Abstract
The honey bee is of paramount importance to humans in both agricultural and ecological settings. Honey bee colonies have suffered from increased attrition in recent years, stemming from complex interacting stresses. Defining common cellular stress responses elicited by these stressors represents a key step in understanding potential synergies. The proteostasis network is a highly conserved network of cellular stress responses involved in maintaining the homeostasis of protein production and function. Here, we have characterized the Heat Shock Response (HSR), one branch of this network, and found that its core components are conserved. In addition, exposing bees to elevated temperatures normally encountered by honey bees during typical activities results in robust HSR induction with increased expression of specific heat shock proteins that was variable across tissues. Surprisingly, we found that heat shock represses multiple immune genes in the abdomen and additionally showed that wounding the cuticle of the abdomen results in decreased expression of multiple HSR genes in proximal and distal tissues. This mutually antagonistic relationship between the HSR and immune activation is unique among invertebrates studied to date and may promote understanding of potential synergistic effects of disparate stresses in this critical pollinator and social insects more broadly.
Similar content being viewed by others
Introduction
Honey bee colonies in the United States have suffered from a higher than usual rate of mortality in the last few years with a complex set of interacting stresses playing a key role. Some stresses thought to be involved include nutritional stress due to loss of appropriate forage, chemical poisoning from pesticides, changes to normal living conditions brought about through large-scale beekeeping practices, and infection by pathogenic microbes1. In seeking to understand how stresses might synergize to impact honey bee health, efforts have been undertaken to more completely define common cellular processes and cell stress pathways that are impacted by multiple stressors.
One such process is proteostasis, which refers to the homeostasis of protein synthesis, folding, function, and degradation both within a cell and in an organism as a whole2. A number of normal and pathologic conditions can lead to disruption of proteostasis. This creates a build-up of unfolded proteins in the cell, triggering a suite of responses designed to limit resulting damage and return the cell to homeostasis2. Within individual cells, proteostasis is maintained by the cellular stress responses of the proteostatic network. These responses include the Heat Shock Response (HSR)3, 4 responding to proteostatic disruption in the cytoplasm, the endoplasmic reticulum Unfolded Protein Response (UPRER) responding to proteostatic perturbation in the endoplasmic reticulum5, and the mitochondrial Unfolded Protein Response (UPRmt) responding to proteostatic perturbation in the mitochondria6. As conditions leading to unfolded proteins can be caused by perturbation of multiple cellular processes and pathways, the proteostastic network provides an optimal hub for sensing and responding to cellular stresses of myriad origin.
The HSR has been well characterized in the invertebrate models Drosophila melanogaster and Caenorhabditis elegans 2, 4. Due to their particular lifestyle, honey bees are exposed to significant routine proteostatic stressors suggesting that the HSR might have unique properties in these insects. Colony-level homeostatic regulation of hive temperature is a key feature of honey bee colony function7,8,9,10,11. Hive temperature is carefully maintained between 32° and 35 °C during normal conditions in colonies in temperate regions. This narrow temperature range is important for brood development and normal colony function12,13,14,15. Complex individual behaviors, including endothermic shivering to increase temperature, are utilized by individual bees to maintain this relatively constant temperature10 (and references therein). In maintaining this narrow range of hive temperature and in performing other specialized tasks, the temperature of individual bees can increase significantly above steady-state to levels that are dangerous to other organisms. For example, the temperature of individual forager bees can reach up to 49 °C in flight16. Honey bees appear highly resistant to heat-shock17,18,19, yet experimental evidence to date is contradictory with regards to the presence of a robust heat-shock response in honey bees17, 18, 20 and our understanding of the specific molecular components involved in performance and regulation of this response is incomplete.
Considering the many known microbial threats to the honey bee, including bacterial, viral, fungal, and parasitic21, we were particularly interested in how the HSR might interact with honey bee immune activation. In studies of invertebrates to date, evidence supports the existance of a positive reciprocal relationship between the HSR and immune activation22. Immune competence, resistance to infection, and levels of immune mediators all increase with temperature and heat shock. In return, infection enhances temperature stress tolerance and expression of HSR target genes. However, it is likely that the individual response to interacting stresses in solitary insects, in which these prior studies were performed, and eusocial insects, such as the honey bee, are divergent. One of the key features leading to the ‘resilience’ of eusocial organisms appears to be the ability to sacrifice non-reproductive individuals for the benefit of the colony23. Thus, individual worker honey bees may have different stress response characteristics in the context of the colony, especially when confronted simultaneously by multiple stresses.
Here, we demonstrate that the core components and function of the HSR pathway are conserved in the honey bee. In addition, our results demonstrate that heat shock decreases the expression of specific immune effectors, namely the antimicrobial peptide genes Hymenoptaecin, Defensin 1, and Abaecin. Inversely, wounding of the abdomen results in decreased expression of multiple HSR target genes in proximal and distal tissues, revealing a mutually antagonistic relationship between the HSR and immune activation in honey bees. This relationship is unique among invertebrates studied to date and may have important consequences for understanding potential synergistic effects of disparate stresses in this critical pollinator and social insects more broadly.
Results
The HSR pathway is conserved in honey bees
We first examined the honey bee genome and identified the gene encoding the canonical Heat Shock Factor (HSF), which is the transcriptional regulator of the HSR, and is found in most eukaryotes. Similarly to D. melanogaster 24, honey bees possess a single gene encoding HSF. Recent work has identified a core set of HSF-dependent genes in the yeast Saccharomyces cerevisiae 25 and in mammals25, 26. We used these to identify putative homologs (Table 1) and found that A. melliferra possess apparent homologs for all of the proposed transcriptional targets of the pathway except Dedd2. However, some differences were evident. Honey bees possess two Hsp90 genes as reported before, Hsp90 and Hsp83l 27. In addition, we found that the honey bee possesses two Hsp70 genes that correspond to three Hsp70 genes (Hspa1a, Hsp70Ab/Hspa1, and Hspa8) described as HSF-core targets in mammals. We also used information in D. melanogaster and Homo sapiens to identify other genes encoding chaperone proteins of the HSP70, HSP90, DNAJ-containing, and alpha-crystallin/sHSP families in the A. mellifera genome. The honey bee genome possesses three additional genes encoding HSP70 in addition to the ones shown in Table 1, including Hsc70-3, Hsc70-5, and Hyuop1-like (all genes encoding HSP70 proteins are listed in Suppl. Table 2). There are two additional genes encoding HSP90 proteins in the honey bee genome in addition to the ones shown in Table 1 (all genes encoding HSP90 proteins are listed in Suppl. Table 3). In addition to the ones shown in Table 1, the honey bee genome also contains 25 further genes encoding proteins containing DNAJ motifs (all genes encoding DNAJ-containing proteins are listed in Suppl. Table 4). There are thirteen genes encoding proteins containing the alpha-crystallin domain characteristic of small heat shock proteins in the honey bee genome (all genes encoding alpha-crystallin domain-containing proteins are listed in Suppl. Table 5). These proteins play an important ‘first line’ role in maintaining proteostasis28. In addition to Hsp10 (shown in Table 1), the honey bee genome has the larger subunit of the mitochondrial, Group I Chaperonin, Hsp60. In eukaryotic organisms, the proteins of the Group II Chaperonin, the TCP-1 Ring Complex (TRiC) are also involved in folding29, and the honey bee possesses genes encoding all eight expected subunits (all genes encoding chaperonin proteins are listed in Suppl. Table 6).
Heat-shock induces a classic HSR in honey bees
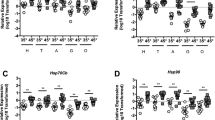
We examined heat-shock dependent induction of putative heat-shock genes in head tissue (predominantly brain and sensory organ tissue, n = 6), midgut (n = 6), thorax tissue (predominantly flight muscle, n = 6), and abdominal wall tissue (predominantly fat body, n = 6 for 35° and n = 5 for 45°) at 35° and 45 °C for 4 hours. Relative to β-actin, we observed robust induction of the homologs of the core HSF target genes, Hsc70-4, Hsp70Ab, Hsp90, Hsp70Cb, Dnaja1, and Hspe1 in all tissues examined (Fig. 1). However, we did not observe induction of the other Hsp90 family member gene, Hsp83l (Fig. 1). Additionally, DnaJ protein homolog 1-like (Dnaj1) was only induced in the head tissue (Fig. 1). β-actin levels were similar irrespective of temperature as assessed by Ct values (Suppl Figure 2). As expected, the transcriptional regulator, Hsf, was not induced after heat shock (Suppl Figure 2). In addition, although we observed a robust induction of these heat-shock target genes in all four tissues, the magnitude of induction differed between tissues.
Core HSR target genes are induced during Heat-Shock. Transcript levels of putative core HSR target genes Hsc70-4, Hsp70Ab, Hsp90, Hsp83l, Hsp70Cb, Hsp10, Dnaj1, and Dnaja1 relative to β-actin in head tissue (H, predominantly brain and sensory organ tissue), midgut (G), thorax tissue (T, predominantly flight muscle), and abdominal wall tissue (A, predominantly fat body) from adult bees captured at the landing board and maintained for four hours in cages at either 35° or 45 °C. Symbols represent expression values of the genes of interest calculated using the ΔΔCT method for individual bees. Mean ± SEM is also shown. Statistical significance was assessed using unpaired t-tests with Welch’s and is noted as *p < 0.05, and **p < 0.01.
Targets of the broader proteostasis network are induced in response to heat-shock
We also explored potential targets from the broader proteostasis network. Hsc70-3, which is a characterized target of the Unfolded Protein Response (UPR), was upregulated by heat shock (Fig. 2). Thus, we wondered if other UPR target genes would be induced as well. Using previously identified honey bee UPR target genes30, we found that a subset of these, Gp93 and p58ipk, (induction in the abdomen displayed a strong trend towards significance, p = 0.082), were triggered by heat shock in most honey bee tissues (Fig. 2). We also found that a greater number of UPR target genes were upregulated in only one or two tissues (Suppl Figure 3). The ER-localized GRP170 homolog, Hyuop1-like, was not affected by heat shock (Suppl Figure 3). We wished to determine whether heat shock induction of UPRER target genes was occurring in conjunction with UPR pathway activation or in a potentially UPR-independent manner. IRE1, a transmembrane receptor involved in one of three UPRER pathways, is usually bound to the ER chaperone HSC70-3 and maintained in a monomeric, inactive form. Upon increase of unfolded proteins in the lumen of the ER, IRE1 is activated by loss of HSC70-3 binding. This leads to IRE1 multimerization and ultimately to activation of its endonuclease domain, which performs non-canonical splicing of the mRNA encoding the bZIP transcription factor XBP1. In its unspliced form, the Xbp1 mRNA (Xbp1u) encodes a truncated protein (XBP1u) with low transactivation activity. Splicing removes a short sequence containing an in-frame stop codon, leading to the translation of the new transcript (Xbp1s), which encodes a longer form of XBP1 (XBP1s) with enhanced transactivation activity, leading to target gene induction. We previously characterized the IRE1 pathway in honey bees and developed a method for measuring its activation. Examination of Xbp1 mRNA splicing in the midguts of bees exposed to heat-shock revealed that neither bees caged at 35 °C nor those incubated at 45 °C had detectable Xbp1 mRNA splicing (Suppl. Figure 3B) after 4 hours, at a time when UPR targets are transcriptionally upregulated.
Select UPRER, UPRmt, and broader cytoplamsic target genes are induced during heat shock. Graphical schematic of broader targets examined (upper left). Transcript levels of UPRER targets genes Hsc70-3, Gp93, p58ipk, the UPRmt targets genes Hsc70-5, Hsp60, and TRiC genes representing the Tricε and Tricδ subunits relative to relative to β-actin in head tissue (H, predominantly brain and sensory organ tissue), midgut (G), thorax tissue (T, predominantly flight muscle), and abdominal wall (A, predominantly fat body) from adult bees captured at the landing board and maintained for four hours in cages at either 35° or 45 °C. Symbols represent expression values of the genes of interest calculated using the ΔΔCT method for individual bees. Mean ± SEM is also shown. Statistical significance was assessed using unpaired t-tests with Welch’s and is noted as *p < 0.05, and **p < 0.01.
In addition, as the mitochondrially localized Hsp10 was upregulated by heat shock (Fig. 1F), we wished to determine whether other UPRmt targets were activated during the heat shock response in honey bees. Heat stress affects protein-folding in the mitochondria and activates the UPRmt 31. Examining other putative honey bee UPRmt target genes, we found that two targets, Hsc70-5 and Hsp60, were induced by this treatment (Fig. 2). Two other UPRmt targets, the HSP90 family member Trap1 and the DNAJ-containing protein Tim14, were not activated during the heat shock response in honey bees (Suppl. Figure 4). While the subunits of the TRiC complex are also involved in folding, they are not upregulated by the HSR upon cytoplasmic proteostatic stress in other organisms32. However, we found that the T-complex chaperonin epsilon (Tricε) (induction in the abdomen displayed a strong trend towards significance, p = 0.056)) and T-complex chaperonin delta (Tricδ) (induction in the abdomen displayed a strong trend towards significance, p = 0.088), subunits were induced in most tissues when bees were exposed to 45 °C (Fig. 2). By contrast, the T-complex chaperonin eta (Tricη) and T-complex chaperonin thelta (Tricθ) subunits were not induced (Suppl Figure 4). In addition to those already tested, we assessed the effect of heat shock on 4 other genes encoding DNAJ proteins based on the localization and known client binding characteristics of their human homologs as described in Kampinga et al.33, testing all the cytoplasmically localized DNAJ-containing proteins with promiscuous or wide client binding function, DnajA3, DnajB11, DnajB12, DnajB6 (Suppl Figure 4).
Proteasome-inhibitors induce HSR in honey bees
Inhibition of the proteasome causes a build-up of misfolded proteins in the cytoplasmic compartment and is commonly used to trigger HSR in a variety of model organisms24. Therefore, we examined heat shock-independent induction of cytoplasmic chaperones in midguts from bees treated with the proteosome inhibitor MG132, which has been used in other invertebrates34, 35, for 24 hours. Relative to β-actin, we observed robust induction of the cytoplasmic Hsp70 family member, Hsc70-4 (Hsp68), when bees were exposed to 500 µM MG132 (n = 9) compared to untreated bees (n = 6) (Fig. 3A).
Pharmacological induction of the HSR. Individual levels of cytoplasmic chaperone Hsc70-4 transcripts relative to β-actin in midgut tissue after MG132 (A) or 17AAG (B) treatment relative to untreated bees. Bar and error bars represent the Mean ± SEM for expression values of the genes of interest calculated using the ΔΔCT method. Statistical significance was assessed using unpaired t-tests with Welch’s and is noted as *p < 0.05, and **p < 0.01.
Honey bees HSR is regulated by HSP90
In other species, HSP90 is the major negative regulator of HSF function. In these organisms, inhibitors which prevent its binding to HSF can cause a HSR in the absence of misfolded proteins. Therefore, we examined heat shock-independent induction of cytoplasmic chaperones in midguts from bees treated with the HSP90 inhibitor, 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG), which has been used in Drosophila 36, for 24 hours. Relative to β-actin, Hsc70-4 (Hsp68) was induced when bees were exposed to 250 µM 17-AAG (n = 9) compared to untreated bees (n = 6) (Fig. 3B).
Heat shock represses expression of immune effectors
In most invertebrates examined to date, heat shock induces the expression of immune genes responsible for defending against microbial infection, likely due to hormetic cross-activation of these stress responses. We were interested in determining whether this relationship between the HSR activation and immune gene expression extended to honey bees. Surprisingly, we observed substantial repression of multiple AMP genes, Hymenoptaecin, Defensin 1, and Abaecin, in the abdominal tissue (containing the fat body) when bees were exposed to 45 °C for 4 hours compared to bees maintained at 35 °C (Fig. 4A–C).
Select AMP gene repression during Heat-Shock. Transcript levels of Hymenoptaecin, Defensin 1, and Abaecin relative to β-actin in abdominal wall (predominantly fat body) from bees maintained for four hours in cages at either 35° or 45 °C. Bar and error bars represent the Mean ± SEM for expression values of the genes of interest calculated using the ΔΔCT method. Statistical significance was assessed using unpaired t-tests with Welch’s and is noted as *p < 0.05, and **p < 0.01.
Septic infection reveals a reciprocal relationship between wounding and heat shock targets
We were interested in determining whether there was a reciprocal relationship between the HSR and microbial infection and established a septic infection model using the Gram-negative bacteria, S. marcescens (Fig. 5A). We observed increased levels of total bacteria (using Universal Bacteria 16 S primers) and Gfp (expressed in the Db11 S. marcescens clone) in the fat body of bees receiving S. marcescens (n = 4) relative to uninjected bees (n = 4) and those injected with Lysogeny Broth (LB) alone (n = 4) (Fig. 5B and C). After injection of Gram-negative bacteria (S. marcescens) or media alone, we observed robust activation of AMPs in the fat body compared to uninjected bees at 4 hours post-injection (Fig. 5D–F). We also observed a significant reduction in the expression of genes encoding the two HSP70 proteins, Hsc70-4 and Hsc70Ab, and Hsp90 (Fig. 5G–I). These results suggest that wounding is sufficient for immune activation and HSR repression regardless of the presence of microbes.
Infection and wounding represses select HSR target genes at the site of injury. Graphical schematic of infection protocol (A). Levels of bacteria (B) or Gfp1 + S. marcescens (Db11) (C) relative to β-actin in abdominal wall (predominantly fat body) in uninjected bees (0) or those injected with either lysogeny broth (LB) or S. marcescens (Db11) four hours after injection. Transcript levels of Hymenoptaecin (D), Defensin 1 (E), and Abaecin (F) or Hsc70-4 (G), Hsp70Ab (H) and Hsp90 (I) relative to β-actin in abdominal wall (predominantly fat body) from these same bees. Bar and error bars represent the Mean ± SEM for expression values of the genes of interest calculated using the ΔΔCT method. Statistical significance was assessed using one way ANOVA with Tukey’s multiple comparison test and is noted as *p < 0.05, and **p < 0.01.
To determine if wounding was sufficient for altering the expression of proteostasis genes, we used a wounding model in which we made a sterile injury in the cuticle of the honey bee abdomen. When we performed this challenge, we again observed robust activation of the AMPs Abaecin, Hymenoptaecin, and Defensin 1 in the fat body of wounded (n = 8) compared to unwounded (n = 8) bees (Suppl. Figure 5A and C). We also observed a significant reduction in the genes encoding the two HSP70 proteins, Hsc70-4 and Hsp70Ab (Suppl. Figure 5D and E), and Hsp90 (Suppl Figure 5F).
Spreading of immune and proteostasis signals beyond the site of the insult have been reported. Examination of gene expression in the midgut tissue of bees from the septic infection model revealed robust activation of the AMP Defensin 1 compared to uninjected bees (Fig. 6A) and a significant reduction in the genes encoding the HSP70 protein, Hsc70-4 (Fig. 6B). Alterations in gene expression distal from the site of wounding implies that some signal is allowing for spread of both immune activation and HSR repression and that this signal does not require the presence of bacteria.
Infection and wounding represses select HSR target genes at the site of injury. Transcript levels of Defensin 1 (A), and Hsc70-4 (B) relative to β-actin in midgut tissue in uninjected bees (0) or those injected with either lysogeny broth (LB) or S. marcescens (Db11) four hour after injection. Bar and error bars represent the Mean ± SEM for expression values of the genes of interest calculated using the ΔΔCT method. Statistical significance was assessed using one way ANOVA with Tukey’s multiple comparison test and is noted as *p < 0.05, and **p < 0.01.
Discussion
Our understanding of the molecular architecture and regulation of the HSR in honey bees is incomplete17, 18, 20. In other organisms, activation of the HSR pathway leads to the activation of a family of leucine-zipper containing transcription factors, HSF, which participate in a medium-term response designed to increase protein production and folding capacity through transcriptional upregulation of proteins involved in these processes3, 4. A number of recent studies have also suggested that within the myriad transcriptional changes induced by heat-shock there is a core set of HSF-dependent genes shared in the HSR from yeast25 to mammals25, 26, although some differences in this core group were found in a similar study in C. elegans 37. Our findings demonstrate that temperatures encountered by honey bees during normal activities result in robust HSR induction and increased expression of the majority of the homologs of the core HSF-dependent found in other systems. Ultimately however, genetic approaches will be necessary to definitively determine HSF1-dependence of these genes in the honey bee HSR.
At least two other pathways contribute to the maintenance of proteostasis in cells, the UPRER, which responds to proteostatic stress in the endoplasmic reticlulum5 and the UPRmt, which responds to proteostatic stress in mitochondria6. We have previously characterized the honey bee UPR[ER 30 and find here that select genes involved in the unfolded protein response in the ER are also triggered by the HSR. We also find that the IRE1 pathway does not appear to be activated by heat shock as assessed by Xbp1 splicing. Xbp1 is upregulated by heat shock in some tissues. However, some evidence suggests that unspliced XBP1 represses target genes and Xbp1 levels do not correlate with increased UPRER target gene expression in our experiments. This suggests that the heat shock response is directly activating these targets via HSF, some unknown intermediary, or one of the other UPR pathways, such as the activating transcription factor 6 (ATF6) or double-stranded RNA-activated protein kinase (PKR)–like ER kinase (PERK) pathway. While the UPRmt has not yet been characterized in this species, we observe activation of honey bee homologs of select genes that are part of this response in other species38. In addition, it is likely that HSR activation shown here impacts other processes involved in proteostasis, such as the ubiquitin proteasome system (UPS)39, which has been characterized in honey bees40, 41. Heat shock likely impacts protein folding in all compartments, however there is accumulating evidence that in some contexts, proteotoxic insults isolated to one cellular compartment communicate this insult to other compartments resulting in cell-wide responses42, 43. Further work will be necessary to fully understand the biological importance and mechanistic underpinnings of proteostasis network crosstalk in this species and other insects.
The honey bee immune system is well characterized44, possessing the machinery for both the Toll and IMD pathways involved in Pathogen-Associated-Molecular-Pattern (PAMP) recognition45. Functional data suggests that septic infection by Gram-negative or Gram-positive bacteria in honey bees results in activation of a systemic immune response in a manner that is not pathogen-specific46. Our data here also suggests a PAMP-independent activation of the immune system in honey bees as we find that the AMPs Hymenoptaecin, Defensin 1, and Abaecin are induced by sterile wounding alone. Immune activation in invertebrates can be triggered by two mechanisms other than these PAMP-dependent pathways. First, sterile mechanical wounding of the epidermis in multiple invertebrate species, including C. elegans 47, D. melanogaster 48, Spodoptera frugiperda 49, Bombus terrestris 50, and honey bees51, 52, can elicit an immune response that includes AMP production, perhaps as prophylactic protection against anticipated infection after wounding. Second, ‘surveillance immunity’ is triggered through the sensing of perturbations in normal cellular processes53, 54. In particular, disruption of proteostasis often triggers this response in invertebrates55. For example, translation inhibition often occurs during microbial infection in worms56,57,58 and flies59, resulting in immune activation. It is likely that mechanical damage, cellular disruption, or both is leading to immune activation in the epidermis and fat body here as well. While we only examined the systemic humoral response in the form of AMP expression in this study, systemic immunity in insects is made of both cellular and humoral elements45 and future studies might explore the effect of heat shock on other aspects of the immune response.
Prior to this report, evidence has suggested a positive reciprocal relationship between the HSR and immune activation in invertebrates22. Immune competence, resistance to infection, and levels of immune mediators all increase with temperature and heat shock. For example, increased temperature makes Galleria mellonella more resistant to infection with fungi60,61,62 and bacteria63. Heat shock was also shown to result in induced expression of humoral immune genes64, 65. Temperature also positively impacted the expression of immune response genes in the alfalfa leafcutting bee66. In C. elegans, heat increases the immune response67 and HSF is also important for immune mediated resistance to infection68, 69. In Drosophila, the p38 pathway mediates defense to bacterial and fungal infections in part through a HSF-dependent mechanism70. Evidence also suggests that activation of the HSR inhibits virus infection71. Reciprocally, in other invertebrate models, infection appears to enhance temperature stress tolerance, stress responses, and expression of stress genes, including HSR target genes. Bacterial infection induced Hsp90 expression in the greater wax moth72 and Hsp70 expression in Musca domestica 73. Hormesis, the beneficial effects of a treatment that are harmful at a higher intensity74, may be the underlying mechanism in these other invertebrates. Sublethal exposure to heat stress or immune stress may induces a cellular or physiological response that results in cross-resistance to the other seemingly unrelated stress.
Our results in the honey bee are consistent with the idea of trade-offs from ecological immunology75,76,77. One tenet of this field is that processes that compete for similar resources, such as energy, can come into conflict at the physiological level resulting in unbalanced emphasis on one or the other system78. The immune system is costly and its activation is often used as one of the competing processes in studies of trade-offs. Cellular mechanisms to maintain proteostasis are also energetically costly79. Thus, energetic trade-offs at the individual level would be expected to allocate energy resources in a manner that leads to a reduction in either immunocompetence or proteostasis. One reason for the differences observed in this study could be that individual worker honey bees have different stress response requirements as a eusocial species. Specifically, in the context of the colony, limited resources are allocated to the individuals most likely to benefit the hive. Originally described in the context of aging and senescence in social insects, the intergenerational transfer theory of aging proposes that allocation of group resources to the non–reproductive individuals, such as workers, will be governed by the amount of resource transfers each individual is likely to provide to the group80, 81. Stressed individuals are less productive and will deliver fewer resource transfers to the group and thus may not be prioritized for receiving limited group resources. For example, the stress of immune activation has been observed to alter learning82 and both individual83 and social84, 85 behavior in honey bees, all of which could impact the ability of individual bees to contribute to the colony. In fact, stresses encountered by individuals of this species often alter physiology and behavior to increase participation in the most risky colony tasks, such as foraging86 or robbing87. For example, a wide array of stresses, including nutritional deficiencies88, 89, infection83, 90,91,92,93,94,95, and immune stimulation96, 97 will lead to precocious foraging and forager physiological features in younger nurse bees98. Early foragers are less successful and die earlier, which results in an increased rate of forager recruitment. While, the ‘resilience’ of eusocial organisms appears to rely on the ability to sacrifice non-reproductive individuals for the benefit of the colony23, even in the group context the supply of individuals is limited and sustained loss can have dramatic impacts on colony fitness99.
All metazoan species possess means for coordinating responses between tissues and organs, especially in cases of stress100. Spreading of immune101 and proteostasis2, 102 signals beyond the site of the insult have been reported in invertebrates. Our discovery that alterations in immune and proteostasis gene expression are occurring in the midgut, which is distal from the site of wounding, implies that some signal is allowing spread of these signals in honey bees as well. For both signal types, communication appears to involve both neural networks103, 104 and soluble hormone signaling105. Communication between the nervous system and distal tissues is bidirectional. For example, in C. elegans, thermosensory neurons contribute to the regulation of HSR in other tissues106 and temperature sensing in disparate tissues can affect behavioral responses to temperature107. More work to understand how the thermosensory system functions in bees at the individual and colony level under normal conditions is warranted. Honey bees participate in activities to maintain a relatively constant hive temperature10 (and references therein) that is critically important for normal brood development and normal colony function. In particular, development outside of the optimal temperature range results in brain abnormalities, cognitive defects, and reduced survival12,13,14,15. In light of the influence of proteostasis on behavioral responses in the roundworm, it is interesting to speculate on how wounding or immune activation in honey bees could impact thermoregulatory behavior via modulation of the proteostasis pathways. For example, Varroa destructor is an ectoparasitic mite that attacks the honey bee108. Its mode of feeding involves puncturing the cuticle to suck hemolymph. A recent report found that Varroa infection and artificial wounding elicited similar transcriptional responses109. In addition, infestation of colonies by V. destructor interferes with proper thermoregulation in honey bee winter clusters, specifically leading to higher thermal fluctuations110. Thus, the role of wounding and the HSR in this phenomenon should be explored.
In the characterization of the honey bee HSR described here, we found that the core components of the pathway are conserved and that temperatures encountered by honey bees during normal activities resulted in robust HSR induction and the increased expression of specific heat shock proteins in a manner that was variable across tissues. In addition, we show that heat shock represses the expression of select immune effectors, the antimicrobial peptide genes, Hymenoptaecin, Defensin 1, and Abaecin. Finally, we show that wounding the abdomen results in decreased expression of multiple HSR target genes in proximal and distal tissues, revealing a reciprocally antagonistic relationship between the HSR pathway and immune activation in honey bees, which is distinctive among invertebrates studied to date. These findings may offer new insight into the potential synergistic effects of disparate stresses in this critical pollinator and social insects more broadly.
Materials and Methods
Honey bee Tissue Collection
Honey bees were collected from the landing board of outbred colonies in New York, New York consisting of a typical mix of Apis mellifera subspecies found in North America, at different times during the months of April-October. Only visibly healthy bees were collected and all source colonies were visually inspected for symptoms of common bacterial, fungal, and viral diseases of honey bees. After cold anesthesia, bees were dissected and we recovered four tissues for analysis; head tissue (predominantly brain and sensory organ tissue including antennae), midgut, thorax tissue (predominantly flight muscle), and abdominal wall (predominantly fat body). Tissues were set aside for gene expression analysis by storing in RNAlater (Invitrogen, San Diego, CA).
Ortholog screening of the honey bee genome
HSR pathway gene candidates from D. melanogaster were used to find orthologs in the honey bee genome using the BLAST family of search functions (www.ncbi.nlm.nih.gov) as described previously30. The KEGG (Kyoto Encyclopedia of Genes and Genomes) database was also used as a guide for comparing pathways between species111. For proteins of interest, we used SignalP 4.1 to predict the presence of a signal sequence, TargetP 1.1 to predict secretory or mitochondrial localization, and Prosite to predict the presence of an ER-retention signal.
Temperature and Chemical Treatments
For all caged experiments, honey bees were selected as above and kept in 177.4 mL (6 oz.) Square-bottomed Drosophila Stock Bottles (VWR) plugged with modified foam tube plugs (Jaece Industries). Bees were maintained in incubators at 35 °C (unless otherwise stated) in the presence of PseudoQueen (Contech, Victoria, British Columbia, Canada) as a source of Queen Mandibular Phermone (QMP) and used as per manufacturer’s instructions. For heat shock, bees were maintained for four hours in cages at 35° or 45 °C. Bees were fed 33% sucrose via a modified 1.5 ml screw-cap tube, with or without the following chemicals at the indicated doses: 500 µM MG13234, 35 (Sigma), 250 µM 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG) (Calbiochem)36 for 24 hours. When DMSO was used as the chemical solute, equivalent amounts of DMSO were added to the food of the control group.
Bacterial Infections
For bacterial infection, Serratia marcescens (strain Db11112) from frozen stocks were plated on Lysogeny Broth (LB) Agarose with Ampicillin and Streptomycin, a colony was selected to be grown in LB without antibiotics overnight, and the resulting culture was diluted in fresh LB without antibiotics. Bacteria was then grown exponentially at 37 °C to OD600 = 1. Db11 cultures or LB alone were then diluted 1:10 in Ringer’s solution. For injections, bees were cold anesthetized, immobilized, and 1 μl was injected between the 2nd and 3rd abdominal segments using a 10 microliter syringe and a disposable sterile 30 G needle. For wounding alone, a disposable sterile 30 G needle was inserted between the 2nd and 3rd abdominal segments, but no material was delivered.
RNA Isolation, reverse-transcription and quantitative PCR for Gene Expression Analysis
Dissected material was placed into RNAlater (Invitrogen, San Diego, CA) for storage and RNA was prepared from bees by manually crushing the tissue of interest with a disposable pestle in Trizol Reagent (Invitrogen, San Diego, CA) and extracting the RNA as per the manufacturer’s instructions. RNA was subsequently DNAseI treated by RQ1 RNase-Free DNase (Promega, Madison, WI) and quantified. cDNA was synthesized using approximately 1 μg of RNA with the iScript cDNA Synthesis Kit (Biorad, Hercules, CA). Typically, 1 μl of cDNA was then used as a template for quantitative PCR to determine the levels of expression of genes of interest using the iQ SYBR Green Supermix (Biorad, Hercules, CA) in an iCycler thermo-cycler (Biorad, Hercules, CA). Primer sequences used in this study are in Supplemental Table 1. When possible, primers amplifying regions spanning introns were used. The difference between the threshold cycle number for β-actin and that of the gene of interest was used to calculate the level of that gene relative to β-actin using the ΔΔCT method.
Xbp1 mRNA Splicing
cDNA from above was used as template for a PCR using primers (Forward, 5′-ctgtgctgtgtcctcactgg-3′ and Reverse 5′- tcaagaggaagtagatggtcagaa-3′) that spanned the predicted splice sites. PCR products were run on a 2.5% Agarose gel to separate spliced from unspliced Xbp1.
Statistical Analysis
All gene expression data were generated by processing and analyzing individual bees (where n denotes the number of bees in each treatment group) and then pooling data for a given experiment. Graphs show representative results from one of multiple trials. For analysis, data was log10 transformed and compared using unpaired t-tests with Welch’s correction as values fit normal distributions. Normality was assessed using Shapiro-Wilk tests. When more than two groups were being compared, data was compared using one way ANOVA with Tukey’s multiple comparison test.
References
Goulson, D., Nicholls, E., Botías, C. & Rotheray, E. L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 (2015).
Taylor, R. C., Berendzen, K. M. & Dillin, A. Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nat Rev Mol Cell Biol 15, 211–217 (2014).
Vabulas, R. M., Raychaudhuri, S., Hayer-Hartl, M. & Hartl, F. U. Protein Folding in the Cytoplasm and the Heat Shock Response. Csh Perspect Biol 2, a004390–a004390 (2010).
Morimoto, R. I. The Heat Shock Response: Systems Biology of Proteotoxic Stress in Aging and Disease. Cold Spring Harbor Symposia on Quantitative Biology 76, 91–99 (2012).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Jovaisaite, V., Mouchiroud, L. & Auwerx, J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. Journal of Experimental Biology 217, 137–143 (2013).
Heinrich, B. The Hot-Blooded Insects. (Harvard University Press, 1993).
Seeley, T. D. Honeybee Ecology: A Study of Adaptation in Social Life. (Princeton Univ. Press, 1985).
Southwick, E. E. The colony as a thermoregulating superorganism 28–47 (CAB International, 1991).
Stabentheiner, A., Kovac, H. & Brodschneider, R. Honeybee Colony Thermoregulation—Regulatory Mechanisms and Contribution of Individuals in Dependence on Age, Location and Thermal Stress. PLoS ONE 5, e8967 (2010).
Owens, C. D. The Thermology of Wintering Honey Bee Colonies. (1971).
Himmer, A. Ein beitrag zur kenntnis des wärmehaushalts im nestbau sozialer hautflügler. Zeitschrift für vergleichende Physiologie 5, 375–389 (1927).
Himmer, A. Die Temperaturverhältnisse bei den sozialen Hymenopteren. Biological Reviews 7, 224–253 (1932).
Groh, C., Tautz, J. & Rössler, W. Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc Natl Acad Sci USA 101, 4268–4273 (2004).
Wang, Q. et al. Low-Temperature Stress during Capped Brood Stage Increases Pupal Mortality, Misorientation and Adult Mortality in Honey Bees. PLoS ONE 11, e0154547 (2016).
Elekonich, M. M. & Roberts, S. P. Honey bees as a model for understanding mechanisms of life history transitions. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol. 141, 362–371 (2005).
Severson, D. W., Erickson, E. H., Williamson, J. L. & Aiken, J. M. Heat stress induced enhancement of heat shock protein gene activity in the honey bee (Apis mellifera). Experientia 46, 737–739 (1990).
Elekonich, M. M. Extreme thermotolerance and behavioral induction of 70-kDa heat shock proteins and their encoding genes in honey bees. Cell Stress and Chaperones 14, 219–226 (2008).
Kovac, H., Käfer, H., Stabentheiner, A. & Costa, C. Metabolism and upper thermal limits of Apis mellifera carnica and A. m. ligustica. Apidologie 45, 664–677 (2014).
Williams, J. B., Roberts, S. P. & Elekonich, M. M. Age and natural metabolically-intensive behavior affect oxidative stress and antioxidant mechanisms. Experimental Gerontology 43, 538–549 (2008).
Evans, J. D. & Schwarz, R. S. Bees brought to their knees: microbes affecting honey bee health. Trends Microbiol 19, 614–620 (2011).
Wojda, I. Temperature stress and insect immunity. Journal of Thermal Biology 68, 96–103 (2017)
Straub, L., Williams, G. R., Pettis, J., Fries, I. & Neumann, P. ScienceDirectSuperorganism resilience: eusociality and susceptibility of ecosystem service providing insects to stressors. Current Opinion in Insect Science 12, 109–112 (2015).
West, J. D., Wang, Y. & Morano, K. A. Small molecule activators of the heat shock response: chemical properties, molecular targets, and therapeutic promise. Chem. Res. Toxicol. 25, 2036–2053 (2012).
Solís, E. J. et al. Defining the Essential Function of Yeast Hsf1 Reveals a Compact Transcriptional Program for Maintaining Eukaryotic Proteostasis. Mol Cell 63, 60–71 (2016).
Mahat, D. B., Salamanca, H. H., Duarte, F. M., Danko, C. G. & Lis, J. T. Mammalian Heat Shock Response and Mechanisms Underlying Its Genome-wide Transcriptional Regulation. Mol Cell 62, 63–78 (2016).
Xu, P. J., Xiao, J. H., Xia, Q. Y., Murphy, B. & Huang, D. W. Apis mellifera has two isoforms of cytoplasmic HSP90. Insect Mol Biol 19, 593–597 (2010).
Strauch, A. & Haslbeck, M. The function of small heat-shock proteins and their implication in proteostasis. Essays In Biochemistry 60, 163–172 (2016).
Horwich, A. L., Fenton, W. A., Chapman, E. & Farr, G. W. Two Families of Chaperonin: Physiology and Mechanism. Annu Rev Cell Dev Biol 23, 115–145 (2007).
Johnston, B. A., Hooks, K. B., McKinstry, M. & Snow, J. W. Divergent forms of endoplasmic reticulum stress trigger a robust unfolded protein response in honey bees. J Insect Physiol 86, 1–10 (2016).
Haynes, C. M., Petrova, K., Benedetti, C., Yang, Y. & Ron, D. ClpP Mediates Activation of a Mitochondrial Unfolded Protein Response in C. elegans. Dev Cell 13, 467–480 (2007).
Richter, K., Haslbeck, M. & Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol Cell 40, 253–266 (2010).
Kampinga, H. H. & Craig, E. A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11, 579–592 (2010).
Griciuc, A. et al. Inactivation of VCP/ter94 Suppresses Retinal Pathology Caused by Misfolded Rhodopsin in Drosophila. PLoS Genet 6, e1001075 (2010).
Guisbert, E., Czyz, D. M., Richter, K., McMullen, P. D. & Morimoto, R. I. Identification of a Tissue-Selective Heat Shock Response Regulatory Network. PLoS Genet 9, e1003466 (2013).
Fujikake, N. et al. Heat Shock Transcription Factor 1-activating Compounds Suppress Polyglutamine-induced Neurodegeneration through Induction of Multiple Molecular Chaperones. Journal of Biological Chemistry 283, 26188–26197 (2008).
Brunquell, J., Morris, S., Lu, Y., Cheng, F. & Westerheide, S. D. The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans. BMC Genomics 17, 559 (2016).
Yoneda, T. et al. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci 117, 4055–4066 (2004).
Finley, D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78, 477–513 (2009).
Felsenberg, J., Dombrowski, V. & Eisenhardt, D. A role of protein degradation in memory consolidation after initial learning and extinction learning in the honeybee (Apis mellifera). Learning & Memory 19, 470–477 (2012).
Felsenberg, J. et al. Two inhibitors of the ubiquitin proteasome system enhance long-term memory formation upon olfactory conditioning in the honeybee (Apis mellifera). Journal of Experimental Biology 217, 3441–3446 (2014).
Kim, H.-E. et al. Lipid Biosynthesis Coordinates a Mitochondrial-to-Cytosolic Stress Response. Cell 166, 1539–1552.e16 (2016).
Perić, M. et al. Crosstalk between cellular compartments protects against proteotoxicity and extends lifespan. Sci. Rep. 6, 28751 (2016).
Evans, J. D. et al. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Biol 15, 645–656 (2006).
Buchon, N., Silverman, N. & Cherry, S. Immunity in Drosophila melanogaster—from microbial recognition to whole- organism physiology. Nat Rev Immunol 14, 796–810 (2014).
Lourenço, A. P., Guidugli-Lazzarini, K. R., Freitas, F. C. P., Bitondi, M. M. G. & Simões, Z. L. P. Bacterial infection activates the immune system response and dysregulates microRNA expression in honey bees. Insect Biochem Molec 43, 474–482 (2013).
Pujol, N. et al. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Current Biology 18, 481–489 (2008).
Patterson, R. A. et al. Serine Proteolytic Pathway Activation Reveals an Expanded Ensemble of Wound Response Genes in Drosophila. PLoS ONE 8, e61773 (2013).
Volkoff, A.-N. et al. Characterization and transcriptional profiles of three Spodoptera frugiperda genes encoding cysteine-rich peptides. A new class of defensin-like genes from lepidopteran insects? Gene 319, 43–53 (2003).
Erler, S., Popp, M. & Lattorff, H. Dynamics of Immune System Gene Expression upon Bacterial Challenge and Wounding in a Social Insect (Bombus terrestris). PLoS ONE 6, e18126 (2011).
Lourenço, A. P., Martins, J. R., Bitondi, M. M. & Simões, Z. L. Trade-off between immune stimulation and expression of storage protein genes. Arch Insect Biochem Physiol 71, 70–87 (2009).
Randolt, K. et al. Immune-related proteins induced in the hemolymph after aseptic and septic injury differ in honey bee worker larvae and adults. Arch Insect Biochem Physiol 69, 155–167 (2008).
Colaço, H. G. & Moita, L. F. Initiation of innate immune responses by surveillance of homeostasis perturbations. FEBS J 283, 2448–2457 (2016).
Pukkila-Worley, R. Surveillance Immunity: An Emerging Paradigm of Innate Defense Activation in Caenorhabditis elegans. PLoS Pathog 12, e1005795 (2016).
Lemaitre, B. & Girardin, S. E. Translation inhibition and metabolic stress pathways in the host response to bacterial pathogens. Nat Rev Micro 11, 365–369 (2013).
Dunbar, T. L., Yan, Z., Balla, K. M., Smelkinson, M. G. & Troemel, E. R. C. elegans detects pathogen-induced translational inhibition to activate immune signaling. Cell Host Microbe 11, 375–386 (2012).
McEwan, D. L., Kirienko, N. V. & Ausubel, F. M. Host Translational Inhibition by Pseudomonas aeruginosa Exotoxin A Triggers an Immune Response in Caenorhabditis elegans. Cell Host Microbe 11, 364–374 (2012).
Reddy, K. C., Dunbar, T. L., Nargund, A. M., Haynes, C. M. & Troemel, E. R. The C. elegans CCAAT-Enhancer-Binding Protein Gamma Is Required for Surveillance Immunity. CellReports 14, 1581–1589 (2016).
Chakrabarti, S., Liehl, P., Buchon, N. & Lemaitre, B. Infection-induced host translational blockage inhibits immune responses and epithelial renewal in the Drosophila gut. Cell Host Microbe 12, 60–70 (2012).
Mowlds, P. & Kavanagh, K. Effect of pre-incubation temperature on susceptibility of Galleria mellonella larvae to infection by Candida albicans. Mycopathologia 165, 5–12 (2007).
Wojda, I., Kowalski, P. & Jakubowicz, T. Humoral immune response of Galleria mellonella larvae after infection by Beauveria bassiana under optimal and heat-shock conditions. J Insect Physiol 55, 525–531 (2009).
Vertyporokh, L., Taszłow, P., Samorek-Pieróg, M. & Wojda, I. Short-term heat shock affects the course of immune response in Galleria mellonella naturally infected with the entomopathogenic fungus Beauveria bassiana. J Invertebr Pathol 130, 42–51 (2015).
Wojda, I. & Taszłow, P. Heat shock affects host-pathogen interaction in Galleria mellonella infected with Bacillus thuringiensis. J Insect Physiol 59, 894–905 (2013).
Wojda, I. & Jakubowicz, T. Humoral immune response upon mild heat-shock conditions in Galleria mellonella larvae. J Insect Physiol 53, 1134–1144 (2007).
Taszłow, P. & Wojda, I. Changes in the hemolymph protein profiles in Galleria mellonella infected with Bacillus thuringiensis involve apolipophorin III. The effect of heat shock. Arch Insect Biochem Physiol 88, 123–143 (2015).
Xu, J. & James, R. R. Temperature stress affects the expression of immune response genes in the alfalfa leafcutting bee, Megachile rotundata. Insect Mol Biol 21, 269–280 (2012).
Prithika, U., Deepa, V. & Balamurugan, K. External induction of heat shock stimulates the immune response and longevity of Caenorhabditis elegans towards pathogen exposure. Innate Immun 22, 466–478 (2016).
Singh, V. & Aballay, A. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc Natl Acad Sci USA 103, 13092–13097 (2006).
Singh, V. & Aballay, A. Heat Shock and Genetic Activation of HSF-1 Enhance Immunity to Bacteria. Cell Cycle 5, 2443–2446 (2014).
Chen, J. et al. Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc Natl Acad Sci USA 107, 20774–20779 (2010).
Merkling, S. H. et al. The heat shock response restricts virus infection in Drosophila. Sci. Rep. 5, 12758 (2015).
Wojda, I. & Kowalski, P. Galleria mellonella infected with Bacillus thuringiensis involves Hsp90. Cent. Eur. J. Biol. 8, 561–569 (2013).
Tang, T. et al. Journal of Insect Physiology. J Insect Physiol 58, 1226–1234 (2012).
Gems, D. & Partridge, L. Stress-Response Hormesis and Aging: ‘That which Does Not Kill Us Makes Us Stronger’. Cell Metabolism 7, 200–203 (2008).
Sadd, B. M. & Schmid-Hempel, P. Principles of ecological immunology. Evol Appl 2, 113–121 (2009).
Hawley, D. & Altizer, S. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Functional Ecology 25, 48–60 (2011).
Boughton, R. K., Joop, G. & Armitage, S. A. O. Outdoor immunology: methodological considerations for ecologists. Functional Ecology 25, 81–100 (2011).
Schwenke, R. A., Lazzaro, B. P. & Wolfner, M. F. Reproduction-Immunity Trade-Offs in Insects. Annu. Rev. Entomol 61, 239–256 (2016).
Powers, E. T. & Balch, W. E. Diversity in the origins of proteostasis networks—a driver for protein function in evolution. Nat Rev Mol Cell Biol 14, 237–248 (2013).
Lee, R. D. Rethinking the evolutionary theory of aging: transfers, not births, shape senescence in social species. Proc Natl Acad Sci USA 100, 9637–9642 (2003).
Amdam, G. & Page, R. Intergenerational transfers may have decoupled physiological and chronological age in a eusocial insect. Ageing research reviews 4, 398–408 (2005).
Mallon, E. B., Brockmann, A. & Schmid-Hempel, P. Immune response inhibits associative learning in insects. Proc Biol Sci 270, 2471–2473 (2003).
Alaux, C., Crauser, D., Pioz, M., Saulnier, C. & Le Conte, Y. Parasitic and immune modulation of flight activity in honey bees tracked with optical counters. Journal of Experimental Biology 217, 3416–3424 (2014).
Richard, F., Aubert, A. & Grozinger, C. Modulation of social interactions by immune stimulation in honey bee, Apis mellifera, workers. BMC biology 6, 50 (2008).
Richard, F.-J., Holt, H. L. & Grozinger, C. M. Effects of immunostimulation on social behavior, chemical communication and genome-wide gene expression in honey bee workers (Apis mellifera). BMC Genomics 13, 1–1 (2012).
Rueppell, O., Bachelier, C., Fondrk, M. & Page, R. Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.). Experimental Gerontology 42, 1020–1032 (2007).
Kuszewska, K. & Woyciechowski, M. Risky robbing is a job for short-lived and infected worker honeybees. Apidologie 45, 537–544 (2014).
Schulz, D. J., Huang, Z. Y. & Robinson, G. E. Effects of colony food shortage on behavioral development in honey bees. Behav Ecol Sociobiol 42, 295–303 (1998).
Toth, A. & Robinson, G. Worker nutrition and division of labour in honeybees. Animal Behaviour 69, 427–435 (2005).
Janmaat, A. F. & Winston, M. L. The influence of pollen storage area and Varroa jacobsoni Oudemans parasitism on temporal caste structure in honey bees (Apis mellifera L.). Insect. Soc. 47, 177–182 (2000).
Woyciechowski, M. & Moroń, D. Life expectancy and onset of foraging in the honeybee (Apis mellifera). Insect. Soc. 56, 193–201 (2009).
Dussaubat, C. et al. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J Invertebr Pathol 113, 42–51 (2013).
Goblirsch, M., Huang, Z. Y. & Spivak, M. Physiological and Behavioral Changes in Honey Bees (Apis mellifera) Induced by Nosema ceranae Infection. PLoS ONE 8, e58165 (2013).
Lecocq, A., Jensen, A. B., Kryger, P. & Nieh, J. C. Parasite infection accelerates age polyethism in young honey bees. Sci. Rep. 6, 22042 (2016).
Benaets, K. et al. Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proc Biol Sci 284 (2017).
Alaux, C. D., Kemper, N., Kretzschmar, A. & Le Conte, Y. Brain, physiological and behavioral modulation induced by immune stimulation in honeybees (Apis mellifera): A potential mediator of social immunity? Brain Behavior and Immunity 26, 1057–1060 (2012).
Bordier, C. et al. Stress response in honeybees is associated with changes in task-related physiology and energetic metabolism. J Insect Physiol 98, 47–54 (2016).
Perry, C. J., Søvik, E., Myerscough, M. R. & Barron, A. B. Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc Natl Acad Sci USA 112, 3427–3432 (2015).
Barron, A. B. Death of the bee hive: understanding the failure of an insect society. Current Opinion in Insect Science 10, 45–50 (2015).
Droujinine, I. A. & Perrimon, N. Interorgan Communication Pathways in Physiology: Focus on Drosophila. Annu. Rev. Genet. 50, 539–570 (2016).
Gendrin, M., Welchman, D. P., Poidevin, M., Herve, M. & Lemaitre, B. Long-Range Activation of Systemic Immunity through Peptidoglycan Diffusion in Drosophila. PLoS Pathog 5, e1000694 (2009).
van Oosten-Hawle, P., Porter, R. S. & Morimoto, R. I. Regulation of Organismal Proteostasis by Transcellular Chaperone Signaling. Cell 153, 1366–1378 (2013).
Aballay, A. Role of the Nervous System in the Control of Proteostasis during Innate Immune Activation: Insights from C. elegans. PLoS Pathog 9, e1003433 (2013).
Mardones, P., Martínez, G. & Hetz, C. Control of systemic proteostasis by the nervous system. Trends Cell Biol 25, 1–10 (2015).
van Oosten-Hawle, P. & Morimoto, R. I. Organismal proteostasis: role of cell-nonautonomous regulation and transcellular chaperone signaling. Genes Dev 28, 1533–1543 (2014).
Prahlad, V., Cornelius, T. & Morimoto, R. I. Regulation of the Cellular Heat Shock Response in Caenorhabditis elegans by Thermosensory Neurons. Science 320, 811–814 (2008).
Sugi, T., Nishida, Y. & Mori, I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nature Neuroscience 14, 984–992 (2011).
Nazzi, F. & Le Conte, Y. Ecology of Varroa destructor, the Major Ectoparasite of the Western Honey Bee, Apis mellifera. Annu. Rev. Entomol 61, 417–432 (2016).
Koleoglu, G., Goodwin, P. H., Reyes-Quintana, M., Hamiduzzaman, M. M. & Guzmán-Novoa, E. Effect of Varroa destructor, Wounding and Varroa Homogenate on Gene Expression in Brood and Adult Honey Bees. PLoS ONE 12, e0169669 (2017).
Schäfer, M. O., Ritter, W., Pettis, J. S. & Neumann, P. Concurrent Parasitism Alters Thermoregulation in Honey Bee (Hymenoptera: Apidae) Winter Clusters. Annals of the Entomological Society of America, 104, 476–482 (2011).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Research 28, 27–30 (2000).
Nehme, N. et al. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog 3, e173 (2007).
Acknowledgements
The authors thank Tara Adames, Julia Discenza, and Marilyn Erazo for assistance with select experiments. We also thank Stephen Chan and Jennifer Mansfield for helpful comments and critical review of the manuscript. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
M.M. and J.W.S. conceived and designed the experiments. M.M., C.C., H.T., B.A.J. and J.W.S. performed honey bee experiments and analyzed the data. All authors contributed to the drafting and revision of the article.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McKinstry, M., Chung, C., Truong, H. et al. The heat shock response and humoral immune response are mutually antagonistic in honey bees. Sci Rep 7, 8850 (2017). https://doi.org/10.1038/s41598-017-09159-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09159-4
This article is cited by
-
Drone honey bees are disproportionately sensitive to abiotic stressors despite expressing high levels of stress response proteins
Communications Biology (2022)
-
Honey bee sHSP are responsive to diverse proteostatic stresses and potentially promising biomarkers of honey bee stress
Scientific Reports (2021)
-
Mild thermal stress does not negatively affect immune gene expression in the bumblebee Bombus terrestris
Apidologie (2021)
-
Reproductive potential does not cause loss of heat shock response performance in honey bees
Scientific Reports (2020)
-
Differential expression of acetylcholinesterase 1 in response to various stress factors in honey bee workers
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.