Abstract
In eukaryotes, RNA silencing, mediated by small interfering RNAs, is an evolutionarily widespread and versatile silencing mechanism that plays an important role in various biological processes. Increasing evidences suggest that various components of RNA silencing pathway are involved in plant defense machinery against microbial pathogens in Arabidopsis thaliana. Here, we show genetic and molecular evidence that Arabidopsis SDE5 is required to generate an effective resistance against the biotrophic bacteria Pseudomonas syringae pv. tomato DC3000 and for susceptibility to the necrotrophic bacteria Erwinia caratovora pv. caratovora. SDE5, encodes a putative mRNA export factor that is indispensable for transgene silencing and the production of trans-acting siRNAs. SDE5 expression is rapidly induced by exogenous application of phytohormone salicylic acid (SA), methyl jasmonate (MeJA), phytopathogenic bacteria, and flagellin. We further report that SDE5 is involved in basal plant defense and mRNA export. Our genetic data suggests that SDE5 and Nonexpressor of PR Gene1 (NPR1) may contribute to the same SA-signaling pathway. However, SDE5 over-expressing transgenic plant exhibits reduced defense responsive phenotype after flagellin treatment. Taken together, these results support the conclusion that SDE5 contributes to plant innate immunity in Arabidopsis.
Similar content being viewed by others
Introduction
Plants have evolved potent inducible immune response to multiple pathogen attacks and bacterial pathogens provide a useful example of how pathogens are encountered at various levels. The first layer of defense responses is originated by perception of conserved molecular features of microbes, termed pathogen-associated molecular patterns (PAMPs)1, 2. PAMPs activate pattern-recognition receptors (PRRs), which in turn initiate diverse downstream signaling events that ultimately result in the activation of a basal resistance that is called PAMP-triggered immunity (PTI)3, 4. Bacterial molecules containing PAMPs include flagellin (the major protein of bacterial flagellum), lipopolysaccharides and the bacterial translation elongation factor, EF-Tu1. Flg22, a conserved 22 amino-acid peptide derived from the N terminus of Pseudomonas syringae flagellin5, is perceived by the receptor flagellin insensitivity 2 (FLS2) and subsequently activates downstream events such as mitogen-activated protein kinase (MAPK) cascades and WRKY transcription factors in Arabidopsis (Arabidopsis thaliana)6, 7. Bacteria counteract PTI by secreting defense-suppressing virulence effectors into host cells. As a counter defense strategy, host plants have evolved a repertoire of immune receptors, called disease resistance (R) proteins that can sense effectors and elicit effector-triggered immunity (ETI)3, 4. Both PTI and ETI are associated with the accumulation of defense signal molecules such as salicylic acid (SA), ethylene (ET), and jasmonic acid (JA). In Arabidopsis, SA-regulated defense responses including Pathogenesis-Related (PR) gene expression require the function of Nonexpressor of PR Gene1 (NPR1) gene, which encodes a 66-kD protein with ankyrin repeats8.
RNA silencing is an RNA-guided, evolutionarily widespread, and versatile silencing mechanism that controls gene expression at the transcriptional (TGS, Transcriptional Gene Silencing) and post-transcriptional (PTGS, Post-transcriptional Gene Silencing) levels. In plants, RNA silencing is triggered by double-stranded RNA (dsRNA), processed into 21- to 24-nt short interfering (si)RNA or micro (mi)RNA by RNaseIII-like enzymes called Dicer-like proteins named DCL1–49, 10. These small RNAs guide suppression of their target gene expression at the level of transcription, RNA stability or translation. RNA-induced silencing complexes invariably contain one member of the Argonaute (AGO) protein family11,12,13,14.
In plants, small RNAs including miRNAs and siRNAs regulate diverse processes including development, abiotic stress tolerance and defenses. Increasing studies indicate that host endogenous small RNAs and small RNA pathway components also participate in plant disease resistance against various pathogens, including bacteria, fungi, oomycetes and viruses. For example, in Arabidopsis, miR393 negatively regulates auxin signaling pathways and contributes to PTI15. Besides miR393, two other miRNA families, miR160 and miR167, are upregulated following Pseudomonas syringae pv. tomato (Pto) DC3000 infection and target members of auxin-response factors16. Although plants contain only several hundred miRNAs, they contain huge numbers of endogenous siRNAs, but only in a few cases the involvement of siRNAs in plant immunity has been described. In Arabidopsis, the natural antisense transcript (NAT)-derived endogenous nat-siRNAATGB2 and AtlsiRNA-1 are induced by the bacterial pathogen Pto DC3000 carrying an effector, AvrRpt2. These siRNAs play an important role in ETI by targeting negative regulators of the cognate R gene RPS2 signaling pathway17, 18. Consistent with a role of these small RNAs in plant immunity, proteins required for small RNA biogenesis and function, such as DCL1, Hua Enhancer1 (HEN1) and AGOs family, have been shown to be required for disease resistance to bacterial pathogens18,19,20,21,22,23,24.
Previous studies indicated that SDE5, a homologue of a human mRNA export protein, is an essential component of the trans-acting siRNA pathway and is required for sense transgene PTGS (S-PTGS) but not inverted repeat transgene-mediated PTGS (IR-PTGS)25, 26. Mutation in SDE5 also resulted in hyper-susceptibility to cucumber mosaic virus but not turnip mosaic virus25. However, the molecular mechanism by which SDE5 participates in plant defense system remains to be elucidated. Here, we report that SDE5 contributes to plant innate immunity in Arabidopsis via ETI pathway and suppresses PTI, while it could be induced by PAMP.
Results
SDE5 gene expression is upregulated upon SA, MeJA and flg22 application and Pto DC3000 inoculation
To determine the expression of SDE5 during plant basal defense, wild-type (WT) seedlings were infiltrated with the virulent Pto DC3000 at 2 × 106 colony-forming units per mL (cfu mL−1) and SDE5 transcript levels were then analyzed using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) at different time points. As shown in Fig. 1A, SDE5 expression was significantly up-regulated at 3 hours post inoculation (hpi). This upregulation was transient as gene expression returned to resting levels by 12 hpi.
Expression analyses of the SDE5 gene in response to pathogen inoculation, PAMP and hormonal treatments. (A) Fifteen-day-old Arabidopsis WT (Col-0) seedlings were vacuum infiltrated with 10 mM MgCl2 (Mock) or Pto strain DC3000 at 2 × 106 cfu mL−1. Samples were collected 0, 1, 3, 6 and 12 h post infiltrated (hpi) and the transcript levels of the SDE5 were determined by quantitative reverse-transcription polymerase chain reaction (qRT-PCR). (B) SDE5 gene expression was monitored at 3 h upon Pto DC3000 inoculation in mutants defective in SA production (sid2) or JA (jar1) and ethylene (ein2) signaling pathways, and also mutants altered in flagellin (fls2) or EF-Tu (efr) perception. (C) SDE5 gene expression was examined at 3 h spraying with plant hormones, SA (250 μM), MeJA (200 μM) using fifteen-day-old WT seedlings. (D) Kinetics of SDE5 gene expression in WT and the fls2 mutant in response to exogenous application of flg22. Fifteen-day-old seedlings grown on MS medium were elicited using water (mock) or 10 μM of flg22 and harvested at 0, 1, 3 and 6 h. ACTIN was used as an internal control. Error bars indicate the mean ± SD for each set of three independent experiments with significant difference at *P < 0.05 and **P < 0.01.
To analyze the signaling pathway that leads to SDE5 expression, we examined pathogen-induced changes of SDE5 transcript levels in WT, as well as in mutants altered in the production of SA (sid2), JA signaling (jar1) and ethylene perception (ein2) following pathogen Pto DC3000 challenge. We also considered mutation in NPR1, one of the central downstream regulators of SA signaling. As shown in Fig. 1B, the Pto DC3000-induced expression of SDE5 is unchanged in ein2 suggesting that the induction of SDE5 by the virulent bacterial pathogen is independent on EIN2. Interestingly, the level of SDE5 expression was significantly reduced in sid2, jar1 and npr1, indicating dependency on SA- and JA-signaling (Fig. 1B). The contribution of two PAMP receptors (FLS2 and EFR), involved in perception of flg22 and the elongation factor EF-Tu, respectively, to SDE5 expression were evaluated using their respective mutants. The Pto DC3000-induced expression of SDE5 was also reduced in the PAMP receptor-defective mutants (fls2 and efr) indicating a contribution of PTI (Fig. 1B). To further validate the involvement of SA and JA in the induction of SDE5, we tested WT plants 3 h after spraying with SA and MeJA and observed that SDE5 expression is 2–2.5-fold up-regulated (Fig. 1C).
The results obtained in Fig. 1B and previous reports27, 28, suggest a critical involvement of the FLS2-dependent signaling pathway in control of SDE5 expression. To confirm the induction of SDE5 during PTI, we next monitored the mRNA levels of SDE5 over a 6 h time course experiments. We found a significant induction of SDE5 expression at 3 h after flg22 peptide treatment (Fig. 1D). As expected, expression was not induced by flg22 in the fls2 mutant indicating that an important role of FLS2 in flg22 mediated SDE5 induction. Taken together, SDE5 expression data indicate a potential role for SDE5 in SA signaling, JA signaling and PTI in Arabidopsis.
Disruption of SDE5 decreases plant basal defense
In order to further elucidate the possible role of SDE5 in plant-pathogen interactions, a reverse genetic approach using mutant alleles of the SDE5 gene containing a T-DNA insertion has been performed. The T-DNA insertion is in the sixth exon of the SDE5 gene and resulted in the loss of detectable SDE5 transcript, indicating that sde5-3 26 is a loss-of function mutant. Another mutant line used in this work was sde5-2 which is known to be a partial loss-of-function mutant in mRNA export25. We also generated SDE5 overexpressing transgenic plants in the sde5-3 background using SDE5 coding sequence under the control of the Cauliflower mosaic virus-derived 35S promoter (Fig. S1). Two transgenic lines showing high expression of SDE5 were selected for further study and named OE-5 and OE-6. Then, we inoculated WT, sde5-2, sde5-3 and two overexpressing lines, OE-5 and OE-6, with a biotrophic pathogen, Pto DC3000, and monitored both bacterial growth (at 2 × 105 cfu mL−1) and disease symptom development (at 2 × 106 cfu mL−1). As shown in Fig. 2A, sde5-3 permitted nearly 10-fold more bacterial growth than the WT plants. The sde5-3 plants also developed significantly more severe disease symptoms than WT plants at 4 dpi (Fig. 2B). However, bacterial growth and disease development in partial loss-of-function mutant sde5-2 were not significant compared with WT. As expected, this disease phenotype has been overcome in OE plants indicating that SDE5 may act as a positive regulator in plant innate immunity.
Altered susceptibility to Pto DC3000 in sde5-3 mutant and transgenic line over-expressing the SDE5 gene. (A) Quantification of bacterial growth 0 or 4 days post-infiltration (dpi) on five-week-old plants after syringe inoculation with concentrations of 2 × 105 cfu mL−1 of the virulent bacterial strain Pto DC3000. The error bars indicate the mean ± SD for each set of three independent experiments with significant difference at *P < 0.05. (B) Disease symptoms in leaves of WT, sde5-2, sde5-3 and SDE5 overexpressing lines (OE-5 and OE-6) caused by Pto DC3000 infiltration. Leaves of five-week-old plants were syringe infiltrated with a concentration of 2 × 106 cfu mL−1 of Pto DC3000, and photographs were taken 4 dpi. Representative leaves are shown. Similar results were obtained in three independent experiments.
SA is a major plant defense hormone, central to the activation of a range of defenses including the induction of PR (pathogen-related) genes, systemic acquired resistance, and hypersensitive response29. Therefore, expression analysis of the well-known SA-dependent effector gene, PR1, was also performed in leaves of WT, sde5-3 mutant and OE-5 plants before and at various time points after inoculation with Pto DC3000. The result indicated that PR1 transcript accumulation was significantly reduced in sde5-3 compared with WT plants (Fig. 3A). Consistent with these findings, analysis of both loss-of-function SDE5 mutants and gain-of-function SDE5-overexpressing plants indicates that SDE5 contributes to SA mediated plant basal defense by modulating responses to bacterial strain Pto DC3000. The biosynthesis of SA is strongly induced upon pathogen infection. This pathogen-induced SA biosynthesis is believed to be controlled by several key components including PAD4, EDS5 and SID230,31,32. To further clarify the roles of SDE5 in SA-mediated basal defense, we examined the expression of PAD4 and SID2, in WT, sde5-3 mutant and OE-5 plants following Pto DC3000 inoculation. No significant difference was found between WT plants and other genotypes tested (Fig. 3B and C), supporting the hypothesis that the effect of SDE5 on PR-1 expression is independent of expression of genes involved with SA production and signaling. To study the epistatic relationships between SDE5 and SA, we also treated SA before Pto DC3000 inoculation in Col-0, sde5-2, sde5-3, OE-5 and npr1, and monitored symptoms. Disease symptom of sde5-3 was not rescued by SA treatment suggesting that SDE5 is acting downstream of SA signaling rather than affecting SA biosynthesis (Fig. S2).
Expression analyses of defense marker gene and SA signaling pathway components upon Pto DC300 inoculation. (A) Analyses of PR1 marker gene in various genetic backgrounds. Fifteen-day-old seedlings of WT, sde5-2, sde5-3 and SDE5 overexpressing lines (OE-5 and OE-6) were vacuum infiltrated with concentrations of 2 × 106 cfu mL−1 of the Pto DC3000 and harvested at 0, 12 and 24 hpi. ACTIN was used as an internal control. (B–D) Expression of genes associated with SA mediated defense signaling pathway such as PAD4 (B), SID2 (C), and NPR1 (D) using seedlings of plants in A. Error bars represent the standard error from three biological replicates. E, Bacterial growth assay of Pto DC3000 in WT, sde5-3, OE-5, npr1, and sde5-3npr1 double mutant plants after syringe inoculation with concentrations 2 × 105 cfu mL−1. The error bars indicate the mean ± SD for each set of three independent experiments with significant difference at *P < 0.05.
The SA-mediated signaling pathway regulated by NPR1 is one of the most important pathways in plant defense33, 34. The observed down-regulation of SDE5 gene expression in npr1 mutant plants indicates that functional NPR1 protein is required for pathogen responsiveness of SDE5 (Fig. 1B). This result prompted us to investigate whether the accumulation of NPR1 transcript levels are altered in WT and sde5-3 mutant upon Pto DC3000 inoculation. However, no significant difference in the NPR1 expression level was observed between the plants tested, indicating that SDE5 may function downstream of NPR1 in SA-signaling (Fig. 3D). Moreover, when challenged with virulent pathogens, the sde5-3/npr1 double mutant was not more susceptible to Pto DC3000 than npr1 (Fig. 3E), indicating that SDE5 and NPR1 might contribute to the same SA-signaling pathway in Arabidopsis.
The sde5-3 mutant confers elevated disease resistance to ECC
Since, SDE5 expression was induced by exogeneous MeJA treatment, we evaluated the contribution of SDE5 to plant responses to the necrotrophic pathogen, Erwinia caratovora pv. caratovora (ECC). We inoculated WT, sde5-2, sde5-3, OE-5 and OE-6 plants with ECC and observed bacterial accumulation at 4 dpi. As shown in Fig. 4A, sde5-3 mutant plants restricted the growth of ECC relative to WT plants, indicating that SDE5 promotes the growth of ECC. ECC susceptibility was restored to WT levels in the OE-5 and OE-6 plants, further confirming that expression of the SDE5 gene promotes infection by this necrotrophic bacterial (Fig. 4A). In an effort to further examine the role of SDE5 in JA-mediated defenses, we determined the expression levels of plant defensin gene PDF1.2, a characteristic molecular response of plants to necrotrophic pathogen attack in different time points after pathogen inoculation. The induction of PDF1.2 was significantly increased in sde5-3 plants following the inoculation with ECC (Fig. 4B). This observation is congruent with the observation that sde5-3 plants showed enhanced disease resistance to this pathogen. Collectively, these results indicate that SDE5 plays positive and negative roles in SA- and JA-mediated pathogen defense, respectively, possibly by participating in the cross-talk between these signaling pathways.
sde5 potentiates the local disease response to ECC infection. (A) Bacterial proliferation assays. Five-week-old WT, sde5-2, sde5-3 and SDE5 overexpressing lines (OE-5 and OE-6) were inoculated with ECC at 2 × 105 cfu mL−1. Leaf discs were collected after 7dpi and observed bacterial accumulation. The graph shows a representative result out of three independent experiments. (B) Quantitative analyses of PDF1.2, a gene associated with necrotrophic pathogen in plant defense. Fifteen-day-old seedlings stated in A vacuum infiltrated with a concentration of 2 × 106 cfu mL−1 of ECC were harvested at 0, 12 and 24 hpi. ACTIN is used as internal control. The error bars indicate the mean ± SD for each set of three independent experiments with significant difference at *P < 0.05.
SDE5 is involved in PAMP triggered immunity in Arabidopsis
SA is required for the full activation of PTI35, 36. To better understand the contribution of SDE5 in PTI, we utilized the type-three secretion system (TTSS)-defective mutant Pto DC3000 hrcC − (Pto hrcC −) strain, which can elicit, but not suppress, PTI responses due to its inability to inject effector proteins within host cells37. The behavior of WT, sde5-2, sde5-3, OE-5 and OE-6 plants was analyzed following foliar inoculation with the Pto hrcC − strain, and, as expected, limited bacterial growth was observed in WT plants compared to the fully virulent bacteria following inoculation at 2 × 105 cfu mL−1. No significant differences were detected between WT and the sde5 mutant lines (Fig. 5A). In contrast, OE-5 and OE-6 plants were more susceptible to Pto hrcC − relative to the WT (Fig. 5A). We next examined the expression pattern of PR1 in response to Pto hrcC − inoculation in various genotype plants. As shown in Fig. 5B, PR1 gene expression was highly induced in sde5-3 plants with significant up-regulation at 24 hpi in comparison to other plants. These results reveal an opposite regulation of PR1 gene expression by SDE5 following infection with virulent Pto DC3000 versus the TTSS-deficient Pto hrcC − strain. These data further support the hypothesis that the responses observed with Pto DC3000 are TTSS-dependent and therefore may involve the activity of bacterial effectors in plant cells. Thus, SDE5 restricts susceptibility to virulent Pto DC3000, but has a less discernible effect on resistance to Pto hrcC − that cannot deliver effectors.
Altered responses were displayed by the sde5 mutants and the transgenic lines over-expressing the SDE5 upon TTSS-defective mutant Pto hrcC − strain. (A) Quantifications of in planta bacterial growth in the Arabidopsis genotypes as indicated were performed at 0 or 7 dpi using at 2 × 105 cfu mL−1 of hrcC −. Plants were placed under high humidity condition (in dew chamber) after infiltration for this experiment. The error bars indicate the mean ± SD for each set of three independent experiments with significant difference at *P < 0.05. Data are representative of four replicates of three independent experiments. (B) Quantitative analyses of PR1 using fifteen-day-old seedlings stated in (A) vacuum infiltrated with a concentration of 2 × 106 cfu mL−1 of Pto hrcC −. Samples were harvested at 0, 12 and 24 hpi. Each bar represents the relative expression of the genes compared with the ACTIN control. Similar results were obtained in independent experiments. Asterisk (*) indicate significant difference at a P value < 0.05.
The observed results indicate that SDE5 may negatively regulate PTI. We further analyzed the formation of cell wall depositions of callose, a PTI response that plays a critical role in the establishment of basal immunity38,39,40 in different genotype upon Pto hrcC − inoculation. Callose accumulated significantly less in OE-5 and OE-6 plants compared with WT and sde5-3 mutant plants, reinforcing a negative role for SDE5 in PTI response (Fig. 6).
Callose deposition significantly reduced in transgenic overexpressing plants upon hrcC − infection. (A) Callose detection was observed using leaves of four-week-old WT, sde5-3 and OE-5 plants infiltrated with Pto hrcC − bacteria (upper panel) and 10 mM MgCl2 (mock; lower panel). Staining was performed at 16 hpi. (B) Quantitative analyses of callose deposition on plants used in A using aniline blue staining. Experiments were performed using four leaves harvested from different plants for each genotype. The error bars indicate the mean ± SD for each set of three independent experiments with significant difference at *P < 0.01.
Next, a model bean (Phaseolus vulgaris) pathogen P. syringae pv. phaseolicola 1448a41, Pph, that is unable to efficiently suppress defense reactions in Arabidopsis was further introduced42, 43. Forsyth et al.44 established that a major determinant of non-host resistance to Pph in Arabidopsis is FLS2. Therefore, the pathosystem Arabidopsis–Pph is considered a classical model to study FLS2-mediated defenses and PTI. We examined the behavior of sde5-3 genotypes upon inoculation with the Pph strain. Significantly enhanced bacterial growth was observed in the transgenic OE-5 plant compared to WT (Fig. S3) at 7 dpi. This is consistent with the susceptibility of OE-5 and OE-6 plants to Pto hrcC − strain and confirms that SDE5 restricts PTI-dependent defense system.
Transgenic overexpressing plant exhibits altered molecular and cellular responses to flagellin application
The up-regulation of SDE5 transcript by exogenous flg22 treatment prompted us to examine the function of SDE5 protein in the FLS2 signaling pathway. Because callose formation is induced in response to PAMPs45, phenotypic assays for flg22-induced callose deposition were performed on sde5-3 genotypes46, 47. Callose accumulation is undetectable in fls2. And, as was observed with Pto hrcC − inoculation, flg22 induced callose accumulation is significantly reduced in transgenic OE-5 and OE-6 plants compared to WT and sde-5 plants (Fig. 7). These results suggest that SDE5 has a negative role in the production and/or deposition of callose probably by regulating FLS2-mediated signaling pathway.
Callose deposition is significantly reduced in transgenic overexpressing plants upon flg22 treatment. (A) Detection of callose papillae on leaves of WT, sde5-3, OE-5 and the fls2 mutant plants infiltrated with water (mock; lower panel. and 10 μM flg22 (upper panel) at 16 hpi. (B) Number of callose papillae was quantified on plants used in A using aniline blue staining. Four leaves harvested from different plants for each genotype. The error bars indicate the mean ± SD for each set of three independent experiments with significant difference at *P < 0.05 and **P < 0.01.
The perception of flagellin by plant cells also leads to important changes in gene expression27, 28. Therefore, we next analyzed the transcript level of two PTI marker genes including Flg22 RECEPTOR KINASE 1 (FRK1) and WRKY29, using mRNA from seedlings of WT, sde5-3, OE-5 and OE-6 lines prepared at 3 h before and after 1 μM flg22 treatment. Both FRK1 and WRKY29 transcripts increased in WT seedlings, but not in fls2 mutant, after flg22 elicitation, confirming that these genes are PAMP-responsive. Transcript accumulation of FRK1 and WRKY29 was similar among WT, sde5-3, OE-5 and OE-6 lines after flg22 treatment (from 0 to 3 h) (Fig. S4). These results indicate that SDE5 involves in the FLS2-signaling pathway at downstream or independently of the induction of these PAMP-responsive genes.
SDE5 is required for mRNA export
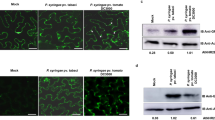
A previous report suggested that SDE5 has some similarity (12% identity and 58% similarity) to the C-terminal domain (PF03943) of human mRNA export factor TAP (or NXF1)48. The TAP C-terminal domain is particularly important for the function of TAP as an mRNA export mediator because it binds to nucleoporin complexes48, 49. Therefore, we speculated that mRNA export may also be affected in the sde5-3 mutant. To test this hypothesis, we performed an in situ hybridization assay50 to localize poly(A) signals in WT and sde5-3 mutant plants. The poly(A) signals were examined by confocal microscopy, using grp7 as a positive control51. As shown in Fig. 8, the fluorescein poly(A) signals were stronger in the nuclei of sde5-3 and grp7 than WT, OE-5 and OE-6, indicating that mRNA export is diminished in sde5-3 plants, resulting in mRNA accumulation in the nucleus. The signal was not observed in not probed sde5-3 mutant. This result indicates that Arabidopsis SDE5 is likely a contributing factor in the mRNA export pathway.
mRNA export is impaired in sde5-3 plants. Small leaf discs from fifteen days old WT, sde5-3, OE-5 and grp7 (cold treated for 2 days) plants were fixed and probed with a fluorescently labeled oligo(dT) probe. The samples were observed under an OLYMPUS 1 × 71 FV500 confocal laser-scanning microscope Green spots represent accumulation of mRNA. In sde5-3 and grp7 cells, mRNA accumulates at much higher level in the nuclei. Each experiment was repeated at least three times, and similar results were obtained. Scale bar = 100 μm.
Discussion
Our results convey that SDE5, a putative RNA export protein and an essential component of the trans-acting small interference RNA (tasiRNA) pathway, may have dual roles in plant defense mechanism. For instance, SDE5 acts as a positive regulator of plant defense system upon fully virulent Pto DC3000 strain (Figs 2 and 3). In contrast, less callose deposition and PR1 expression in SDE5-overexpressing lines upon flg22 application or Pto hrcC − inoculation indicate that SDE5 may act as a negative regulator of the flagellin signaling pathway (Figs 6 and 7).
Our results show that the SDE5 expression is rapidly induced in response to phytopathogenic bacteria and PAMP treatment (flagellin). Using a reverse genetics approach, we clearly demonstrated that SDE5 acts as a positive regulator of plant defense in response to biotrophic pathogen Pto DC3000. The knockout mutant exhibits higher levels of susceptibility than WT to Pto inoculations, and, conversely, SDE5 over-expressing transgenic lines exhibit fewer discernible symptoms and a significantly lower level of bacteria development compared to WT and knockout plants (Fig. 2). In addition, SA-regulated defense marker gene, PR1 expression was significantly downregulated in sde5-3 relative to WT and OE-5 plants (Fig. 3A).
Signaling cross-talk between plant hormones, such as SA, ET and JA, fine tunes the plant defense response52. In general, it is believed that SA signaling plays an important role in resistance to biotrophic pathogens and ET/JA signaling plays a crucial role in resistance to necrotrophic pathogens53. And both synergistic and antagonistic interactions between SA and ET/JA signaling pathways have been reported54. Up-regulation of JA-mediated responses and inhibition of SA-inducible defenses result in enhanced resistance to necotrophs but increased susceptibility to biotrophs55. Data reported in this study also indicates that SDE5 regulates disease susceptibility to the necrotrophic pathogen, ECC and the expression of PDF1.2 in an opposite way to the biotrophic pathogen (Fig. 4). Depending on the type of invader, a particular subset of defense responses might be activated, such as SA- and JA-mediated signaling pathways, to specifically fend off specific classes of pathogens.
To examine the role of SDE5 in PTI-mediated restriction of bacterial growth, we challenged Arabidopsis with two pathogens, Pto hrcC − and Pph. The Pto hrcC − bacteria trigger PTI and lack effectors to suppress it. FLS2-dependent PTI makes a critical contribution to the resistance of Arabidopsis to Pph, which lacks effectors required to efficiently block PTI43, 44. When either Pto hrcC − or Pph were introduced into WT, sde5-3 and over-expressing lines, the OE-5 and OE-6 plants showed increased susceptibility compared to WT control and sde5-3 plants (Figs 5 and S3). These results align with the observation of reduced cell wall depositions of callose and lower PR1 gene expression in the OE-5 and OE-6 plants (Figs 5 and 6). On the other hand, sde5-3 showed an opposite tendency, as indicated by the increased PR1 expression. Thus, SDE5 may have a negative role in the PAMP-signaling pathway resulting in reduced plant defense responses. Although SDE5 negatively regulates callose deposition, it did not affect the expression of early induced PTI marker genes such as FRK1 and WRKY29, indicating that SDE5 might regulate a later stage of plant defense signaling (Fig. S4). In addition, the results presented here convey the involvement of SDE5 in PTI through a flagellin-dependent signaling pathway. Further research would be required in order to explore the molecular mechanism controlled by SDE5 in plant defense signaling.
Previous studies revealed that siRNA-related components, such as AGO4, DRB4 and HPR1, involve in plant defense system. A mutation in the AGO4, that is associated with siRNAs showed enhanced susceptibility to the bacterial pathogen Pto DC300019. An important observation presented by the Lopez et al.56 showed that the co-existence of an enhanced disease resistance to a biotrophic bacteria, like Pto DC3000, with an enhanced susceptibility to necrotrophic fungi in RNA Polymerase V (Pol V)-defective mutant. Pol V is crucial for the RNA-directed DNA methylation (RdDM) pathway that is an epigenetic control mechanism driven by siRNAs. A mutation in double-stranded RNA binding protein 4 (DRB4) had a more severe effect on RPS2- and RPM1-mediated resistance to Pto DC3000 expressing avrRpt2 or avrRpm1, respectively57. Biochemical studies also suggest that DRB4 is required for the stability of RPS2 and RPM1 proteins and thereby resistance mediated by these R proteins57. Similarly, an mRNA export factor in Arabidopsis, HPR1, which is also involved in the production of endogenous and exogenous siRNA, acts as a positive regulator in plant defense signaling23. Based on these findings, we hypothesize that SDE5 functions in plant defense system in the same pathway as other siRNA components above upon biotrophic pathogen infection.
Many studies also have shown that the mutants impaired in mRNA export exhibit enhanced susceptibility to pathogens58. Such as, in Arabidopsis, mutations in MOS3 (modifier of snc1) and MOS11 (suppressor of npr1-1, constitutive 1) lead to defects in mRNA export58, 59. Similar to sde5-3 plants, both mos3 and mos11 single mutants are more susceptible to the virulent strain than WT58, 59. Pan et al.23 reported that HPR1, another mRNA trafficking protein, contributes to the basal defense against virulent pathogens. And accordingly, similar to hpr1, mos3 and mos11, mRNA export was affected in the sde5-3 mutant (Fig. 8). Consistent with these observations, we further conclude that SDE5, HPR1, MOS3 and MOS11 probably belong to the same pathway. Interestingly, prl1 mutant, a loss-of-function mutant of a second SDE5 homologue in Arabidopsis (at AT58720)60, exhibited enhanced susceptibility to various kinds of virulent and avirulent pathogens61. Thus, it would be speculated that SDE5 and PRL1 may function together to regulate innate immunity in Arabidopsis.
In this study, we found that SDE5, a homologue of a human mRNA export protein, is upregulated upon pathogen inoculation, exogenous PAMP and hormonal application and sde5 mutant plants are defective in mRNA export from the nucleus to cytoplasm. Loss of SDE5 function leads to increased susceptibility to the biotrophic pathogen Pseudomonas syringae and enhanced resistance toward necrotrophic Erwinia caratovora pv caratovora (ECC). In addition, knockout mutants and over-expressing transgenic plants also exhibit delayed defense responses after flg22 treatment. Taken together, our results establish that SDE5 contributes to plant innate immunity.
Although, we showed that SDE5 is involved in plant disease resistance, knowledge regarding the role of the mRNA export in plant defense responses is just emerging. Therefore, further functional analysis will be required to determine the detail molecular basis of mRNA export and defense responses in plants.
Methods
Plant lines, growth conditions and chemical treatments
Arabidopsis plants used in all experiments were derived from ecotype Columbia-0 (Col-0). Mutant lines were sde5-2 25, sde5-3 26, sid2 32, ein2 62, jar1 63 and npr1 8, and fls2 obtained from the Arabidopsis Biological Resource Center. Genotyping of the Transfer DNA (T-DNA) insertion lines was performed by PCR, using allele-specific primers. The double mutant was produced by crossing the above mutants and genotyped using the primer sets listed in Supplemental Table S1. Plants were grown either on soil or on plates containing Murashige and Skoog (MS) medium (Sigma-Aldrich) with 1% sucrose and 0.6% agar (Sigma-Aldrich) in a growth chamber (16 h of dark and 8 h of light) unless otherwise indicated. SA (250 μM), MeJA (200 μM), flg22 (10 μM) and sterile water (for mock) treatment was carried out in fifteen-day-old seedlings grown in MS medium with 1% sucrose. Seedlings were collected as indicated time after treatment, immediately frozen in liquid nitrogen, and stored at −80 °C until RNA purification.
Bacterial strains, growth conditions and inoculations
The P. syringae strains and ECC strain SCC164 used in this study were grown at 28 °C on King’s B (KB) medium supplemented with the appropriate antibiotics: 50 μg/ml rifampicin and 50 μg/ml kanamycin (for Pto DC3000) or 50 μg/ml rifampicin (for Pto hrcC −, Pph and ECC). Inoculation was performed as described65. In brief, five-week-old leaves were infiltrated with a needless syringe on the abaxial side at the indicated densities. Mock-treated plants were infiltrated with 10 mM MgCl2 alone. Disease symptoms and quantification of bacterial growth was performed at the indicated times. These experiments were repeated at least three times with similar results.
Plasmid constructs and plant transformation
Constructs overexpressing SDE5 were generated using the Gateway cloning system (Invitrogen). The SDE5 coding sequence was amplified by PCR using cDNA synthesized from total RNAs of Arabidopsis WT Col-0 seedlings as the template. The amplified fragment was cloned into the pENTR/D-TOPO vector and inserts were confirmed by sequencing. The entry clones were subsequently transformed into the destination vector pSITE-4CA (to overexpress the protein fused to RFP). These constructs were transformed into WT plants through Agrobacterium tumefaciens (GV3101 strain)-mediated floral dip method66. Homozygous transgenic lines were selected and transgene expression was analyzed by qRT-PCR and by confocal microscopy to the 35 S::SDE5-RFP transgenic plants.
Callose staining
Callose detection was performed as described65. In brief, four-week-old leaves were syringe-infiltrated with 2 × 106 cfu/ml of Pto hrcC −, 10 μM flg22, 10 mM MgCl2 (Mock for bacterium) and sterile water (Mock for flg22) and collected after 16 h. Whole leaves were collected, stained with 0.1% methyl blue, mounted in 50% glycerol, and examined with OPTIKA fluorescence microscope. Four leaves were prepared for each treatment. Three independent biological assays were performed. Representative views of these pictures were randomized, and the number of callose deposits was counted blind.
Gene expression analyses
Total cDNA was synthesized from total RNA of fifteen-day-old seedlings either pathogen inoculation or chemical treatment as indicated using the SuperScript III first strand synthesis system (Invitrogen), according to the manufacturer’s instructions. qRT-PCR was performed using a Bio-Rad CFX96 Real-Time System. Amplification curves and gene expression were normalized using ACTIN as an internal standard. The primers used for qRT-PCR were listed in Supplemental Table S1. Triplicate biological and technical replications were performed. Data were analyzed using BioRad CFX Manager 2.0 Software.
Poly(A) mRNA in situ localization assay
Poly(A) mRNA in situ hybridization was conducted essentially as described previously50, 51. Briefly, leaf samples of 2-week-old seedlings were fixed in a fixation buffer (3 mM NaH2PO4, 7 mM Na2HPO4, 120 mM NaCl, 2.7 mM KCl, 80 mM EGTA, 0.1% Tween 20, 5% formaldehyde, 10% DMSO, and 50% heptane), were subsequently incubated in 1:1 ethanol:xylene, and were washed with ethanol, methanol and finally with 1:1 methanol:fixation buffer. The samples were post-fixed in the fixation buffer for 30 min at room temperature, and were rinsed with Hyb Plus hybridization buffer (Sigma-Aldrich). After prehybridization in hybridization buffer for 1 h at 50 °C, 10 pmol of 45-mer oligo(dT) labeled with fluoresceine at the 5′-end was added and hybridized at 50 °C in darkness. The samples were then washed in 2 × SSC (1 × SSC is 0.15 M NaCl and 0.015 M sodium citrate), 0.1% SDS at 50 °C and in 0.2 × SSC, 0.1% SDS at 50 °C in darkness. The samples were immediately observed under an OLYMPUS 1 × 71 FV500 confocal laser-scanning microscope (Olympus America Inc.) with a 488-nm excitation laser and a 522/DF35 emission filter at identical laser strength. Each experiment was repeated at least three times, and similar results were obtained.
References
Boller, T. & Felix, G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406 (2009).
Zipfel, C. & Felix, G. Plants and animals: a different taste for microbes? Curr. Opin. Plant Biol. 8, 353–360 (2005).
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329, doi:10.1038/nature05286 (2006).
Chisholm, S. T., Coaker, G., Day, B. & Staskawicz, B. J. Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814 (2006).
Felix, G., Duran, J. D., Volko, S. & Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18, 265–276 (1999).
Asai, T. et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977–983 (2002).
Gomez-Gomez, L. & Boller, T. Flagellin perception: a paradigm for innate immunity. Trends Plant Sci. 7, 251–256 (2002).
Cao, H., Glazebrook, J., Clark, J. D., Volko, S. & Dong, X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88, 57–64 (1997).
Hyun, T. K., Uddin, M. N., Rim, Y. & Kim, J. Y. Cell-to-cell trafficking of RNA and RNA silencing through plasmodesmata. Protoplasma 248, 101–116 (2011).
Voinnet, O. Origin, biogenesis, and activity of plant microRNAs. Cell 136, 669–687 (2009).
Matzke, M. A. & Birchler, J. A. RNAi-mediated pathways in the nucleus. Nat. Rev. Genet. 6, 24–35 (2005).
Olmedo-Monfil, V. et al. Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 464, 628–632 (2010).
Zheng, Z., Qamar, S. A., Chen, Z. & Mengiste, T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 48, 592–605 (2006).
Zilberman, D., Cao, X. & Jacobsen, S. E. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 299, 716–719 (2003).
Navarro, L. et al. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312, 436–439 (2006).
Fahlgren, N. et al. High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS One 2, e219 (2007).
Katiyar-Agarwal, S. et al. A pathogen-inducible endogenous siRNA in plant immunity. Proc Natl Acad Sci USA 103, 18002–18007 (2006).
Katiyar-Agarwal, S., Gao, S., Vivian-Smith, A. & Jin, H. A novel class of bacteria-induced small RNAs in Arabidopsis. Genes Dev. 21, 3123–3134 (2007).
Agorio, A. & Vera, P. ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell 19, 3778–3790 (2007).
Li, F. et al. MicroRNA regulation of plant innate immune receptors. Proc Natl Acad Sci USA 109, 1790–1795 (2012).
Navarro, L., Jay, F., Nomura, K., He, S. Y. & Voinnet, O. Suppression of the microRNA pathway by bacterial effector proteins. Science 321, 964–967 (2008).
Zhang, X. et al. Arabidopsis Argonaute 2 regulates innate immunity via miRNA393*-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol. Cell 42, 356–366 (2011).
Pan, H., Liu, S. & Tang, D. HPR1, a component of the THO/TREX complex, plays an important role in disease resistance and senescence in Arabidopsis. Plant J. 69, 831–843 (2012).
Boccara, M. et al. The Arabidopsis miR472-RDR6 silencing pathway modulates PAMP- and effector-triggered immunity through the post-transcriptional control of disease resistance genes. PLoS Pathog 10, e1003883 (2014).
Hernandez-Pinzon, I. et al. SDE5, the putative homologue of a human mRNA export factor, is required for transgene silencing and accumulation of trans-acting endogenous siRNA. Plant J. 50, 140–148 (2007).
Jauvion, V., Elmayan, T. & Vaucheret, H. The conserved RNA trafficking proteins HPR1 and TEX1 are involved in the production of endogenous and exogenous small interfering RNA in Arabidopsis. Plant Cell 22, 2697–2709 (2010).
Navarro, L. et al. The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 135, 1113–1128 (2004).
Zipfel, C. et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428, 764–767 (2004).
Durrant, W. E. & Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 42, 185–209 (2004).
Jirage, D. et al. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc. Natl. Acad. Sci., USA 96, 135883–113588 (1999).
Jirage, D. et al. Constitutive salicylic acid-dependent signaling in cpr1 and cpr6 mutants requires PAD4. Plant J. 26, 395–407 (2001).
Wildermuth, M. C., Dewdney, J., Wu, G. & Ausubel, F. M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565 (2001).
Loake, G. & Grant, M. Salicylic acid in plant defence-the players and protagonists. Curr. Opin. Plant Biol. 10, 466–472 (2007).
Spoel, S. H. et al. Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell 137, 860–872 (2009).
Mishina, T. E. & Zeier, J. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 50, 500–513 (2007).
Tsuda, K., Sato, M., Glazebrook, J., Cohen, J. D. & Katagiri, F. Interplay between MAMP-triggered and SA-mediated defense responses. Plant J. 53, 763–775 (2008).
Yuan, J. & He, S. Y. The Pseudomonas syringae Hrp regulation and secretion system controls the production and secretion of multiple extracellular proteins. J. Bacteriol. 178, 6399–6402 (1996).
DebRoy, S., Thilmony, R., Kwack, Y. B., Nomura, K. & He, S. Y. A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in plants. Proc Natl Acad Sci USA 101, 9927–9932 (2004).
Hauck, P., Thilmony, R. & He, S. Y. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc Natl Acad Sci USA 100, 8577–8582 (2003).
Kim, M. G. & Mackey, D. Measuring cell-wall-based defenses and their effect on bacterial growth in Arabidopsis. Methods Mol Biol 415, 443–452 (2008).
Dangl, J. L., Hauffe, K. D., Lipphardt, S. L., Hahkbrock, K. & Scheel, D. Parsley protoplasts retain differential responsiveness to U.V. light and fungal elicitor. The EMBO Journal 6, 2551–2556 (1987).
Ham, J. H., Kim, M. G., Lee, S. Y. & Mackey, D. Layered basal defenses underlie non-host resistance of Arabidopsis to Pseudomonas syringae pv. phaseolicola. Plant J. 51, 604–616 (2007).
Arnold, D. L., Lovell, H. C., Jackson, R. W. & Mansfield, J. W. Pseudomonas syringae pv. phaseolicola: from ‘has bean’ to supermodel. Mol. Plant Pathol. 12, 617–627 (2011).
Forsyth, A. et al. Genetic dissection of basal resistance to Pseudomonas syringae pv. phaseolicola in accessions of Arabidopsis. Mol Plant Microbe Interact 23, 1545–1552 (2010).
Galletti, R. et al. The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol. 148, 1695–1706 (2008).
Clay, N. K., Adio, A. M., Denoux, C., Jander, G. & Ausubel, F. M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323, 95–101 (2009).
Saijo, Y. et al. Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J. 28, 3439–3449 (2009).
Kang, Y. & Cullen, B. R. The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev. 13, 1126–1139 (1999).
Schmitt, I. & Gerace, L. In vitro analysis of nuclear transport mediated by the C-terminal shuttle domain of Tap. J. Biol. Chem. 276, 42355–42363 (2001).
Gong, Z. et al. A DEAD box RNA helicase is essential for mRNA export and important for development and stress responses in Arabidopsis. Plant Cell 17, 256–267 (2005).
Kim, J. S. et al. Glycine-rich RNA-binding protein 7 affects abiotic stress responses by regulating stomata opening and closing in Arabidopsis thaliana. Plant J. 55, 455–466 (2008).
Pieterse, C. M., Leon-Reyes, A., Van der Ent, S. & Van Wees, S. C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 5, 308–316 (2009).
Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227 (2005).
Leon-Reyes, A. et al. Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol. 149, 1797–1809 (2009).
Gao, Q. M., Venugopal, S., Navarre, D. & Kachroo, A. Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiol. 155, 464–476 (2011).
Lopez, A., Ramirez, V., Garcia-Andrade, J., Flors, V. & Vera, P. The RNA silencing enzyme RNA polymerase v is required for plant immunity. PLoS Genet. 7, e1002434 (2011).
Zhu, S. et al. Double-stranded RNA-binding protein 4 is required for resistance signaling against viral and bacterial pathogens. Cell Rep 4, 1168–1184 (2013).
Germain, H. et al. MOS11: a new component in the mRNA export pathway. PLoS Genet. 6, e1001250 (2010).
Zhang, Y. & Li, X. A putative nucleoporin 96 is required for both basal defense and constitutive resistance responses mediated by suppressor of npr1-1, constitutive 1. Plant Cell 17, 1306–1316 (2005).
Nemeth, K. et al. Pleiotropic control of glucose and hormone responses by PRL1, a nuclear WD protein, in Arabidopsis. Genes Dev. 12, 3059–3073 (1998).
Palma, K. et al. Regulation of plant innate immunity by three proteins in a complex conserved across the plant and animal kingdoms. Genes Dev. 21, 1484–1493 (2007).
Guzmán, P. & Ecker, J. R. Exploiting the triple response of Arabidopsis to identify ethylene insensitive mutants. Plant Cell 2, 513–523 (1990).
Staswick, P. E., Su, W. & Howell, S. H. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89, 6837–6840 (1992).
Rantakari, A. et al. Type III secretion contributes to the pathogenesis of the soft-rot pathogen Erwinia carotovora: partial characterization of the hrp gene cluster. Mol Plant Microbe Interact 14, 962–968 (2001).
Kim, M. G., Geng, X., Lee, S. Y. & Mackey, D. The Pseudomonas syringae type III effector AvrRpm1 induces significant defenses by activating the Arabidopsis nucleotide-binding leucine-rich repeat protein RPS2. Plant J. 57, 645–653 (2009).
Clough, S. J. & Bent, A. F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Acknowledgements
This work was supported by grants from Next-Generation BioGreen21 Program (SSAC: PJ01109102) and by Cooperative Research Program for Agriculture Science & Technology Development, (project no. PJ010953042016), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
M.N.U., D.M.M., J.H.B. and M.G.K. wrote the manuscript with input from other authors. M.N.U., S.A., R.C., J.Y.C., and S.J.P. performed experiments. W.Y.K., H.K., and S.Y.L. edited the paper, gave support and conceptual advice. All authors discussed the contents and agreed on the contents of the paper and post no conflicting interest.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uddin, M.N., Akhter, S., Chakraborty, R. et al. SDE5, a putative RNA export protein, participates in plant innate immunity through a flagellin-dependent signaling pathway in Arabidopsis . Sci Rep 7, 9859 (2017). https://doi.org/10.1038/s41598-017-07918-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07918-x
This article is cited by
-
Molecular characterization of HEXOKINASE1 in plant innate immunity
Applied Biological Chemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.