebellum in patients with OSA38, 84 compared with those in healthy controls, despite some inconsistencies22. Because the cerebellum is sensitive to carbon dioxide85 and because there is a negative correlation between cerebellar GMV and AHI or ODI20, our results might be associated with repetitive intermittent hypoxia or disturbed nocturnal sleep. According to our results, GM atrophy occurs in cerebellar hemisphere lobules IV, V, VI, VIII, and IX. Lobule VI is a crucial node in sensorimotor control86. Additionally, strong resting-state functional connectivity exists among different lobules and various brain networks, such as among lobule IX, vermal VIIIb and the DMN and among lobules VI, VIIb, and VIII and the SN87. Thus, the cerebellum is extremely important for the proper functioning of both motor and cognitive networks, and its alteration may contribute to behavioral changes associated with OSA.
A comparison of the findings of our study with those of two previous meta-analyses reveals partial overlap, indicating the involvement of the DMN, SN and limbic system in OSA. Therefore, our findings provide support for the involvement of these brain systems in OSA from a network perspective. Moreover, a prior review on OSA have highlighted that sleep disturbance and hypoxia could not explain all impacts on brain systems in ways that may contribute to cognitive deficits88. Our findings expand those observations by highlighting additional disturbances in brain regions that support affect processing. For example, while a recent review emphasized the importance of posterior DMN in OSA89, our review that includes more recently published studies highlights the importance of anterior DMN regions, which are regions more important for emotion modulation and motivation than sensory/perceptual processing.
Effects of demographic variation in GM changes
ESS scores were negatively correlated with GM in the bilateral OFC (patients who had higher ESS scores tended to have smaller GM values in the OFC). Additionally, the results of the EDS subgroup analysis were identical to those of the pooled analysis. The ESS score subjectively measures the severity of OSA and reflects the degree of daytime sleepiness with some limitations. Daytime sleepiness is a consequence of sleep loss during the night, and sleep loss per se may lead to abnormal brain activity in the ACC90, OFC91, and cerebellum92 or GM volume reductions in the cerebellum and MTG93. These abnormalities may consequently cause the cognitive and affective problems observed in patients with OSA. For example, sleepiness could predict vigilance performance in patients with OSA94, and the severity of EDS predicted incident depression in the patients95.
Interestingly, no correlation was found between AHI or ODI and GM changes. Previous VBM studies reported significant negative16, 20, positive19, 23 or no43 correlations between AHI/ODI and GMV in different brain areas. These findings suggest that the integrated influence of these divergent findings may have been balanced out in the meta-analysis, thus revealing no consistent pattern of effect. The present results are also in accordance with the viewpoint that both AHI and ODI can only partly explain the phenotypes of OSA96. While these scales are important for evaluating acute illness severity over recent days or weeks, they may not accurately reflect the severity of sleeplessness and hypoxia over months and years of OSA, which may be the primary cause of GM alterations.
In addition, although no study included in our meta-analysis used RDI, this index extends the notion of AHI by emphasizing the role of RERA, a condition of arousal from sleep caused by increased respiratory effort seen in some OSA patients. To our best of our knowledge, only one VBM study used both AHI (34 ± 20 events/h) and RDI (38 ± 24 events/h) to define OSA patients; however, the authors of that study did not perform a correlation analysis to examine the relationships between these two indexes and GMV changes27. Another study exploring the effect of obesity on brain diffusion found that both RDI and AHI showed positive correlations with the apparent diffusion coefficient (ADC) in several brain areas, although RDI showed a more extensive impact, suggesting that RDI might be more sensitive than AHI to microstructural changes97. Considering this finding, despite some debates regarding the role of RERA in OSA, future meta-analyses might benefit from comparing OSA patients with and without RERA once more studies using RDI are published. Another area where greater work is needed is the effort to resolve the long-standing debate about the relative importance and differential impacts of sleep apnea and sleep fragmentation in causing GM changes in patients with OSA.
Our regression analysis revealed a positive correlation between the age of the patient group and GM in the bilateral ACG. In prior OSA studies, the correlations between age and GMV were not consistent. Macey et al.27 reported that age was significantly and negatively correlated with the total GMV in controls but not in the patients. Torelli et al.32 reported a negative correlation between age and GMV in the hippocampus/amygdala in patients with OSA. One study reported a negative result21. Our regression analysis indicated that older patients tended to have less GM atrophy in the ACG. This finding may be related to the GM pattern changes that occur during normal aging. As most of the OSA patients included in our meta-analysis were middle-aged, the age range was much smaller than typically used in studies comparing age effects, and age may be confounded with illness duration, age associations raise issues that need to be addressed in longitudinal studies.
The BMI of patients with OSA was also positively associated with GM in the bilateral ACG. Obesity is related to OSA and sleep loss. Regarding the relationship between sleep loss and BMI, sleep loss can affect the connections in the SN when accompanied by a higher intake of dietary fat98. One recent study using a cohort of 1,333 OSA patients found that more than 80% of patients were overweight or obese, and obesity was a more important factor in contributing to OSA than sex (male)15. Although our regression analysis suggested a positive relationship between BMI and GMV among OSA patients, in another study, obese (BMI > 30) subjects (but not overweight (BMI = 25–30) subjects) exhibited GM atrophy in the ACG and OFC compared with normal-BMI subjects99. Therefore, the influence of body fat on brain GM should be clarified in future studies.
OSA is more prevalent among males and is likely more severe among males100. Our subgroup analysis of studies including only male participants yielded results similar to the analysis of male and female patients combined, except for two clusters (left cerebellum (lobule VIII) and right MTG) that were no longer significant in the male-only analysis. This result might be due to the smaller sample of patients, or it may suggest some sex differences in the brain impact of OSA. For example, the percentage of male patients was negatively correlated with GM in the OFC, indicating that a greater proportion of males in the patient group resulted in more GM atrophy in this region. This finding again underscores the importance of sex differences in OSA.
The above findings regarding demographic effects raise questions regarding the relevance of OSA phenotypes, which have largely been ignored by individual VBM studies but are more readily examined via meta-analyses. As there is still no consensus regarding standardized diagnostic criteria for OSA, with AHI cut-off criteria considered an interim standard101, many researchers regard OSA as a heterogeneous disease in terms of symptoms, etiology, comorbidities and outcomes. At present, factors including AHI, EDS, sex, age, obesity and race/ethnicity are all recognized as phenotypes or important factors impacting OSA102, 103. Our study demonstrated that these phenotypes based on clinical or demographic information had both significant (e.g., age and sex) and non-significant (e.g., AHI and ODI) relationships with GMV. The lack of relationships between GM measurements and current and recent clinical features of OSA raises the possibility that cumulative long-term effects, which appear variable in relation to demographic factors, may impact brain anatomy in a way that single-point assessments fail to reflect, especially for scales that rate clinical features during only the most recent few days.
Given the effects we observed, MRI may be suitable for clinical evaluations of OSA, especially for patients showing neuropsychiatric symptoms. Such changes may indicate a need for more aggressive therapy or support for therapeutic compliance to prevent further adverse brain changes that could significantly reduce quality of life and hopefully to reverse some abnormalities.
Limitations and Conclusions
Our study has some limitations. First, this meta-analysis was coordinate-based, and this approach has some inherent inaccuracies compared with image-based meta-analyses. Second, the results may be biased because different studies use different statistical thresholds and different criteria for OSA diagnoses. Third, the jack-knife sensitivity analysis revealed that the findings of GM reductions in the right premotor cortex, left cerebellar lobules and right MTG were less robust than alterations in other brain regions; thus, these findings should be interpreted with caution. Fourth, although most researchers carefully excluded comorbid diseases or sleep disorders and treatment history, participant heterogeneity remained that may have biased the results, such as those regarding the severity, duration and cause of OSA; the degree of nocturnal desaturation; the presence of predominant apneas or hypopneas; and sleep quality. Fifth, the small cohorts of some of the included studies might have biased the results.
Taken together, we identified GM reductions in the limbic lobe, frontal lobe, temporal lobe, and cerebellum in OSA patients compared with those in healthy controls. The most reliable findings were seen in the bilateral ACG/ApCG and bilateral SFG (medial rostral part). Additionally, the age, BMI, ESS, and sex (male) of the patient group were associated with GM atrophy. The intra- and inter-networks of DMN, SN, and motor networks and the limbic system were observed in OSA. Further exploration is required to verify our conclusions through multimodality neuroimaging studies and longitudinal studies and to determine their clinical relevance for clinical practice in the diagnosis and management of patients with OSA. Of note, this study adds to Psychoradiology (https://radiopaedia.org/articles/psychoradiology), an emerging subspecialty of radiology, which seems primed to play a major clinical role in guiding diagnostic and treatment planning decisions in patients with mental disorder104, 105.
References
Al Lawati, N. M., Patel, S. R. & Ayas, N. T. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog. Cardiovasc. Dis. 51, 285–293 (2009).
Heinzer, R., Marti-Soler, H. & Haba-Rubio, J. Prevalence of sleep apnoea syndrome in the middle to old age general population. Lancet Resp. Med. 4, e5–e6, doi:10.1016/S2213-2600(16)00006-0 (2016).
Jordan, A. S., McSharry, D. G. & Malhotra, A. Adult obstructive sleep apnoea. Lancet 383, 736–747 (2014).
Beebe, D. W., Groesz, L., Wells, C., Nichols, A. & McGee, K. The neuropsychological effects of obstructive sleep apnea: A meta-analysis of norm-referenced and case-controlled data. Sleep 26, 298–307 (2003).
Kerner, N. A. & Roose, S. P. Obstructive sleep apnea is linked to depression and cognitive impairment: Evidence and potential mechanisms. Am. J. Geriatr. Psychiatry 24, 496–508 (2016).
Emamian, F. et al. The association between obstructive sleep apnea and alzheimer’s disease: A meta-analysis perspective. Front. Aging Neurosci. 8, 78, doi:10.3389/fnagi.2016.00078 (2016).
Flemons, W. W. et al. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22, 667–689 (1999).
Berry, R. B. et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 8, 597–619 (2012).
Schwartz, A. R. et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am. Rev. Respir. Dis. 144, 494–498 (1991).
Johns, M. W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14, 540–545 (1991).
Tonon, C. et al. Proton magnetic resonance spectroscopy study of brain metabolism in obstructive sleep apnoea syndrome before and after continuous positive airway pressure treatment. Sleep 30, 305–311 (2007).
Uysal, A. et al. Nocturnal hypoxemia biomarker predicts sleepiness in patients with severe obstructive sleep apnea. Sleep Breath. 18, 77–84 (2014).
Tanaka, S. & Shima, M. Assessment of screening tests for sleep apnea syndrome in the workplace. J. Occup. Health 52, 99–105 (2010).
Fietze, I. et al. Night-to-night variation of the oxygen desaturation index in sleep apnoea syndrome. Eur. Respir. J. 24, 987–993 (2004).
Ernst, G. et al. Difference between apnea-hypopnea index (AHI) and oxygen desaturation index (ODI): Proportional increase associated with degree of obesity. Sleep Breath. 1–9, doi:10.1007/s11325-016-1330-3 (2016).
Canessa, N. et al. Obstructive sleep apnea: Brain structural changes and neurocognitive function before and after treatment. Am. J. Respir. Crit. Care Med. 183, 1419–1426 (2011).
Morrell, M. J. & Glasser, M. The brain in sleep-disordered breathing a vote for the chicken? Am. J. Respir. Crit. Care Med. 183, 1292–1294 (2011).
Kim, H. et al. Effects of long-term treatment on brain volume in patients with obstructive sleep apnea syndrome. Hum. Brain Mapp. 37, 395–409 (2016).
Huynh, N. T., Prilipko, O., Kushida, C. A. & Guilleminault, C. Volumetric brain morphometry changes in patients with obstructive sleep apnea syndrome: Effects of CPAP treatment and literature review. Front. Neurol. 5, 58–58 (2014).
Celle, S. et al. Undiagnosed sleep-related breathing disorders are associated with focal brainstem atrophy in the elderly. Hum. Brain Mapp. 30, 2090–2097 (2009).
Joo, E. Y. et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep 33, 235–241 (2010).
Fatouleh, R. H. et al. Functional and structural changes in the brain associated with the increase in muscle sympathetic nerve activity in obstructive sleep apnoea. Neuroimage Clin. 6, 275–283 (2014).
Lin, W. C. et al. Longitudinal brain structural alterations and systemic inflammation in obstructive sleep apnea before and after surgical treatment. J. Transl. Med. 14, 139 (2016).
Zimmerman, M. E. & Aloia, M. S. A review of neuroimaging in obstructive sleep apnea. J. Clin. Sleep Med. 2, 461–471 (2006).
Celle, S. et al. Desperately seeking grey matter volume changes in sleep apnea: A methodological review of magnetic resonance brain voxel-based morphometry studies. Sleep Med. Rev. 25, 112–120 (2016).
Weng, H. H. et al. Mapping gray matter reductions in obstructive sleep apnea: An activation likelihood estimation meta-analysis. Sleep 37, 167–175 (2014).
Macey, P. M. et al. Brain morphology associated with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 166, 1382–1387 (2002).
O’Donoghue, F. J. et al. Cerebral structural changes in severe obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 171, 1185–1190 (2005).
Morrell, M. J. & Twigg, G. Neural consequences of sleep disordered breathing: The role of intermittent hypoxia. Adv. Exp. Med. Biol. 588, 75–88 (2006).
Yaouhi, K. et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J. Sleep Res. 18, 36–48 (2009).
Morrell, M. J. et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax 65, 908–914 (2010).
Torelli, F. et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage 54, 787–793 (2011).
Tahmasian, M. et al. Structural and functional neural adaptations in obstructive sleep apnea: An activation likelihood estimation meta-analysis. Neurosci. Biobehav. Rev. 65, 142–156 (2016).
Li, H. J. et al. Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI. Neuropsychiatr. Dis. Treat. 11, 207–214 (2015).
Santarnecchi, E. et al. Altered cortical and subcortical local coherence in obstructive sleep apnea: A functional magnetic resonance imaging study. J. Sleep Res. 22, 337–347 (2013).
Prilipko, O. et al. Task positive and default mode networks during a parametric working memory task in obstructive sleep apnea patients and healthy controls. Sleep 34, 293–301 (2011).
Ayalon, L., Ancoli-Israel, S., Aka, A. A., McKenna, B. S. & Drummond, S. P. A. Relationship between obstructive sleep apnea severity and brain activation during a sustained attention task. Sleep 32, 373–381 (2009).
Ayalon, L., Ancoli-Israel, S., Klemfuss, Z., Shalauta, M. D. & Drummond, S. P. Increased brain activation during verbal learning in obstructive sleep apnea. Neuroimage 31, 1817–1825 (2006).
Macey, K. E. et al. Inspiratory loading elicits aberrant fMRI signal changes in obstructive sleep apnea. Respir. Physiol. Neurobiol. 151, 44–60 (2006).
Macey, P. M. et al. Functional magnetic resonance imaging responses to expiratory loading in obstructive sleep apnea. Respir. Physiol. Neurobiol. 138, 275–290 (2003).
Henderson, L. A. et al. Neural responses during Valsalva maneuvers in obstructive sleep apnea syndrome. J. Appl. Physiol. (1985) 94, 1063–1074 (2003).
Harper, R. M. et al. fMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J. Appl. Physiol. (1985) 94, 1583–1595 (2003).
Zhang, Q. et al. Altered resting-state brain activity in obstructive sleep apnea. Sleep 36, 651–659 (2013).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 62, e1–e34, doi:10.1016/j.jclinepi.2009.06.006 (2009).
Yang, X. et al. Brain gray matter alterations and associated demographic profiles in adults with autism spectrum disorder: A meta-analysis of voxel-based morphometry studies. Aust. N. Z. J. Psychiatry 50, 741–753 (2016).
Radua, J. et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry 27, 605–611 (2012).
Wang, X. L. et al. Neural correlates during working memory processing in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 56, 101–108 (2015).
Radua, J. & Mataix-Cols, D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry 195, 393–402 (2009).
Morrell, M. J. et al. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 4, 451–454 (2003).
Innes, C. R., Kelly, P. T., Hlavac, M., Melzer, T. R. & Jones, R. D. Decreased regional cerebral perfusion in moderate-severe obstructive sleep apnoea during wakefulness. Sleep 38, 699–706 (2015).
Chan, K. C. et al. Neurocognitive dysfunction and grey matter density deficit in children with obstructive sleep apnoea. Sleep Med. 15, 1055–1061 (2014).
Critchley, H. D. et al. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 126, 2139–2152 (2003).
Frysinger, R. C. & Harper, R. M. Cardiac and respiratory relationships with neural discharge in the anterior cingulate cortex during sleep-waking states. Exp. Neurol. 94, 247–263 (1986).
Joo, E. Y., Jeon, S., Kim, S. T., Lee, J. M. & Hong, S. B. Localized cortical thinning in patients with obstructive sleep apnea syndrome. Sleep 36, 1153–1162 (2013).
Carter, C. S., Botvinick, M. M. & Cohen, J. D. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev. Neurosci. 10, 49–57 (1999).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007).
Walter, H. et al. Understanding intentions in social interaction: The role of the anterior paracingulate cortex. J. Cogn. Neurosci. 16, 1854–1863 (2004).
Cavada, C., Compañy, T., Tejedor, J., Cruz-Rizzolo, R. J. & Reinoso-Suárez, F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb. Cortex 10, 220–242 (2000).
Thomas, M. et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J. Sleep Res. 9, 335–352 (2000).
Cavada, C. & Schultz, W. The mysterious orbitofrontal cortex. Foreword. Cereb. Cortex 10, 205 (2000).
Killgore, W. D. S., Lipizzi, E. L., Kamimori, G. H. & Balkin, T. J. Caffeine effects on risky decision making after 75 hours of sleep deprivation. Aviat. Space Environ. Med. 78, 957–962 (2007).
Tempesta, D., De Gennaro, L., Natale, V. & Ferrara, M. Emotional memory processing is influenced by sleep quality. Sleep Med. 16, 862–870 (2015).
Li, H. J. et al. Abnormal resting-state functional connectivity within the default mode network subregions in male patients with obstructive sleep apnea. Neuropsychiatr. Dis. Treat. 12, 203–212 (2016).
Evans, K. C., Shea, S. A. & Saykin, A. J. Functional MRI localisation of central nervous system regions associated with volitional inspiration in humans. J. Physiol. 520(Pt 2), 383–392 (1999).
McKay, L. C., Evans, K. C., Frackowiak, R. S. & Corfield, D. R. Neural correlates of voluntary breathing in humans. J. Appl. Physiol. (1985) 95, 1170–1178 (2003).
Kimmerly, D. S., Morris, B. L. & Floras, J. S. Apnea-induced cortical BOLD-fMRI and peripheral sympathoneural firing response patterns of awake healthy humans. PLoS One 8, e82525, doi:10.1371/journal.pone.0082525 (2013).
Sweeney, J. A. et al. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J. Neurophysiol. 75, 454–468 (1996).
Wager, T. D. & Smith, E. E. Neuroimaging studies of working memory: A meta-analysis. Cogn. Affect Behav. Neurosci. 3, 255–274 (2003).
Fischer, S., Nitschke, M. F., Melchert, U. H., Erdmann, C. & Born, J. Motor memory consolidation in sleep shapes more effective neuronal representations. J. Neurosci. 25, 11248–11255 (2005).
Davey, J. et al. Exploring the role of the posterior middle temporal gyrus in semantic cognition: Integration of anterior temporal lobe with executive processes. Neuroimage 137, 165–177 (2016).
Xu, J. et al. Tractography-based parcellation of the human middle temporal gyrus. Sci. Rep. 5, 18883 (2015).
Song, S. et al. Frequency specificity of fMRI in mesial temporal lobe epilepsy. PLoS One 11, e0157342, doi:10.1371/journal.pone.0157342 (2016).
Lencer, R. et al. Altered transfer of visual motion information to parietal association cortex in untreated first-episode psychosis: Implications for pursuit eye tracking. Psychiatry Res 194, 30–38 (2011).
Zhang, H. et al. Brain gray matter alterations in first episodes of depression: A meta-analysis of whole-brain studies. Neurosci. Biobehav. Rev. 60, 43–50 (2016).
Prilipko, O. et al. The effects of CPAP treatment on task positive and default mode networks in obstructive sleep apnea patients: An fMRI study. PLoS One 7, e47433, doi:10.1371/journal.pone.0047433 (2012).
Xie, J. et al. Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J. Am. Heart Assoc. 5, doi:10.1161/jaha.115.003162 (2016).
Catani, M. & Thiebaut de Schotten, M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44, 1105–1132 (2008).
Schmahmann, J. D. & Pandyat, D. N. The cerebrocerebellar system. Int. Rev. Neurobiol. 41, 31–60 (1997).
Xu, F. & Frazier, D. T. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum 1, 35–40 (2002).
Strick, P. L., Dum, R. P. & Fiez, J. A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 32, 413–434 (2009).
Jack, A. & Pelphrey, K. A. Neural correlates of animacy attribution include neocerebellum in healthy adults. Cereb. Cortex 25, 4240–4247 (2015).
DelRosso, L. M. & Hoque, R. The cerebellum and sleep. Neurol. Clin. 32, 893–900 (2014).
Gazes, Y. et al. Dual-tasking alleviated sleep deprivation disruption in visuomotor tracking: An fMRI study. Brain Cogn. 78, 248–256 (2012).
Peng, D. C. et al. Altered intrinsic regional brain activity in male patients with severe obstructive sleep apnea: A resting-state functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 10, 1819–1826 (2014).
Parsons, L. M. et al. Neuroimaging evidence implicating cerebellum in the experience of hypercapnia and hunger for air. Proc. Natl. Acad. Sci. USA 98, 2041–2046 (2001).
Belkhiria, C. et al. Exploration and identification of cortico-cerebellar-brainstem closed loop during a motivational-motor task: An fMRI study. Cerebellum doi:10.1007/s12311-016-0801-1 (2016).
Sang, L. et al. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 61, 1213–1225 (2012).
Olaithe, M., Bucks, R. S., Hillman, D. R. & Eastwood, P. R. Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med. Rev. doi:10.1016/j.smrv.2017.03.005(2017).
Khazaie, H. et al. Functional reorganization in obstructive sleep apnoea and insomnia: A systematic review of the resting-state fMRI. Neurosci. Biobehav. Rev. 77, 219–231 (2017).
Wu, J. et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am. J. Psychiatry 156, 1149–1158 (1999).
Gao, L. et al. Frequency-dependent changes of local resting oscillations in sleep-deprived brain. PLoS One 10, e0120323, doi:10.1371/journal.pone.0120323 (2015).
Bell-McGinty, S. et al. Identification and differential vulnerability of a neural network in sleep deprivation. Cereb. Cortex 14, 496–502 (2004).
Liu, C., Kong, X.-z., Liu, X., Zhou, R. & Wu, B. Long-term total sleep deprivation reduces thalamic gray matter volume in healthy men. Neuroreport 25, 320–323 (2014).
Adams, N., Strauss, M., Schluchter, M. & Redline, S. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am. J. Respir. Crit. Care Med. 163, 1626–1631 (2001).
LaGrotte, C. et al. The relative association of obstructive sleep apnea, obesity and excessive daytime sleepiness with incident depression: A longitudinal, population-based study. Int. J. Obes. (Lond.) 40, 1397–1404 (2016).
Saaresranta, T. et al. Clinical phenotypes and comorbidity in european sleep apnoea patients. PLoS One 11, e0163439, doi:10.1371/journal.pone.0163439 (2016).
Kilicarslan, R. et al. The effect of obesity on brain diffusion alteration in patients with obstructive sleep apnea. Sci. World J. 2014, 768415, doi:10.1155/2014/768415 (2014).
Fang, Z. et al. Altered salience network connectivity predicts macronutrient intake after sleep deprivation. Sci. Rep. 5, 8215 (2015).
Raji, C. A. et al. Brain structure and obesity. Hum. Brain Mapp. 31, 353–364 (2010).
Deng, X. et al. Age-group-specific associations between the severity of obstructive sleep apnea and relevant risk factors in male and female patients. PLoS One 9, e107380, doi:10.1371/journal.pone.0107380 (2014).
Eastwood, P. R. et al. Obstructive sleep apnoea: From pathogenesis to treatment: Current controversies and future directions. Respirology (Carlton, Vic.) 15, 587–595 (2010).
Zinchuk, A. V., Gentry, M. J., Concato, J. & Yaggi, H. K. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med. Rev. doi:10.1016/j.smrv.2016.10.002 (2016).
Subramani, Y. et al. Understanding phenotypes of obstructive sleep apnea: Applications in anesthesia, surgery, and perioperative medicine. Anesth Analg 124, 179–191 (2017).
Lui, S., Zhou, X. J., Sweeney, J. A. & Gong, Q. Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology 281, 357-372 (2016).
Kressel, H. Y. Setting Sail: 2017. Radiology 282, 4–6 (2017).
Acknowledgements
This study was supported by the National Natural Science Foundation (Grant Nos 81621003, 81761128023, 81220108013, 81227002, 81030027 and 81401398) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT16R52) of China. Dr. Gong would also like to acknowledge the support from his American CMB Distinguished Professorship Award (Award No. F510000/G16916411) administered by the Institute of International Education, USA. The authors want to thank Miss Lei Zhang and Dr. Jianling Gu from Departement of Sociology and Psychology, School of Public Administration, Sichuan University, for their helpful assisstance to the present study.
Author information
Authors and Affiliations
Contributions
Qiyong Gong contributed to the conception of the study. Yan Shi, Lizhou Chen and Qiyong Gong contributed significantly to the analysis and manuscript preparation. Yan Shi and Lizhou Chen performed the data analyses, wrote the manuscript and prepared Tables 1–3. Taolin Chen prepared Figs 1–3. Lei Li, Jing Dai, Xiaoqi Huang, Su Lui, Taolin Chen and John A. Sweeney helped perform the analysis with constructive discussions. John A. Sweeney helped improve the manuscript for clarity and readability. All authors reviewed the article and approved its publication.
Corresponding author
Ethics declarations
Competing Interests
Dr. Sweeney is a consultant for Takada Pharmaceuticals. None of the remaining authors have financial conflicts of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, Y., Chen, L., Chen, T. et al. A Meta-analysis of Voxel-based Brain Morphometry Studies in Obstructive Sleep Apnea. Sci Rep 7, 10095 (2017). https://doi.org/10.1038/s41598-017-09319-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09319-6
This article is cited by
-
The Association of Upper Airway Anatomy with Brain Structure: The Multi-Ethnic Study of Atherosclerosis
Brain Imaging and Behavior (2024)
-
Association between the Pittsburgh sleep quality index and white matter integrity in healthy adults: a whole-brain magnetic resonance imaging study
Sleep and Biological Rhythms (2023)
-
Deep Learning Analyses of Brain MRI to Identify Sustained Attention Deficit in Treated Obstructive Sleep Apnea: A Pilot Study
Sleep and Vigilance (2022)
-
18F-FDG PET, cognitive functioning, and CSF biomarkers in patients with obstructive sleep apnoea before and after continuous positive airway pressure treatment
Journal of Neurology (2022)
-
Associations between frontal lobe structure, parent-reported obstructive sleep disordered breathing and childhood behavior in the ABCD dataset
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.