Abstract
We investigated body mass index (BMI) trajectories in the first 2 years of life in 1170 children from an Asian mother-offspring cohort in Singapore, and examined their predictors and associations with childhood cardio-metabolic risk measures at 5 years. Latent class growth mixture modelling analyses were performed to identify distinct BMI z-score (BMIz) trajectories. Four trajectories were identified: 73.2%(n = 857) of the children showed a normal BMIz trajectory, 13.2%(n = 155) a stable low-BMIz trajectory, 8.6%(n = 100) a stable high-BMIz trajectory and 5.0%(n = 58) a rapid BMIz gain after 3 months trajectory. Predictors of the stable high-BMIz and rapid BMIz gain trajectories were pre-pregnancy BMI, gestational weight gain, Malay and Indian ethnicity, while predictors of stable low-BMIz trajectory were preterm delivery and Indian ethnicity. At 5 years, children with stable high-BMIz or rapid BMIz gain trajectories had increased waist-to-height ratios [B(95%CI) 0.02(0.01,0.03) and 0.03(0.02,0.04)], sum of skinfolds [0.42(0.19,0.65) and 0.70(0.36,1.03)SD units], fat-mass index [0.97(0.32,1.63)SD units] and risk of obesity [relative risk 3.22(1.73,6.05) and 2.56 (1.19,5.53)], but not higher blood pressure. BMIz trajectories were more predictive of adiposity at 5 years than was BMIz at 2 years. Our findings on BMIz trajectories in the first 2 years suggest important ethnic-specific differences and impacts on later metabolic outcomes.
Similar content being viewed by others
Introduction
Childhood obesity is a major health concern worldwide1, because of its association with later cardio-metabolic outcomes such as coronary heart disease and Type 2 diabetes2, 3. These associations suggest that weight and body mass in early childhood may affect health risks in later life. Most epidemiological studies examining associations between childhood body mass index (BMI) and later cardio-metabolic outcomes have focused on BMI at only one time point4, 5. Childhood growth trajectory has recently been advocated as a predictor of future cardio-metabolic risk6, 7. Trajectory patterns account for dynamic changes in size that vary over time during the child’s development, providing an important dimension for consideration, in addition to just assessing size at one point in time.
Recent progress in statistical techniques makes it possible to study the potential heterogeneity of BMI changes in early childhood. Individual children may belong to distinct BMI trajectories8, 9 which may confer different risks towards the subsequent development of obesity or cardio-metabolic disease later in life. Techniques such as latent class growth mixture modelling (LCGMM) allow for estimation of such trajectories and their within-class variance, thereby allowing for greater heterogeneity in statistical model10, 11, unlike other trajectory models such as group-based trajectory modelling, which fix the within-class variation in each trajectory to zero8.
While many studies have prospectively explored BMI trajectories during childhood and adolescence12,13,14,15,16, few have examined BMI trajectories in the first 1000 days after conception (age 0 to 2 years)17, 18, which may be a sensitive window for the development (and hence potential prevention) of later obesity and cardio-metabolic disease19, 20. Even fewer studies have been conducted in Asian populations, whose susceptibility to metabolic disease often exceeds that in Western populations21. We are not aware of any accepted guidelines to identify clinically important weight gains22 or growth trajectories in children aged ≤2 years. Identifying groups of young children following trajectories associated with high risk of developing obesity or cardio-metabolic disease could potentially help in targeting early intervention. Using data from a prospective mother-offspring Asian cohort in Singapore, we aimed to identify distinct BMI trajectories in the first 2 years of life, and examine their predictors and their associations with cardio-metabolic risk measures at age 5 years. We also hypothesized that BMI trajectories in the first 2 years may be more predictive than static BMI measurement at 2 years.
Methods
Study population
The Growing Up in Singapore Towards healthy Outcomes (GUSTO) study has been previously described in detail23. Briefly, pregnant women were recruited in their first trimester at two major public hospitals in Singapore with obstetric services (KK Women’s and Children’s Hospital and the National University Hospital) between June 2009 and September 2010. Eligible women were Singapore citizens or permanent residents who were of Chinese, Malay, or Indian ethnicity with homogeneous parental ethnic backgrounds, and did not receive chemotherapy or psychotropic drugs and did not have diabetes mellitus. Of 3751 women approached, 2034 were eligible, 1247 were recruited and 1170 had singleton deliveries (Supplemental Fig. 1). The reasons of ineligibility have been previously described in detail23. Informed written consent was obtained from the women, and the study was approved by the National Healthcare Group Domain Specific Review Board and SingHealth Centralized Institutional Review Board. All methods were performed in accordance with the relevant guidelines and regulations, and ethical approval was granted by the National Healthcare Group Domain Specific Review Board and SingHealth Centralized Institutional Review Board.
Maternal data
Socio-demographic data (age, self-reported ethnicity, educational attainment, income level and parity) were obtained at recruitment. Pregnant women underwent a 2-hour, 75-gram oral glucose tolerance test after an overnight fast at 26–28 weeks of gestation, as detailed previously24; those diagnosed with gestational diabetes based on World Health Organization’s (WHO) criteria [FPG ≥ 7.0 mmol/L or 2-hour glucose ≥7.8 mmol/L] were placed on a diet or treated with insulin. Gestational age (GA) was assessed by trained ultrasonographers at the first dating scan after recruitment and was reported in completed weeks.
Maternal pre-pregnancy weight was self-reported at study enrolment. Measurements of weight and height for mothers during pregnancy were obtained using SECA 803 Weighing Scale and SECA 213 Stadiometer (SECA Corp, Hamburg, Germany). These measurements were used to calculate body mass index (BMI) in kg/m2. Gestational weight gain (GWG) was calculated as the difference between last measured weight before delivery (between 35–37 weeks of gestation) and pre-pregnancy weight, and was corrected for gestational duration using maternal weight-gain-for-gestational age z-score charts by Hutcheon et al.25. Maternal blood pressure (BP) at 26–28 weeks of gestation was taken by trained research coordinators with an oscillometric device (MC3100, HealthSTATS International Pte Ltd, Singapore).
Infant feeding
Mothers were asked about infant milk feeding using interviewer-administered questionnaires at home visits when the infants were 3, 6, 9, and 12 months of age. Feeding practices were classified into exclusive, predominant, and partial breastfeeding at each of those ages. Both direct breastfeeding and expressed breast milk intakes were classified as breastfeeding. Infants were defined as having low, intermediate or high breastfeeding, as detailed previously26.
Child anthropometric measurements
Measurements of child weight and length/height were obtained at birth, 3, 6, 9, 12, 15 and 18 months and 2 years and 5 years of age, as detailed previously27, 28. At 5 years, we measured waist circumference, four skinfold thicknesses (triceps, biceps, subscapular and suprailiac), fat and lean mass [in a subset of children (n = 274) whose parents provided written consent] based on quantitative magnetic resonance imaging, as detailed previously29. These measurements were used to calculate BMI, sum of skinfolds (SSF), waist-to-height ratio (WHtR), fat mass index (FMI) and lean mass index (LMI) (calculated as fat or lean mass divided by square of height). Age- and sex-specific BMI z-scores (BMIz) were calculated using WHO refs 30, 31. Child obesity at 5 years was defined as age- and sex-specific BMIz two standard deviations higher than the median of the WHO ref. 31.
Based on standardized protocols32, child BP at 5 years was measured by trained research coordinators using a Dinamap CARESCAPE V100 (GE Healthcare, Milwaukee, WI), with the arm resting at the chest level. An average of two blood pressure readings were calculated if the difference between readings was <10 mmHg; otherwise, a third reading was taken and the average of the three readings used instead. Child prehypertension was defined as systolic (SBP) or diastolic (DBP) BP above the 90th percentile for the child’s sex, age and height. As there is currently no reference for blood pressure percentiles in the Singapore population, we utilized reference values published by the American Academy of Pediatrics33.
Statistical analysis
Stage 1: Modelling BMIz trajectories in the first 2 years using LCGMM
We analyzed child BMIz trajectories in the first 2 years of life using LCGMM10, 11. LCGMM is a longitudinal technique based on structural equation modelling that incorporates both continuous and categorical latent (unobserved) variables. The technique assumes that individuals in the sample need not come from a single underlying population, but rather from multiple, latent subgroups. Each identified subgroup has its own specific parameters (e.g., intercept, slope, quadratic), which are unobserved. Furthermore, LCGMM accounts for within-class variation in all growth parameters, implying within-class heterogeneity in addition to the between-class heterogeneity among the identified subgroups.
Quadratic-shaped trajectories were fitted, allowing for curved developmental patterns, with an increasing number of latent trajectories, assuming a constant variance–covariance structure (correlated random intercept, linear, quadratic function). The proportions of missing BMIz data at each time point and across all time points in the first 2 years are shown in Supplemental Table 1; 87.5% of children had at least 4 measurements of BMI in the first 2 years. We used the maximum likelihood robust estimator to account for missing data by full information maximum likelihood. This process approximates missing data by estimating a likelihood function for each individual based on variables that are present, such that all the available data points are used34. We identified the optimal number of latent trajectories based on two model-fit indices: the Bayesian Information Criterion (BIC) and the Bootstrap Likelihood Ratio Test (BLRT). A lower BIC value indicates a better model fit, while the BLRT provides a p-value indicating whether a model with one fewer trajectory groups (k-1 model) should be rejected in favour of a model with k trajectories. Posterior probabilities of belonging to each trajectory were also examined, with subjects assigned to the trajectory for which they had the highest posterior probability. We required each trajectory to contain a minimum of 5% of subjects, so that it would be large enough to be clinically important. Distinct trajectories were coded as a categorical variable (with k number of categories) and were named based on their visual appearance. As the trajectories were similar in nature in both girls and boys (as found in other studies13, 14), all analyses were performed on the total sample. In addition, when corrected postnatal age for infants born preterm35 was used in deriving the trajectories, the patterns were exactly the same as those derived using uncorrected postnatal age. In light of this, all analyses were performed using uncorrected postnatal age. To illustrate the robustness of the extracted trajectories, we repeated the analyses restricted to children with no missing BMIz data in the first 2 years (n = 536). All LCGMM analyses were conducted using Mplus version 7.4 (Muthén and Muthén, Los Angeles, CA).
Stage 2: Predictors and cardio-metabolic consequences of BMIz trajectories
Associations between maternal (age, income level, pre-pregnancy BMI (ppBMI), height, GWG, GDM status, parity and GA at delivery) and infant (ethnicity and breastfeeding) factors and BMIz trajectories were first examined using ordinal logistic regression. However, the proportional odds assumption was violated (Brant test p < 0.05) for many of the predictors (ppBMI, height, GWG, income level, ethnicity and GA at delivery), rendering the model unsuitable for analysis and interpretation. We therefore used multinomial logistic regression, with the most commonly occurring trajectory chosen as the reference category. As self-reported pre-pregnancy weight may have limited validity, we also carried out sensitivity analyses by replacing ppBMI with maternal BMI at booking (mean 8.7 ± 2.8 weeks of gestation).
We studied the association between BMIz trajectories and cardio-metabolic measures at 5 years (i.e., WHtR, SSF, FMI, LMI, SBP and DBP) using multivariable linear regression. As the distributions of child WHtR, SSF, FMI and LMI were skewed, the data were log-transformed and standardized to z-scores with a mean 0 and SD of 1. The log-transformation reduced the skewness and the problem of non-normality. Poisson regression models with robust variance were used to calculate the relative risk of obesity or prehypertension at 5 years for each distinct BMIz trajectory.
For comparison, we also estimated the relative risk of obesity or prehypertension at 5 years for the (static) BMIz measurement at 2 years, categorized into four levels: <5th, 5th–<85th, 85th–<95th or ≥95th percentiles and compared those adjusted relative risk estimates to those associated with BMIz trajectories. To assess the variance of continuous cardio-metabolic outcomes explained by trajectory vs static BMIz groupings beyond baseline covariates, a basic model was first fitted by including predictors of cardio-metabolic outcomes (maternal income level, ppBMI, height, GWG, parity, GA at delivery, breastfeeding, child ethnicity, and sex) as independent variables in a linear regression analysis. Subsequently, the trajectory or static BMIz groupings were added separately to those baseline models; the increment in variance explained beyond that of baseline covariates was assessed using the adjusted R2 values. The area under the receiver operating characteristics (ROC) curve was also used to compare the predictive value of trajectory vs static BMIz groupings for obesity and prehypertension at 5 years.
All models were adjusted for maternal income level, ppBMI, GWG, parity, GA at delivery, breastfeeding, child ethnicity, and sex to reduce confounding, and exact age at measurement to improve precision. Recent studies have found a relationship between maternal height and offspring adiposity in childhood36; therefore, we also considered maternal height as a potential confounding variable in the analysis. Multiple imputation was used to account for missing covariates (maternal income level, n = 77; ppBMI, n = 105; height, n = 27; GWG, n = 33; breastfeeding, n = 142) with 20 imputations based on the Markov-chain Monte Carlo technique, using MI IMPUTE to impute the missing values and MI ESTIMATE to analyze the imputed datasets. These analyses were performed using Stata 13 software (StataCorp LP, TX).
Data availability
Data are available from the National University of Singapore LORIS Database for researchers who meet the criteria for access to confidential data.
Results
BMIz trajectories in the first 2 years
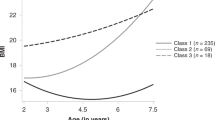
Based on the BIC, BLRT and posterior probabilities, the “best-fitting model” (lowest BIC, significant BLRT p-value and posterior probability ≥0.70 for each subgroup) was the four-class model; the fit information indices are presented in Supplemental Table 2. Table 1 describes the demographic and clinical characteristics separately by BMIz trajectories. A large majority of the children (73.2%, n = 857) exhibited a normal BMIz trajectory, centred on BMIz = 0. The other three trajectories had distinct shapes: 13.2% (n = 155) had a stable low BMIz trajectory (average BMIz = −1 SD), 8.6% (n = 100) exhibited a stable high BMIz trajectory (average BMIz = + 1SD) and 5.0% (n = 58) showed a rapid BMIz gain after 3 months trajectory in the first 2 years of life (Fig. 1). Sensitivity analyses using subjects with no missing BMIz data in the first 2 years (n = 536) showed the same trajectory patterns, further illustrating the robustness of the extracted BMIz trajectories (Supplemental Figure 2). Amongst children with no missing BMIz data, cross-tabulation analyses showed similar group assignments as in the full dataset (Supplemental Table 3). Supplemental Table 4 shows the corresponding percentiles at 2 years for each BMIz trajectory group; these percentiles closely approximate the thresholds based on standard categories (i.e., 5th, 50th, 85th and 95th percentiles).
BMIz trajectories in the first 2 years of life in the GUSTO cohort. Red line = stable low BMIz trajectory (n = 155); Green line = Normal BMIz trajectory (n = 857); Purple line = stable high BMIz trajectory (n = 100); Blue line = Rapid BMIz gain after 3 months trajectory (n = 58). Values indicate mean BMIz at each time point.
Predictors of BMIz trajectories in the first 2 years
The likelihood ratio test statistic of the multinomial logistic regression model yielded a p value of <0.01, indicating a significant association of the combined predictors with BMIz trajectory outcomes. Children born preterm [odds ratio (95% CI): 2.23 (1.28–3.36)] and of Indian ethnicity [2.36 (1.54–3.63) vs Chinese ethnicity] were more likely to be in the stable low BMIz trajectory. Children of Malay [3.49 (1.48–8.25)] and Indian ethnicity [6.30 (2.66–14.73)] were more likely to be in the rapid BMIz gain trajectory than those of Chinese ethnicity, while those born to multiparous mothers were more likely to be in the stable high BMIz trajectory [1.67 (1.07–2.61)] vs primiparous mothers. A 1-SD increase in maternal ppBMI or GWG was associated with a higher likelihood of belonging to the rapid BMIz gain and stable high BMIz trajectories, and a lower likelihood of belonging to the stable low BMIz trajectory (Table 2). Similarly, a 1-SD increase in maternal height was associated with a lower likelihood [0.79 (0.66–0.94)] of her child’s being in the stable low BMIz trajectory. Sensitivity analyses showed booking BMI, in place of ppBMI, was also a significant predictor of BMIz trajectories in the first 2 years (Supplemental Table 5).
Associations of early BMIz trajectories with later (5 years) cardio-metabolic measures
After adjusting for potential confounders, the stable low BMIz trajectory was significantly associated with lower WHtR [β (95% CI): −0.02SD units (−0.03, −0.01)], SSF [−0.43SD units (−0.62, −0.24)], FMI [−0.55SD units (−0.94, −0.15)], LMI [−0.68SD units (−1.07, −0.29)], SBP [−2.86 mmHg (−4.92, −0.80)] and DBP [−1.61 mmHg (−3.01, −0.20)], compared to the normal BMIz trajectory. Both the stable high BMIz and rapid BMIz gain trajectories were associated with higher WHtR and SSF, but not with SBP and DBP, compared to the normal BMIz trajectory (Table 3). Only the rapid BMIz gain trajectory was associated with higher FMI [0.97SD units (0.32, 1.63)], and only the stable high BMIz trajectory was associated with higher LMI [0.45SD units (0.05, 0.86)]. In addition, children in the rapid BMIz gain trajectory had significantly higher adiposity (WHtR, SSF, FMI) at 5 years compared to children in the stable high BMIz trajectory (Supplemental Table 6). Compared to the normal BMIz trajectory, both stable high BMIz [relative risk (95% CI): 3.22 (1.73, 6.05)] and rapid BMIz gain [2.56 (1.19–5.53)] trajectories were associated with an increased risk of obesity, while the stable low BMIz trajectory was associated with a decreased risk of obesity [0.12 (0.02, 0.85)] at 5 years. No significant associations were observed between early BMIz trajectories and later prehypertension (Table 4).
Comparing early BMIz trajectories and static BMIz in predicting cardio-metabolic measures at 5 years
Early BMIz trajectory was more predictive of body composition measures (FMI and LMI) and obesity at 5 years than was (static) BMIz at 2 years. For FMI and LMI, the adjusted R2 values were 26.5% and 17.6%, respectively, in fully-adjusted models with BMIz trajectories; these R2 values were higher than those for static BMIz at 2 years (16.9% and 14.6%, respectively) (Supplemental Table 7). Furthermore, all BMIz trajectories in the first 2 years (compared to the normal BMIz trajectory) were significantly associated with obesity at 5 years (a negative association for the stable low BMIz trajectory and positive associations for the stable high BMIz and rapid BMIz gain trajectories), while BMIz ≥95th percentile at 2 years (compared to BMIz 5th – 85th percentile) was the only static measure found to be significantly associated with obesity at 5 years [relative risk (95% CI): 6.96 (3.67–13.20)] (Table 4). The area under the ROC curve was also higher in fully-adjusted models for BMIz trajectories, compared to those for static BMIz at 2 years (Supplemental Table 7).
Discussion
We have identified four distinct BMIz trajectories in the first 2 years of life in a multi-ethnic cohort of Asian children. We observed ethnic-specific differences and several factors predictive of the BMIz trajectories. Our findings suggest that different BMIz trajectories in the first 2 years reflect differential changes in body composition (fat vs lean mass) and are more predictive of body composition and obesity in later childhood than a single time point assessment of BMIz at 2 years.
The trajectory patterns observed in our study are consistent with those reported in previous studies by Magee et al.13 and Ventura et al.15, which also identified four BMI trajectories in childhood using LCGMM. The combined findings thus suggest that these four distinct BMI trajectories are real, rather than an artefact of the data-driven nature of the LCGMM technique. Other studies by Kwon et al.18 and Giles et al.17 also identified four BMI trajectories with similar patterns; those studies utilized a different technique known as group-based trajectory modelling, however, which assumes no within-class variation (i.e., variance in each subgroup is fixed to zero)8. LCGMM allows for greater heterogeneity because of its estimation of within-class variance for each trajectory10, 11.
The comparative strength and limitations of the LCGMM method over other alternative approaches (e.g., tracing growth on a growth chart) are the subject of some debate37. Clinicians often follow a “rule-of-thumb” method, in which excessive growth is indicated by crossing major percentile lines on a standard growth chart38. That method is simple and straightforward to implement, while LCGMM is relatively computer-intensive. The choice of the correct model and number of classes in LCGMM is also not always straightforward. The “rule-of-thumb” method however, assumes growth to be a linear function of size at different ages, which could subject the observed associations to statistical artefacts. LCGMM is capable of modelling non-linear growth curves, estimating individual trajectories and identifying distinctive subgroups in the population37. We believe these advantages outweigh its relative complexity.
We found that maternal adiposity-related factors (ppBMI, GWG) were associated with BMIz trajectories reflecting larger child size (stable high-weight and rapid weight gain). The positive relation between maternal adiposity-related factors and child size has been reported in other populations39, 40, as well as in our own cohort27, 41. Our findings provide further evidence that over-nutrition in utero may lead to high-risk BMI trajectories during early childhood. Although the mechanism is still not well-understood, it is believed that this may occur through increased transfer of maternal energy substrates, such as glucose, lipids and amino acids to the fetus28, 42, with the combined increase in all fuels likely contributing to the development of high-risk trajectories during early childhood28. We did not find an association between breastfeeding and the BMIz trajectories, consistent with earlier studies by Pryor et al.14, Giles et al.17 and Garden et al.43. While meta-analyses have suggested that breastfeeding is associated with a reduced risk of childhood overweight and obesity, this evidence is largely based on observational studies; reverse causality and residual confounding by behavioral or socio-economic attributes may explain the results of those studies.
We also observed ethnic differences in BMIz trajectories, with Malay children more likely than Chinese children to have rapid BMIz gain trajectories and Indian children more likely to have stable low BMIz or rapid BMIz gain trajectories. We have previously described that Indian children had smaller birth sizes and lower BMI in infancy compared to Chinese children28; this may potentially explain the relationship between Indian ethnicity and a low BMIz trajectory, which is the result of BMI tracking. Similar ethnic differences in rapid BMI gain trajectories have also been reported between African-American and white children in the U.S12. We have no clear biological explanation for the observed ethnic differences in rapid BMIz gain trajectories; an interplay between genetic and epigenetic factors, as well as a shared obesogenic environment, may be operating.
Maintenance of a stable high BMIz trajectory or accelerated BMIz gain was strongly associated with higher adiposity and increased obesity risk, while maintenance of a stable low BMIz trajectory protected against increased adiposity and obesity risk at 5 years, in line with earlier findings17, 18. This relationship may potentially be explained by BMI tracking, which has been demonstrated from infancy to middle childhood44. In addition, all BMIz trajectories in the first 2 years were significantly associated with obesity at 5 years (a negative association for stable low BMIz trajectory and positive associations for stable high BMIz and rapid BMIz gain trajectories), while BMIz ≥95th percentile at 2 years was the only static measure with a significant association. A drawback of previous studies is the utilization of BMI at a single time-point as a predictor of subsequent obesity and cardiovascular risk4, 5. Such static assessments of BMI provide a snapshot of the “intensity” of adiposity, while ignoring the dynamics of BMI over time, such as duration, age of onset, and rate of rise of adiposity, which may have profound effects. Our findings provide evidence that both duration (reflected by stable high BMIz and stable low BMIz trajectories) and age of onset (reflected by rapid BMIz gain trajectory) of excess adiposity may be important factors in predicting subsequent cardio-metabolic risk. Our findings suggest that identification of developmental BMIz trajectories in the first 2 years may be helpful in identifying high-risk groups early in life, and thus in tailoring preventive interventions.
Strengths of our study include its prospective design, which is crucial for assessing the predictors of BMIz trajectories and their associations with cardio-metabolic measures later in childhood. Given the paucity of data in Asian populations on growth trajectories and their relation to later health outcomes, our findings fill an important gap and provide new insights into the role of early BMI development and its potential contribution to future metabolic disease risk in Asian populations. Our study also included several measures of BMI in the first 2 years, providing greater granularity in estimating BMIz trajectories, as well as several measures of adiposity at age 5 years, including WHtR, skinfolds and fat mass.
Limitations include the fact that maternal pre-pregnancy weight was self-reported at study enrolment, which may be affected by errors in recall. However, our data showed a strong correlation between self-reported pre-pregnancy weight and measured booking weight (ρ = 0.96), and sensitivity analyses using booking BMI as a predictor showed similar observations. Maternal smoking during pregnancy was not included as a predictor, owing to low prevalence in our study sample (2%), but was unrelated to child adiposity in previous analyses45. We were also unable to account for childhood dietary patterns or physical activity at 5 years, which may reflect exposure to an obesogenic environment. Half of the women approached were not eligible for inclusion in the cohort, and we had no wish to generalize our findings to ineligible women and children. We have previously shown that the ethnic background of those recruited and not recruited differed between these two groups, reflecting our a priori plans to recruit disproportionately from the minority ethnic groups23. Moreover, the strong associations of the stable high BMIz and rapid BMIz gain trajectories with increased adiposity and obesity risk observed in our study were in line with recent findings17, 18, also suggesting the robustness of our study findings. Finally, our study did not measure other cardio-metabolic biomarkers such as blood glucose, insulin, lipids, triglycerides and C-peptide, and thus lacking outcomes such as dysglycemia or dyslipidemia. In future studies, data on these outcomes would help clarify the health implications of our findings.
In conclusion, BMIz trajectories in the first 2 years were associated with cardio-metabolic measures later in childhood, which in turn are known predictors of cardio-metabolic outcomes in adult life. The potential public health and clinical implications of our findings are worth noting. First, identification of developmental BMIz trajectories in the first 2 years may be helpful in identifying high-risk groups. Second, the assessment of prenatal predictors of BMIz trajectories may enhance our understanding of etiologic pathways of cardio-metabolic disease. Lastly, the predictors of early childhood BMIz trajectories, especially modifiable ones (e.g., pre-pregnancy BMI and gestational weight gain), may help in developing effective preventive clinical and public health interventions for cardio-metabolic disease. Future follow-up of our cohort will be important to assess whether the associations we observed persist later in life.
References
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 384, 766–781, doi:710.1016/S0140-6736(1014)60460–60468. Epub 62014 May 60429 (2014).
Bass, R. & Eneli, I. Severe childhood obesity: an under-recognised and growing health problem. Postgrad Med J. 91, 639–645, doi:610.1136/postgradmedj-2014-133033. Epub 132015 Sep 133033 (2015).
Sahoo, K. et al. Childhood obesity: causes and consequences. J Family Med Prim Care. 4, 187–192, doi:110.4103/2249-4863.154628 (2015).
Baker, J. L., Olsen, L. W. & Sørensen, T. I. Childhood body-mass index and the risk of coronary heart disease in adulthood. New England journal of medicine 357, 2329–2337 (2007).
Twig, G. et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N Engl J Med. 374, 2430–2440, doi:2410.1056/NEJMoa1503840. Epub 1502016 Apr 1503813 (2016).
Huang, R. C., Burrows, S., Mori, T. A., Oddy, W. H. & Beilin, L. J. Lifecourse Adiposity and Blood Pressure Between Birth and 17 Years Old. Am J Hypertens. 28, 1056–1063, doi:1010.1093/ajh/hpu1266. Epub 2015 Jan 1019 (2015).
Huang, R. C. et al. Lifecourse childhood adiposity trajectories associated with adolescent insulin resistance. Diabetes Care. 34, 1019–1025, doi:1010.2337/dc1010-1809. Epub 2011 Mar 1014 (2011).
Nagin, D. S. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychological methods 4, 139 (1999).
Wang, M. & Bodner, T. E. Growth mixture modeling identifying and predicting unobserved subpopulations with longitudinal data. Organizational Research Methods 10, 635–656 (2007).
Muthén, B. The potential of growth mixture modelling. Infant and Child Development 15, 623–625, doi:10.1002/icd.482 (2006).
Muthen, B. & Muthen, L. K. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 24, 882–891 (2000).
Li, C., Goran, M. I., Kaur, H., Nollen, N. & Ahluwalia, J. S. Developmental trajectories of overweight during childhood: role of early life factors. Obesity (Silver Spring). 15, 760–771 (2007).
Magee, C. A., Caputi, P. & Iverson, D. C. Identification of distinct body mass index trajectories in Australian children. Pediatr Obes. 8, 189–198, doi:110.1111/j.2047-6310.2012.00112.x. Epub 02012 Nov 00119 (2013).
Pryor, L. E. et al. Developmental trajectories of body mass index in early childhood and their risk factors: an 8-year longitudinal study. Arch Pediatr Adolesc Med. 165, 906–912, doi:910.1001/archpediatrics.2011.1153 (2011).
Ventura, A. K., Loken, E. & Birch, L. L. Developmental trajectories of girls’ BMI across childhood and adolescence. Obesity (Silver Spring). 17, 2067–2074, doi:2010.1038/oby.2009.2123. Epub 2009 May 2067 (2009).
Ziyab, A. H., Karmaus, W., Kurukulaaratchy, R. J., Zhang, H. & Arshad, S. H. Developmental trajectories of Body Mass Index from infancy to 18 years of age: prenatal determinants and health consequences. J Epidemiol Community Health. 68, 934–941, doi:910.1136/jech-2014-203808. Epub 202014 Jun 203803 (2014).
Giles, L. C. et al. Growth trajectories in early childhood, their relationship with antenatal and postnatal factors, and development of obesity by age 9 years: results from an Australian birth cohort study. Int J Obes (Lond). 39, 1049–1056, doi:1010.1038/ijo.2015.1042. Epub 2015 May 1026 (2015).
Kwon, S., Janz, K. F., Letuchy, E. M., Burns, T. L. & Levy, S. M. Association between body mass index percentile trajectories in infancy and adiposity in childhood and early adulthood. Obesity (Silver Spring). 25, 166–171, doi:110.1002/oby.21673. Epub 22016 Nov 21672 (2017).
Blake-Lamb, T. L. et al. Interventions for Childhood Obesity in the First 1,000 Days A Systematic Review. Am J Prev Med. 50, 780–789, doi:710.1016/j.amepre.2015.1011.1010. Epub 2016 Feb 1022 (2016).
Woo Baidal, J. A. et al. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med. 50, 761–779, doi:710.1016/j.amepre.2015.1011.1012. Epub 2016 Feb 1022 (2016).
Kurpad, A. V., Varadharajan, K. S. & Aeberli, I. The thin-fat phenotype and global metabolic disease risk. Curr Opin Clin Nutr Metab Care. 14, 542–547, doi:510.1097/MCO.1090b1013e32834b32836e32835e (2011).
Ogden, C. L., Carroll, M. D., Kit, B. K. & Flegal, K. M. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA 311, 806–814, doi:10.1001/jama.2014.732 (2014).
Soh, S. E. et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 43, 1401–1409, doi:1410.1093/ije/dyt1125. Epub 2013 Aug 1402 (2014).
Aris, I. M. et al. Associations of gestational glycemia and prepregnancy adiposity with offspring growth and adiposity in an Asian population. Am J Clin Nutr. 102, 1104–1112, doi:1110.3945/ajcn.1115.117614. Epub 112015 Sep 117630 (2015).
Hutcheon, J. A. et al. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr. 97, 1062–1067, doi:1010.3945/ajcn.1112.051706. Epub 052013 Mar 051706 (2013).
Cai, S. et al. Infant feeding effects on early neurocognitive development in Asian children. Am J Clin Nutr. 101, 326–336, doi:310.3945/ajcn.3114.095414. Epub 092014 Dec 095410 (2015).
Aris, I. M. et al. Postnatal height and adiposity gain, childhood blood pressure and prehypertension risk in an Asian birth cohort. Int J Obes 14, 40 (2017).
Aris, I. M. et al. Infant body mass index peak and early childhood cardio-metabolic risk markers in a multi-ethnic Asian birth cohort. Int J Epidemiol 20 (2016).
Chen, L. W. et al. Body composition measurement in young children using quantitative magnetic resonance: A comparison with air displacement plethysmography. Pediatric Obesity Under review (2017).
de Onis, M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 450, 76–85 (2006).
de Onis, M. et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 85, 660–667 (2007).
Lim, W. Y. et al. Maternal Blood Pressure During Pregnancy and Early Childhood Blood Pressures in the Offspring: The GUSTO Birth Cohort Study. Medicine (Baltimore). 94, e1981, doi:1910.1097/MD.0000000000001981 (2015).
The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 114, 555–576 (2004).
Little, T. D., Jorgensen, T. D., Lang, K. M. & Moore, E. W. On the joys of missing data. J Pediatr Psychol. 39, 151–162, doi:110.1093/jpepsy/jst1048. Epub 2013 Jul 1098 (2014).
Engle, W. A. Age terminology during the perinatal period. Pediatrics. 114, 1362–1364 (2004).
Azcorra, H., Dickinson, F. & Datta Banik, S. Maternal height and its relationship to offspring birth weight and adiposity in 6- to 10-year-old Maya children from poor neighborhoods in Merida, Yucatan. Am J Phys Anthropol. 161, 571–579, doi:510.1002/ajpa.23057. Epub 22016 Jul 23028 (2016).
Tu, Y. K., Tilling, K., Sterne, J. A. & Gilthorpe, M. S. A critical evaluation of statistical approaches to examining the role of growth trajectories in the developmental origins of health and disease. Int J Epidemiol. 42, 1327–1339, doi:1310.1093/ije/dyt1157. Epub 2013 Sep 1314 (2013).
Taveras, E. M. et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 165, 993–998, doi:910.1001/archpediatrics.2011.1167 (2011).
Davey Smith, G., Steer, C., Leary, S. & Ness, A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC). Arch Dis Child. 92, 876–880, Epub 2007 Jun 2026 (2007).
Reilly, J. J. et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 330, 1357. Epub 2005 May 1320 (2005).
Lin, X. et al. Ethnic Differences in Effects of Maternal Pre-Pregnancy and Pregnancy Adiposity on Offspring Size and Adiposity. J Clin Endocrinol Metab. 100, 3641–3650, doi:3610.1210/jc.2015–1728. Epub 2015 Jul 3622 (2015).
Whitaker, R. C. & Dietz, W. H. Role of the prenatal environment in the development of obesity. J Pediatr. 132, 768–776 (1998).
Garden, F. L., Marks, G. B., Simpson, J. M. & Webb, K. L. Body mass index (BMI) trajectories from birth to 11.5 years: relation to early life food intake. Nutrients. 4, 1382–1398, doi:1310.3390/nu4101382 (2012).
Krassas, G. E. & Tzotzas, T. Do obese children become obese adults: childhood predictors of adult disease. Pediatr Endocrinol Rev. 1, 455–459 (2004).
Zhou, Y. et al. Sleep duration and growth outcomes across the first two years of life in the GUSTO study. Sleep Med. 16, 1281–1286, doi:1210.1016/j.sleep.2015.1207.1006. Epub 2015 Jul 1217 (2015).
Acknowledgements
The GUSTO study group includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Carolina Un Lam, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Claudia Chi, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, E Shyong Tai, Elaine Tham, Elaine Quah Phaik Ling, Evelyn Xiu Ling Loo, Falk Mueller- Riemenschneider, George Seow Heong Yeo, Helen Chen, Heng Hao Tan, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joanne Yoong, Joao N. Ferreira, Jonathan Tze Liang Choo, Jonathan Y Bernard, Joshua J. Gooley, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Kuan Jin Lee, Leher Singh, Lieng Hsi Ling, Lin Lin Su, Lourdes Mary Daniel, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Melvin Khee-Shing Leow, Michael Meaney, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Paulin Tay Straughan, Pratibha Agarwal, Queenie Ling Jun Li, Rob M. van Dam, Salome A. Rebello, See Ling Loy, S. Sendhil Velan, Seng Bin Ang, Shang Chee Chong, Sharon Ng, Shiao-Yng Chan, Shirong Cai, Sok Bee Lim, Stella Tsotsi, Chin-Ying Stephen Hsu, Sue Anne Toh, Swee Chye Quek, Victor Samuel Rajadurai, Walter Stunkel, Wayne Cutfield, Wee Meng Han, Yin Bun Cheung, Yiong Huak Chan, and Zhongwei Huang. This work was supported by the Translational Clinical Research (TCR) Flagship Programme on Developmental Pathways to Metabolic Disease, NMRC/TCR/004-NUS/2008; NMRC/TCR/012- NUHS/2014 funded by the National Research Foundation (NRF) and administered by the National. Medical Research Council (NMRC), Singapore. KMG is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement no. 289346.
Author information
Authors and Affiliations
Contributions
Izzuddin M. Aris analyzed the data, interpreted the results, contributed to the discussion and wrote the manuscript. Ling Wei Chen interpreted the results and wrote the manuscript. Mya Thway Tint, Wei Wei Pang and Shu E. Soh contributed to the discussion and were responsible for recruitment of the GUSTO cohort. Seang-Mei Saw, Lynette Pei-Chi Shek, Kok-Hian Tan, Peter D. Gluckman, Yap-Seng Chong and Fabian Yap contributed to the discussion and were responsible for the conception and recruitment of the GUSTO cohort. Keith M. Godfrey, Michael S. Kramer and Yung Seng Lee interpreted the results, contributed to the discussion, and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
Keith M Godfrey, Yap Seng Chong and Yung Seng Lee have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. Keith M Godfrey and Yap Seng Chong are part of an academic consortium that has received research funding from Abbot Nutrition, Nestec and Danone. All other authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aris, I.M., Chen, LW., Tint, M.T. et al. Body mass index trajectories in the first two years and subsequent childhood cardio-metabolic outcomes: a prospective multi-ethnic Asian cohort study. Sci Rep 7, 8424 (2017). https://doi.org/10.1038/s41598-017-09046-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09046-y
This article is cited by
-
Impact of preconception and antenatal supplementation with myo-inositol, probiotics, and micronutrients on offspring BMI and weight gain over the first 2 years
BMC Medicine (2024)
-
Tracking of serum lipids in healthy children on a year-to-year basis
BMC Cardiovascular Disorders (2023)
-
Association of BMI trajectories with cardiometabolic risk among low-income Mexican American children
Pediatric Research (2023)
-
Humero-femoral index and diabetes risk in the US population– a case study
Journal of Diabetes & Metabolic Disorders (2023)
-
Early childhood body mass index trajectory and overweight/obesity risk differed by maternal weight status
European Journal of Clinical Nutrition (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.