Abstract
Aberrant regulation of uterine cell growth can lead to endometrial cancer and infertility. To understand the molecular mechanisms of estrogen-induced uterine cell growth, we removed the estrogen receptor α (Esr1) from mouse uterine stromal cells, where the embryo is implanted during pregnancy. Without ESR1 in neighboring stroma cells, epithelial cells that line the inside of the uterus are unable to grow due to a lack of growth factors secreted from adjacent stromal cells. Moreover, loss of stromal ESR1 caused mice to deliver fewer pups due in part due to inability of some embryos to implant in the uterus, indicating that stromal ESR1 is crucial for uterine cell growth and pregnancy.
Similar content being viewed by others
Introduction
In female mammals, 17β-estradiol (E2), an endogenous estrogen, is primarily produced by the granulosa cells of the ovaries. E2 exerts its activity through estrogen receptors α and β (ESR1 and ESR2)1. Upon E2 binding, ESRs are dimerized, translocated from the cytoplasm into the nucleus, recruited onto targeted DNA sequences, where they initiate or repress transcription of E2-target genes in both non-reproductive and reproductive organs2. Female reproductive tissues including mammary glands, ovaries, oviducts, and the uterus, express both ESR1 and ESR23. In the uterus, ESR1 is the major subtype and is expressed in all cell layers: epithelia (monolayer of cells lining the uterine lumen), stroma (connective tissue in the endometrial lining between epithelia and myometrium), and myometrium (muscle cell layer).
Estrogens induce cell proliferation and growth in both reproductive and non-reproductive tissues (such as osteoblasts and hepatocytes). It has been shown that E2 selectively stimulates proliferation of uterine epithelial cells in adult ovariectomized mice4,5,6. Tissue recombination studies using isolated epithelial and stromal cells from wild-type or Esr1 −/− neonatal uterine tissues transplanted under the kidney capsule showed that ESR1 is not required in uterine epithelial cells for their proliferation. We have confirmed this observation using an adult epithelial cell specific knockout mouse model (Wnt7a Cre/+; Esr1 f/f)7, 8, with an intact (no tissue disruption and recombination) uterine tissue structure. The results from these two studies support a mechanism in which E2 treatment and activation of ESR1 in the stromal cells produces mitogenic factors, including insulin-like growth factor 1 (IGF1)9, 10, and transforming growth factor11, which stimulate their subsequent signaling cascades in uterine epithelial cells, leading to cell proliferation. However, a functional requirement of stromal ESR1 in the normal uterine environment in vivo has not yet been explored.
In addition to cell proliferative events accompanying E2 treatment, we also evaluated the role of stromal ESR1 in female reproductive functions in this study. In normal mouse reproduction, the presence of a copulatory plug is observed the morning after mating is designated 0.5 days post coitus (dpc). At 0.5 dpc, the oocytes are fertilized by the sperm and during 3.0 dpc the embryos develop into morulas or blastocysts within the oviducts (known as Fallopian tubes in humans). In rodents, the blastocysts transit the oviduct to the uterus where they implant exclusively onto the anti-mesometrial pole of the uterine wall at approximately 4.0 dpc12. Embryo attachment requires secretion of leukemia inhibitory factor (LIF), an implantation facilitating cytokine, from uterine glands located in the anti-mesometrial pole of the uterus13. After embryo implantation, the uterine endometrium undergoes a decidual response (called decidualization), in which the stromal cells proliferate and differentiate into decidua14. The decidual cells surrounding embryos provide nutrients and support for the developing fetus before the placenta starts to fully function. The placenta forms on the mesometrial pole of the uterus, where the blood vessels are supplied via the uterine broad ligament. These implantation and decidualization processes are orchestrated by ovarian steroid hormones (E2 and progesterone; P4) through ESR1 and progesterone receptor (PGR)15, 16.
We previously showed that female mice with a global deletion of ESR1 (Esr1 −/−) are infertile, in part due to an implantation defect15. Using a female reproductive tract epithelial cell ESR1 null mouse model (Wnt7a Cre/+; Esr1 f/f), our group and others have demonstrated that uterine epithelial ESR1 is crucial for embryo implantation, decidual response, and fertility8, 17. From these previous findings, we hypothesized that a lack of stromal ESR1 could lead to aberrant uterine cell proliferation, while not affecting embryo implantation or uterine decidual response. To test our hypothesis, we have generated a mouse model lacking stromal ESR1, specifically at the anti-mesometrial pole of the uterus. Here we report that stromal ESR1 is required for epithelial cell proliferation. Surprisingly, stromal ESR1 in the uterine anti-mesometrium is also crucial for optimal embryo implantation and artificially induced-decidualization.
Results
Stromal ESR1 underlying uterine epithelial cells is essential for E2-induced proliferative response
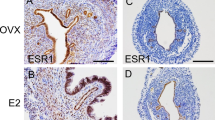
Amhr2 Cre/+ mice were bred with Esr1 f/− to specifically delete ESR1 in uterine stromal cells. Deletion of Esr1 in the uterine tissues was confirmed using ESR1 immunohistochemical (IHC) analysis in 12-week-old mice. In the control uteri (Esr1 f/−), ESR1 protein was detected throughout uterine cross-sections, including epithelial, stromal, and muscle cell layers (Fig. 1). In the Amhr2 Cre/+; Esr1 f/− uteri, ESR1 protein was ablated in the stromal cells of the uterine anti-mesometrial pole whereas the expression of ESR1 remained intact in the epithelial cell layer as well as in the stromal cells in the mesometrial pole (Fig. 1). We observed variable degrees of ESR1 deletion in individual Amhr2 Cre/+; Esr1 f/− mice. This is likely due to uneven expression of the Cre-recombinase amongst stromal cells in the Amhr2 Cre/+ mouse line18,19,20. Therefore, the extent of deletion of ESR1 in the circular smooth muscle cells varied between individual animals (Supplementary Fig. S1).
Selective deletion of ESR1 anti-mesometrial mouse uterine stromal cells. ESR1 deletion was confirmed using immunohistochemical (IHC) staining of ESR1 in whole uterine sections; mesometrium (M) and anti-mesometrium (AM), in ovarian intact adult (12-week-old) Esr1 f/− and Amhr2 Cre/+; Esr1 f/− females. LE = luminal epithelial cells, GE = glandular epithelial cells, and Myo = myometrium. Representative images shown.
Using our previous mouse model in which Esr1 is selectively deleted in uterine epithelial cells, we reported that uterine epithelial ESR1 was not required for E2 to induce uterine epithelial cell proliferation8. No E2 induced epithelial proliferation occurs in global Esr1-null uteri21, demonstrating that uterine ESR1 is needed to mediate epithelial proliferation, thus we hypothesized that stromal ESR1 was required for paracrine regulation of epithelial cell proliferation. We collected the tissues 24 h after E2 treatment of ovariectomized 8–12-week-old females to observe E2-induced uterine wet weight increase, which reflects uterine growth (late response). In Esr1 f/− uteri, E2 significantly increased the uterine wet weight compared to the vehicle treated controls (Fig. 2A). However, there was no uterine weight increase in the E2 treated Amhr2 Cre/+; Esr1 f/− uteri compared to the vehicle control. Evaluation of cellular proliferative responses to E2, as reflected by expression of Ki67 proliferative marker, revealed few Ki67 positive cells in vehicle treated uteri of both genotypes. As expected, the luminal epithelial cells of E2 treated Esr1 f/− uteri were positive for Ki67 (Fig. 2B). However, the luminal epithelial cells exclusively in the mesometrial but not in the anti-mesometrial pole of the E2 treated Amhr2 Cre/+; Esr1 f/− uteri were positive for the Ki67 staining (Fig. 2B and higher magnification in Fig. 2C). A limited number of glandular epithelial cells were positive for Ki67 staining in both Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri (Fig. 2B to C). Therefore, the Ki67-positive cells in the glandular epithelia were excluded from quantification of Ki67-positive cells. We found that the percentage of luminal epithelial cells that were Ki67-positive in the anti-mesometrial pole after 24 h E2 treatment was significantly less in Amhr2 Cre/+; Esr1 f/− than in Esr1 f/− uteri, whereas the percentage of epithelial cells that were proliferating in the mesometrial pole was similar in both Amhr2 Cre/+; Esr1 f/− and Esr1 f/− uteri (Fig. 2D). To determine whether the expression level of ESR1 in mesometrial and anti-mesometrial uterine stromal cells of Amhr2 Cre/+; Esr1 f/− corresponds with the E2-induced proliferative response of epithelial cells, we compared ESR1 and Ki67 in adjacent sections. We found that ESR1 was ablated in anti-mesometrial stromal cells next to non-proliferating epithelial cells in Amhr2 Cre/+; Esr1 f/− uteri (Fig. 2C and Supplementary Fig. S2). This finding suggests that loss of ESR1 in stromal cells prevents proliferation of immediately adjacent epithelial cells, indicating that signals emanating from stromal cells directly adjacent to responding epithelial cells transduce the required stimulus for proliferation. This stimulus is apparently unable to diffuse throughout the tissue, but rather works in a juxtacrine manner on neighboring epithelial cells.
Uterine response to E2 treatment (24 h) in the absence of anti-mesometrial stromal ESR1. Adult (8–12-week-old) Esr1 f/− and Amhr2 Cre/+; Esr1 f/− females were ovariectomized and treated with vehicle or E2 for 24 h. (A) Uterine wet weight after 24 h of E2 treatment. *p < 0.05; significant difference between vehicle and E2 treated samples within genotype. N = 3 mice/genotype/treatment. (B) Uterine epithelial cell proliferation determined by Ki67 IHC staining in Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri. (C) Higher magnification of Amhr2 Cre/+; Esr1 f/− treated with E2 for 24 h. Uterine sections were stained with Ki67 and ESR1 antibodies in adjacent sections. Note that epithelial cell proliferation, as indicated by the appearance of Ki67, is primarily observed in the M where ESR1 is expressed in the adjacent stromal cells. (D) Percentage of Ki67-positive cells of total luminal epithelial cells in M vs. AM regions. *p < 0.05; significant difference between vehicle and E2 treated samples within genotype and region. # p < 0.05; significant difference between Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri after E2 treatment in the AM region, unpaired t-test. N = 4–8 mice/genotype/treatment. All graphs represent mean ± SEM. M = Mesometrium, AM = Anti-mesometrium. Representative images shown.
Loss of stromal ESR1 leads to blunted E2-induced cell cycle-related transcripts and proteins
We previously reported that several genes associated with cell-cycle progression, including Igf1, CCAAT enhancer binding protein beta (Cebpb), cyclin-dependent kinase inhibitor 1a (Cdkn1a), and mitotic arrest deficient 2-like protein 1 (Mad2l1), were E2 responsive genes and that their expression was epithelial ESR1-independent, therefore, potentially mediated by stromal ESR18, 22. To confirm that the expression of these transcripts and encoded proteins were stromal ESR1-dependent, we euthanized ovariectomized 8–12-week-old animals and collected the uterine tissues 6 h after the injection of E2. Igf1, Mad2l1, and Cdkn1a were significantly increased by E2 treatment compared to vehicle treated uteri in both Esr1 f/− and Amhr2 Cre/+; Esr1 f/− groups (Fig. 3A). However, the E2 induction of these transcripts in Amhr2 Cre/+; Esr1 f/− uteri was blunted in comparison to the response of E2 treated Esr1 f/− uteri; the Igf1 was the most blunted (Fig. 3A). Mantena et al. have demonstrated that Cebpb is rapidly induced in uterine stromal cells by E2 and contributes to uterine epithelial cell proliferation23. Therefore, we reasoned that the deletion of stromal ESR1 would alter Cebpb expression in the uterus. However, we found that E2 induced similar levels of Cebpb transcript in Esr1 f/− and Amhr2 Cre/+; Esr1 f/− (Fig. 3A). To evaluate whether expression of the Cebpb gene in the whole uterus masked any differences in induction in stromal cells, expression of CEBPB protein was examined in uterine sections using IHC analysis. After E2 treatment, CEBPB was highly expressed in both epithelial and stromal cells in both the mesometrium and anti-mesometrium of the Esr1 f/− uteri (Fig. 3B). In Amhr2 Cre/+; Esr1 f/− uteri, CEBPB was highly induced in the mesometrial area, whereas the expression was minimally detected in the anti-mesometrial area (Fig. 3B).
Cell proliferation-related uterine transcripts and protein in ovariectomized 8–12-week-old Esr1 f/− and Amhr2 Cre/+; Esr1 f/− females treated with E2 for 6 h. (A) Real-time PCR was performed and the relative expression values of Igf1, Mad2l1, Cdkn1a, Cebpb, Klf4, Mcm2, Mcm4, and Klf15 were normalized to Rpl7. *, ***p < 0.05, 0.001; significant difference between vehicle and E2 treated samples within genotype. # p < 0.05; significant difference between E2 treated samples between genotype; unpaired t-test. (B) CEBPB protein expression after 6 h of E2 treatment in Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri using IHC analysis. All graphs represent mean ± SEM. N = 3–5 mice/genotype/treatment. M = Mesometrium, AM = Anti-mesometrium. Representative images shown.
In addition to these E2-responsive genes, other factors including kruppel like factor 4 (Klf4) and minichromosome maintenance complex components (Mcm2 and Mcm4) are also involved in E2-induced uterine proliferation24. We found that Klf4 was significantly induced by E2 treatment in both Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri (Fig. 3A). However, Mcm2 and Mcm4 transcripts tended to be induced by E2 but not at significant levels. As expected, Klf15 was not increased by E2 treatment as Klf15 expression was previously shown to be regulated by P4 24. These results suggest that uterine stromal ESR1 mediates the expression of some cell-cycle regulated genes and protein in response to E2 treatment.
We previously reported that deletion of ESR1 from epithelial cells had no effect on the expression of progesterone receptor (PGR), a hallmark E2-induced protein in the uterus (after 24 h of treatment8). We collected uterine tissues and evaluated the PGR protein levels using IHC analysis to determine how loss of anti-mesometrial stromal ESR1 affected uterine PGR expression. We found that E2 treatment compared to vehicle significantly increased PGR signal intensity in the cytoplasmic compartment in the mesometrial pole of both Esr1 f/− and Amhr2 Cre/+; Esr1 f/− animals (Fig. 4A; yellow arrowheads and Fig. 4B). In Esr1 f/− uteri, E2 had a tendency to increase anti-mesometrial cytosolic PGR signal intensity (p = 0.2437). However, in the absence of anti-mesometrial stromal ESR1 in Amhr2 Cre/+; Esr1 f/− animals, anti-mesometrial cytosolic PGR signal intensities in vehicle and E2 treatment were similar (p = 0.5765). In addition, the proportion of PGR-positive stromal cells was significantly increased in E2-treated Esr1 f/− uteri in both mesometrial and anti-mesometrial poles (Fig. 4C). However, E2 treatment only increased PGR-positive cells in the mesometrial stromal cells of Amhr2 Cre/+; Esr1 f/−. From these findings, we conclude that ESR1 must be present in all uterine stromal cells for E2 to fully induce epithelial cell proliferation and properly regulate the pattern of PGR expression.
E2-induced progesterone receptor (PGR) expression in the uterus. Adult (8–12-week-old) female mice were ovariectomized and treated with vehicle or E2 for 24 h. (A) Top panel: Cross-sections of the whole uteri were stained with PGR antibodies. Bottom panels: PGR expression pattern in the mesometrial (M) vs. anti-mesometrial (AM) poles in Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri. Representative images shown. (B) Relative signal intensities of nuclear (Nuc) and cytosolic (Cyto) compartments in the uterine luminal epithelial cells of Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri after vehicle and E2 treatment for 24 h. (C) Percentage of PGR-positive cells of total stromal cells in M vs. AM regions. *p < 0.05; significant difference between vehicle and E2 treated samples within genotype and region. All graphs represent mean ± SEM. N = 3 mice/genotype/treatment.
Loss of stromal ESR1 in the uterus causes severe fertility defect
To evaluate whether the anti-mesometrial stromal ESR1 is functionally required for female fertility, we determined the number of pups born to adult female mice during a 6-month period. The total number of pups delivered by Amhr2 Cre/+; Esr1 f/− dams (0.7 ± 0.6 pups/dam) was significantly less than the number delivered by Esr1 f/− dams (38.3 ± 3.5 pups/dam) (Fig. 5A). Eight out of ten Amhr2 Cre/+; Esr1 f/− dams evaluated did not deliver any pups. Because Amhr2 Cre/+ was also expressed in the ovaries, we then investigated whether the subfertility phenotype in Amhr2 Cre/+; Esr1 f/− females was due to impaired ovulation. Comparable numbers of oocytes were ovulated following gonadotropin stimulation of prepubertal Esr1 f/− and Amhr2 Cre/+; Esr1 f/− females (Fig. 5B). Since ovulation occurs normally, and our recent findings indicate that blastocyst development is not affected by the deletion of stromal ESR1 in the oviduct25, we reasoned that the subfertility phenotype in Amhr2 Cre/+; Esr1 f/− females was due to impaired uterine function as a result of deletion of anti-mesometrial stromal ESR1. Amhr2 Cre/+; Esr1 f/− adult females had significantly fewer implantation sites at 4.5 dpc, than Esr1 f/− females (Fig. 5C). However, Amhr2 Cre/+; Esr1 f/− implantation sites exhibited no apparent morphological defects (Fig. 5D).
Fertility study of Amhr2 Cre/+; Esr1 f/− and Esr1 f/− mice. (A) Number of total pups per dam during 6-month breeding trial with wild-type males. Each data point represents an individual dam. ****p < 0.0001; unpaired t-test. N = 9–10 mice/genotype. (B) Ovulatory responses of 3–5-week-old females to gonadotropins indicated by total number of ovulated oocytes/mice. N = 6 mice/genotype. (C) Total number of 4.5 dpc implantation sites in 8–12-week-old female mice after natural mating. ****p < 0.0001; unpaired t-test. N = 3–4 mice/genotype. (D) Hematoxylin and eosin staining of implantation sites (4.5 dpc) in uterine cross sections. N = 3–4 mice/genotype. E = embryo, M = Mesometrium, AM = Anti-mesometrium. (E) Expression of implantation markers (Lif, Ihh, Wnt5a, and Hbegf) and E2-target transcripts (Muc1, Ltf, and Clca3) at 3.5 dpc in Esr1 f/− and Amhr2 Cre/+; Esr1 f/− 8–12-week-old female mice. *p < 0.05; unpaired t-test. N = 5–6 mice/genotype. All graphs represent mean ± SEM. Representative images shown.
To determine the potential cause of impaired implantation observed at 4.5 dpc, we evaluated several key uterine receptivity genes expressed during early gestation (3.5 dpc), at which time nidatory levels E2 are secreted to prepare the uteri for embryo implantation26. We found the hallmark implantation markers, Lif 13 and indian hedgehog (Ihh)27, tended to be expressed at higher levels in Amhr2Cre/+; Esr1 f/− (p = 0.1237 and 0.0685, respectively) compared to Esr1 f/− uteri (Fig. 5E). Surprisingly, significant elevation of E2-responsive genes such as mucin 1 (Muc1)28, lactotransferrin (Ltf)28, and chloride channel calcium-activated 3 (Clca3)29 was observed in Amhr2Cre/+; Esr1 f/− compared to Esr1 f/− uteri. These transcripts are normally suppressed during the 3.5 dpc pre-implantation period. Proper Wnt5a expression levels are crucial for embryo homing and optimal implantation30. Here, we demonstrated that Wnt5a transcript was significantly elevated in Amhr2Cre/+; Esr1 f/− compared to Esr1 f/− uteri at 3.5 dpc. However, heparin-binding EGF-like growth factor (Hbegf, another implantation marker31) was expressed at comparable levels in Amhr2Cre/+; Esr1 f/− and Esr1 f/− uteri. These findings indicate that stromal ESR1 in the anti-mesometrium is required for the suppression of some E2-regulated transcripts during implantation. However, when embryos did implant in Amhr2Cre/+; Esr1 f/− uteri, implantation sites appear normal.
Stromal ESR1 ablation contributes to a lack of uterine stromal cell proliferation
Successful embryo implantation requires the cessation of uterine epithelial cell proliferation and subsequent stromal cell proliferation32. Because we observed impaired uterine receptivity, we investigated whether lacking ESR1 in the stromal cells contributed to defective uterine stromal cell proliferation or prevention of the cessation of the epithelial cell proliferation. To mimic the hormonal profile during embryo implantation, 8–12-week-old animals were ovariectomized and treated with E+Pe (see Methods for detail). Mice lacking anti-mesometrial stromal ESR1 did not show a uterine weight increase after E+Pe treatment (Fig. 6A). Moreover, uterine stromal cell proliferation was blunted in the absence of stromal ESR1 in the anti-mesometrial region (Fig. 6B). However, the uterine epithelial cells ceased proliferation similarly in Esr1 f/− and Amhr2 Cre/+; Esr1 f/− E+Pe treated uteri (Fig. 6B). In the absence of stromal ESR1, Lif expression was similarly induced in Esr1 f/− and Amhr2 Cre/+; Esr1 f/− E+Pe treated uteri (Fig. 6C). We then evaluated whether a lack of stromal cell proliferation was due to a loss of CEBPB expression. In the E+Poil control treatment group, there were fewer cells expressing CEBPB protein in the Amhr2 Cre/+; Esr1 f/− compared to Esr1 f/− uteri (Fig. 6D). However, upon E+Pe treatment, CEBPB was expressed similarly between Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri (Fig. 6D). These results suggest that anti-mesometrial stromal ESR1 is required for the stromal cell proliferation and defective stromal cell proliferation is not due to a lack of CEBPB expression.
Proliferation of the uterine stromal cells after a series of E2 and P4 treatments (E+Pe) to mimic the hormonal profile during implantation and at 4.5 dpc. (A) Uterine wet weights of Esr1 f/− and Amhr2 Cre/+; Esr1 f/− 8–12-week-old females mice treated with E + Poil or E + Pe. *p < 0.05; significant difference between E+Pe treated Esr1 f/− vs. Amhr2 Cre/+; Esr1 f/− females. (B) EdU incorporation assay of Esr1 f/− and Amhr2 Cre/+; Esr1 f/− female mice that were treated with E+Poil or E+Pe. Cells with green signal represent EdU positive (DNA synthesis) cells. Cells with blue signal represent the nuclei stained with Hoescht. (C) Real-time PCR analysis of Lif. Values were normalized to Rpl7. **p < 0.01; significant difference between E+Poil and E+Pe treated samples within genotype. All graphs represent mean ± SEM. (D) CEBPB IHC staining of Esr1 f/− and Amhr2 Cre/+; Esr1 f/− female mice treated with E+Poil or E+Pe. Arrowheads indicate glandular epithelial cells. N = 4–6 mice/genotype/treatment. M = Mesometrium, AM = Anti-mesometrium. (E) Expression of CEBPB and ESR1 proteins of implantation sites from Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uterine cross sections at 4.5 dpc using IHC analysis. Representative images shown. N = 3–4 mice/genotype. E = embryo.
In addition, we evaluated 4.5 dpc implantation sites from 8–12-week-old mice to determine whether stromal ESR1 deletion in the anti-mesometrium altered CEBPB expression. At 4.5 dpc, CEBPB is expressed homogenously in the uterine epithelial and stromal cells surrounding the implantation sites (Fig. 6E), regardless of the expression of ESR1 in the stroma. Expression of ESR1 was not detected in the primary decidual zone in either Esr1 f/− or Amhr2 Cre/+; Esr1 f/− uteri. These results indicate that CEBPB is expressed independently from ESR1 expression in the implantation sites and that the implantation defect observed in the Amhr2 Cre/+; Esr1 f/− females is not due to a lack of CEBPB.
Ablation of anti-mesometrial stromal ESR1 caused impaired uterine decidual response after artificial stimulation
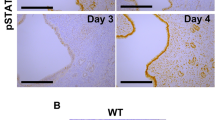
Pawar et al. have shown that a lack of epithelial ESR1 impairs decidualization17. To clarify whether anti-mesometrial stromal ESR1 was also required for uterine decidualization, we evaluated the decidual response using a well-established method of injection of inert oil into the uterine lumen to artificially stimulate decidualization17. In 8–12-week-old Esr1 f/− animals, artificial stimulation resulted in decidualization of 6 out of 10 uteri, whereas 2 out of 9 Amhr2 Cre/+; Esr1 f/− females responded (Fig. 7A). The uterine weight increase resulting from decidual stimulation was significantly greater in Esr1 f/− than in Amhr2 Cre/+; Esr1 f/− uteri (Fig. 7B). Decidual markers and cell-cycle regulated genes, including bone morphogenic protein 2 (Bmp2), prolactin family 8, subfamily a, member 2 (Prl8a2), cyclin B1 (Ccnb1), and cyclin dependent kinase A1 (Cdc2a), were significantly induced in stimulated horns compared to un-stimulated horns in Esr1 f/− uteri (Fig. 7C). The expression of these genes tended to be increased in responding horns of Amhr2 Cre/+; Esr1 f/− animals, however, statistical analysis could not be performed as only 2 of 9 Amhr2 Cre/+; Esr1 f/− animals responded to the artificial stimulation. As we observed an impaired decidual response in Amhr2 Cre/+; Esr1 f/− animals, we next evaluated whether stromal cell proliferation was affected by a lack of stromal ESR1 by using an EdU incorporation assay. EdU was mainly detected in the mesometrial pole of Esr1 f/− uteri, whereas a smaller area of EdU incorporation was seen in the anti-mesometrial pole of the decidualized Amhr2 Cre/+; Esr1 f/− uterus. (Fig. 7D). However, EdU incorporation was not or was minimally detected in stimulated non-responding Amhr2 Cre/+; Esr1 f/− uteri (Supplementary Fig. S3).
Impaired decidual response to artificial stimulation in the absence of stromal ESR1 in the anti-mesometrium. Artificial stimulation was used to induce decidual response in adult (8–12-week-old) Esr1 f/− and Amhr2 Cre/+; Esr1 f/− female mice (details for the treatment regime are described in Methods). (A) Gross morphology of uteri showing un-stimulated vs. stimulated uterine horns. Data below indicate the numbers of animals responding to the stimulation in Esr1 f/− (6/10) or Amhr2 Cre/+; Esr1 f/− (2/9) animals. Non-responsive Amhr2 Cre/+; Esr1 f/− uteri (7/9) are also shown. (B) Uterine weight increased in the stimulated horns compared to un-stimulated horns (N = 9–10 animals/group). *p < 0.05, significant difference, unpaired t-test. (C) Transcript levels of decidual markers and cell-cycle regulators (Bmp2, Prl8a2, Ccnb1, and Cdc2a) in Esr1 f/− (N = 6 of 10 animals) vs. Amhr2 Cre/+; Esr1 f/− (N = 2 of 9 animals) uteri that responded to artificial stimulation. # p < 0.05, significant difference between un-stimulated and stimulated horns within genotype, unpaired t-test. All graphs represent mean ± SEM. (D) Uterine cross-section of Esr1 f/− and Amhr2 Cre/+; Esr1 f/− mice after artificial stimulation. Images illustrate ESR1 IHC and EdU incorporation assay in un-stimulated and stimulated horns. Green indicates cells in S-phase of DNA synthesis. Blue represents Hoescht stained nuclei.
Because the uterus responds differently to artificial stimulation and embryo-initiated decidualization33, to determine whether stromal ESR1 is required for natural decidualization, we assessed the decidual response 5.5 and 7.5 dpc after natural mating of Esr1 f/− and Amhr2 Cre/+; Esr1 f/− adult females. We found that PGR was expressed similarly in Amhr2 Cre/+; Esr1 f/− and Esr1 f/− uteri regardless of ESR1 expression status in the anti-mesometrium (Fig. 8A). Moreover, proliferation of the decidual cells in the anti-mesometrium was comparable between Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri at 5.5 and 7.5 dpc using Ki67 IHC analysis (Fig. 8B). There was no significant difference in expression of decidual gene markers, including epidermal growth factor receptor (Egfr), FK506 binding protein 5 (Fkbp5), prostaglandin-endoperoxide synthase 2 (Ptgs2), wingless-type MMTV integration site family, member 4 (Wnt4), Bmp2, and Prl8a2 between Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri at 5.5 dpc (Fig. 8C). These findings suggest that ESR1expression in anti-mesometrial stromal cells is required for normal decidualization in response to artificial stimulation, but that decidualization can occur after implantation.
Expression of ESR1, PGR, Ki67 and marker genes for decidualization at 5.5 dpc and decidual cell proliferation at 7.5 dpc in the absence of anti-mesometrial stromal ESR1 in 8–12-week-old females. IHC analysis of (A) ESR1 and PGR and (B) Ki67 in Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri collected at 5.5 and 7.5 dpc. N = 3–4 mice/genotype. (C) Transcript levels of decidualization markers in the uteri collected from decidual zones at 5.5 dpc, including Egfr, Ptgs2, Pgr, Wnt4, Bmp2, and Prl8a2. Graphs represent mean ± SEM. N = 3–4 mice/genotype. Representative images shown.
Loss of stromal ESR1 in uterine anti-mesometrium leads to an increased resorption of embryos post-decidualization
In the absence of stromal ESR1 in the anti-mesometrium, there was a 50% increase in resorption sites compared to Esr1 f/− controls (Fig. 9A). To determine the cause of embryo resorption during this post-decidualization period of pregnancy, we assessed the expression of angiogenic genes in 10.5 dpc implantation sites. Several studies show that uterine decidual response is marked by an increase in uterine vasculature that subsequently provides nutrients to developing embryos14. Decidual vascularization is mainly regulated by vascular endothelial growth factors (VEGFs) and angiopoietins34. The hallmark features of decidual vascularization consist of angiogenic genes34, including Vegfa, Vegfb, Vegfc, FMS-like tyrosine kinase 1 (Flt1, also known as Vegf receptor 1 or Vegfr1), kinase insert domain protein receptor (Kdr, also known as Vegfr2), Flt4 (or Vegfr3), angiopoietin 2 (Angpt2), adrenomedullin (Adm), anti-angiogenic factors such as thrombospondin (Thbs1)35, and gap junction protein alpha 1 (Gja1, also known as connexin 43)36. At 10.5 dpc, the implantation sites of Amhr2Cre/+; Esr1 f/− showed significantly higher levels of Vegfb, Kdr, and Thbs1 compared to those of Esr1 f/− females, while other angiogenic genes (Vegfa, Vegfc, Flt1, Flt4, Angpt2, Adm and Gja1) were expressed at similar levels (Fig. 9B). This finding indicates that there is slight difference in the expression of angiogenic and anti-angiogenic markers in the absence of anti-mesometrial stromal ESR1, which may perturb optimal angiogenesis, leading to a failure of embryo development, and increased embryo resorption.
Resorption sites and the transcripts of angiogenesis markers at 10.5 dpc in Esr1 f/− (N = 3) and Amhr2 Cre/+; Esr1 f/− (N = 8) adult (8–12-week-old) female mice. (A) Percentage of resorption sites at 10.5 dpc in Esr1 f/− vs. Amhr2 Cre/+; Esr1 f/uteri. (B) Transcript levels of angiogenic factors (Vegfa, Vegfb, Vegfc, Flt1, Kdr, Flt4, Angpt2, and Adm), anti-angiogenic factor (Thbs1), and gap junction protein alpha 1 (Gja1) of implantation sites at 10.5 dpc. *p < 0.05; unpaired t-test. All graphs represent mean ± SEM.
Discussion
We report here that the expression of ESR1 in uterine stromal cells is necessary for E2-induced epithelial cell proliferation. Initially, based on the previous evidence that epithelial ESR1 regulated implantation and decidualization8, 17, 22, we hypothesized that stromal ESR1 was not necessary for embryo implantation and decidualization. However, the results presented in this study demonstrate that loss of stromal ESR1 caused over-expression of E2-regulated genes that are normally suppressed during early pregnancy to provide a receptive uterine environment, leading to decreased embryo implantation. Stromal ESR1 also appeared to be required for uterine decidual response to artificial stimulation. Additionally, we observed aberrant expression of some angiogenic factors at 10.5 dpc, which could lead to disrupted angiogenesis in the absence of stromal ESR1 in the anti-mesometrium. Together, our findings indicate that uterine epithelial cell proliferation is modulated by stromal ESR1 and that E2 orchestrates its function through both epithelial and stromal ESR1 in order to provide the optimal uterine environment for embryo implantation and uterine decidualization.
Consistent with previous findings, the Cre activity of Amhr2Cre/+ animals was only active in the anti-mesometrial pole of the uterus18,19,20, therefore, in Amhr2 Cre/+ Esr1 f/− animals, the deletion of stromal ESR1 was observed only in the anti-mesometrium leaving the expression of stromal ESR1 in the mesometrium intact. Such a model system has exceptional specificity, since in the same animal only a portion of the tissue is affected by Cre expression, while other portions essentially function as an internal control. Note that we observed a higher level of ESR1 expression in anti-mesometrial epithelial cells than in mesometrial epithelial cells, however, only in some animals (3/8 Amhr2 Cre/+; Esr1 f/− animals evaluated, Supplementary Fig. S1). This phenomenon might occur because luminal epithelial cells that developed in a region lacking stromal ESR1 were developmentally altered and had characteristics of glandular epithelial cells, which normally expressed more ESR1 than epithelial cells.
Along with several groups, our laboratory, has demonstrated that E2 mediates its proliferative effect through stromal ESR1 via paracrine activity by inducing secretion of growth factors, such as Igf1, and cell-cycle related proteins, including Mad2l1, Cdkn1a, and Cebpb 7,8,9, 11, 22, 23, 37, 38. These findings are consistent with our previous report that the ablation of epithelial ESR1 does not affect the expression level of these growth factors and cell cycle regulated genes8. However, in our stromal ESR1 deletion model, E2-induced Igf1, Mad2l1, Cdkn1a, and Cebpb were attenuated, but not absent. This discrepancy likely reflects selective deletion of anti-mesometrial stromal ESR1 and retention of mesometrial ESR1 to mediate the observed responses.
Here, we observed prominent epithelial cell proliferation in the mesometrium after E2 treatment, whereas the proliferation was blunted in the anti-mesometrium. These findings illustrate the local requirement of stromal ESR1 activity for epithelial proliferation, indicating a juxtacrine mechanism in which the stromal factors have a localized action and primarily affect neighboring epithelial cell. This unique responsive pattern in the Amhr2 Cre/+; Esr1 f/− uteri was observed with Ki67, CEBPB, and PGR protein expression after E2 treatment. By selectively deleting ESR1 in the anti-mesometrium, we have created a unique tool, in which both positive and negative controls are present in the same tissue, to test the effect of stromal ESR1 in E2-regulated cell proliferation.
Our studies provide compelling data regarding the role of ESR1 during normal uterine proliferation. Such findings have the potential to advance understanding of abnormalities of the endometrium, such as endometriosis and endometrial cancer. It is well-established that endometrial cancer type I is estrogen-dependent, ESR1-positive, and is the most common form of endometrial cancer (>80% of the endometrial cancer cases). Moreover, IGF1 has been shown to be the major driver of endometrial hyperplasia progression and endometrial cancer formation in women39. Additionally, Ghazal et al. has recently demonstrated that estrogen increases IGF1R expression and subsequently induces stromal cell proliferation in endometrial tissues from women with endometriosis40. Our studies showed that local production of IGF1 was regulated by the stromal cells underlying luminal epithelial cells via the stimulation of estrogen signaling through stromal ESR1. Together, these findings advance general understanding regarding the roles of estrogen during normal endometrial growth. Furthermore, our studies indicate the potential source and location of growth factor production that could be targeted by therapeutic agents against endometrial growth abnormalities such as endometriosis, endometrial hyperplasia, and cancer.
Ablation of anti-mesometrial stromal ESR1 led to a decreased fecundity, without affecting the number of ovulated oocytes and blastocysts in the uterus25. Pawar et al. and our laboratory have demonstrated that epithelial ESR1 is necessary for embryo implantation8, 17. Implantation of embryos normally occurs exclusively in the anti-mesometrial pole of the uterus12. We found that ablation of anti-mesometrial stromal ESR1 affected uterine receptivity, partly due to increased expression of E2-regulated genes, such as Muc1, Ltf, and Clca3. This finding suggests that the stromal ESR1 is involved in regulating the gene expression in epithelial cells. Lacking stromal ESR1 in the anti-mesometrium decreases the number of implantation sites at 4.5 dpc by 50%. Cessation of uterine epithelial cell proliferation and increase stromal cell proliferation are also crucial steps for normal uterine receptivity32. Using a hormonal profile mimicking implantation (E+Pe), we found that lacking ESR1 in the anti-mesometrial stromal cells caused diminished stromal cell proliferation. Together, these findings suggest that Amhr2 Cre/+; Esr1 f/− females are less receptive to embryo attachment/implantation due to a lack of stromal cell proliferation in the specific area of the uterus linked to the loss of anti-mesometrial stromal ESR1.
LIF, a key mediator of uterine receptivity, is expressed in glandular epithelial cells and regulated by epithelial ESR18, 13, 17. Loss of glandular LIF expression impairs receptivity13. As expected, Lif transcript was comparable in both Esr1 f/− and Amhr2 Cre/+; Esr1 f/− uteri in the E+Pe model, but tended to be increased in the uterus at 3.5 dpc. This result suggests that ablation of stromal ESR1 does not significantly affect the production of Lif in the glandular epithelial cells. This comparable production of Lif in Amhr2 Cre/+; Esr1 f/− and Esr1 f/− uteri may facilitate the initiation of embryo attachment/implantation in the absence of proper stromal cell proliferation. However, uterine receptivity is not determined by the proliferation status of epithelial and stromal cells or the production of LIF alone, other factors such as HBEGF and WNT5A are also crucial implantation signals30, 31. We found that Wnt5a transcript was significantly increased in Amhr2 Cre/+; Esr1 f/− uteri, which could potentially disrupt embryo homing and subsequently lead to implantation failure.
Mantena et al. have demonstrated that both E2 and P4 treatment regulate uterine CEBPB expression23. In their report, E2 rapidly increased uterine CEBPB expression in ovariectomized mice within 1 h of treatment whereas P4 induced expression after 24 h. However, the expression of CEBPB during decidualization on day 6 of pregnancy is solely regulated by PGR, as CEBPB protein is attenuated by a PGR antagonist (RU486). We also found rapid induction of CEBPB by E2, which is required for epithelial cell proliferation, but only in the mesometrial region, indicating that stromal ESR1 mediated the induction. However, CEBPB expression in the uterine anti-mesometrium as a result of E+Pe treatment or decidual response was independent of stromal cell ESR1 expression.
Previous findings using the original Esr1 −/− mouse line41 showed that global deletion of ESR1 did not affect uterine decidual responses in an artificial decidualization model42, 43. However, our recent unpublished data using the Ex3αERKO mouse line21 indicates that global loss of Esr1 prevents decidualization. Additionally, recent findings suggest that ESR1 in uterine epithelial cells is in fact modulating the decidualization process17. Protein expression analysis of ESR1 in mouse uteri during pregnancy clearly showed that ESR1 is not expressed in the primary decidual zone44, which suggests that stromal ESR1 in the uterine anti-mesometrium is not required for the decidual response. However, after artificial stimulation, uteri with stromal ESR1 deletion in the anti-mesometrium showed impaired decidual responses as measured by uterine weight increase and cell proliferation. These results confirm recent findings showing ESR1 is required for normal decidualization of cultured human stromal cells45.
From our findings, we surmise that the regulation of uterine epithelial cell proliferation in response to E2 mediated by ESR1 is through a local cell-cell communication between the stromal cells and adjacent epithelial cells. In addition, this communication is crucial for normal embryo attachment/implantation and decidual response to artificial stimulation.
Methods
Animals and experimental procedures
We generated a mouse model with stromal cell selective deletion of ESR1 (encoded by the Esr1 gene) using Amhr2 Cre/+ animals19, 46 bred with our Esr1 f/− animals21. Female Esr1 f/− mice were considered control animals for experiments. Amhr2 Cre/+ animals exhibit higher expression levels of Cre activity in anti-mesometrial uterine stromal cells than in mesometrial uterine stromal cells18,19,20. Adult females (8–12-week-old) were ovariectomized and housed for 14 days to eliminate the endogenous circulating ovarian steroid hormones. Animals were randomly grouped and subcutaneously injected with vehicle control (sterile normal saline) or 17β-estradiol (E2, Steraloids, Newport, RI) at a dose of 0.25 μg/mouse in saline. To evaluate transcript and protein expression initially regulated by E2 (early responses), we euthanized the mice 6 h after the injection of E2 and collected the uterine tissues. To determine the E2-induced protein expression and uterine wet weight increase reflecting uterine growth (late responses), we collected the tissues 24 h after E2 treatment. In some experiments, animals were injected with E2 and P4 (Sigma) called “E+Pe” to mimic the hormonal profile during implantation as previously described47. The control group of this experiment was injected with the series of hormones similar to the E+Pe treatment group except the last nidatory dose of E2 was replaced with sesame oil, called “E+Poil”. The animals were injected intraperitoneally with 100 μL of 5-ethynyl-2-deoxyuridine (EdU, Invitrogen, Carlsbad, CA) at a dose of 2 mg/mL in phosphate-buffered saline (PBS) 2 h prior to sacrifice. At the time of collection, uteri were weighed, and one uterine horn was snap frozen and stored at −80 °C for RNA extraction. The contralateral horn was collected in 10% buffered formalin solution for histological analysis. Animals were handled according to National Institute of Environmental Health Sciences (NIEHS) Animal Care and Use Committee guidelines and in compliance with NIEHS-approved animal protocol. All methods were performed in accordance with the relevant guidelines and regulations.
Artificial decidualization
Adult (8–12-week-old) female mice were ovariectomized (n = 9–10 animals/genotype). Two weeks after ovariectomy, the mice were treated with E2 (100 ng/mouse) subcutaneously for 3 consecutive days (Day 1 or D1) to D3. On D6-D11, the mice were treated daily with P4 (1 mg/mouse) together with E2 (6.7 ng/mouse). Artificial decidualization was stimulated on D8 by a intraluminal injection of 50 μL of sesame oil into the right uterine horn, the left horn was not injected to be used as a negative control. EdU was injected intraperitoneally 2 h before sacrifice. The animals were euthanized on D11 (72 h after intraluminal oil injection). The uteri (decidualized and non-decidualized horns) were weighed and fixed in formalin for histological analysis. Sections of uteri were snap frozen for RNA analysis. Uterine weight change reflects the weight increase in decidualized horns compared to non-decidualized horns. Six of ten Esr1 f/− females responded to decidual stimulation, whereas two of nine Amhr2 Cre/+; Esr1 f/− females responded. Therefore, only uteri from 6 Esr1 f/− and 2 Amhr2 Cre/+; Esr1 f/− animals were included for the RNA data analysis.
Fertility study and collection of implantation sites
To evaluate the ovulatory response, pubertal (3–5-week-old) Esr1 f/− and Amhr2 Cre/+; Esr1 f/− females were injected with 5 U of pregnant mare’s serum gonadotropin (PMSG, EMD Millipore, Billerica, MA) in sterile normal saline. Human chorionic gonadotropin (hCG, EMD Millipore) was injected 48 h after PMSG injection. At 18 h post hCG injection, the ovulated oocytes were collected from the oviduct, the number of oocytes were counted and recorded. In the fertility study, adult (8-week-old) Esr1 f/− and Amhr2 Cre/+; Esr1 f/− females were mated with a male proven breeder (C57BL6/J, Jackson Laboratory) continuously for 6 months. Numbers of pups per litter per dam over the 6-month period were recorded.
To collect the uteri at different stages of pregnancy as well as implantation sites, adult (8–12-week-old) Esr1 f/− and Amhr2 Cre/+; Esr1 f/− females were mated with the stud male (B6/D2F1/J, Jackson Laboratory) over night. The next morning, the observed presence of a copulatory plug was designated as 0.5 dpc. At 3.5, 4.5, 5.5, 7.5 and 10.5 dpc, uteri were collected from both Esr1 f/− and Amhr2 Cre/+; Esr1 f/− mice. To visualize implantation sites at 4.5 dpc, Chicago Blue dye (Sigma) was injected into the tail vein as previously described48. The visible blue bands indicate the implantation sites. Some of the implantation sites were collected for RNA extraction, the rest of the implantation sites were collected in formalin for histological analysis. At 5.5, 7.5 and 10.5 dpc, the implantation sites are visible without the blue dye injection. The sites were collected for RNA and histological analysis.
Real-time RT-PCR analysis
After collection, RNA was extracted from uteri (or implantation sites) using TriZol reagent (Invitrogen) according to manufacturer’s protocol. Genomic DNA contamination was eliminated by incubating the RNA samples with DNaseI (Invitrogen). Two μg of RNA was used as a template for cDNA synthesis using SuperScript II (Invitrogen). Real-time PCR and analysis was performed as described previously8. Expression values were normalized to ribosomal protein L7 (Rpl7) and calculated as fold change over vehicle control (or over E+Poil) of the Esr1 f/− group. The primer sequences of Rpl7, Lif (Leukemia inhibitory protein), Ihh (indian hedgehog), Igf1 (insulin-like growth factor-1), Mad2l1 (mitotic arrest deficient-like 1), Cdkn1a (Cyclin-Dependent Kinase Inhibitor 1 A), Cebpb (CCAAT Enhancer Binding Protein Beta), Klf4 and Klf15 (Kruppel like factors), Mcm2 (minichromosome maintenance complex component 2), and Ltf (lactotransferrin) were reported previously8, 22. The primer sequences for Adm (adrenomedullin), Antpt2 (angiopoietin 2), Bmp2 (bone morphogenic protein 2), Ccnb1 (cyclin B1), Cdc2a (cyclin dependent kinase A1), Clca3 (chloride channel calcium-activated 3), Egfr (epidermal growth factor receptor), Fkbp5 (FK506 binding protein 5), FMS-like tyrosine kinase 1 (Flt1 also known as vascular endothelial growth factor receptor 1 or Vegfr1), Flt4 (or Vegfr3), Gja (gap junction protein alpha), Hbegf (heparin-binding epidermal growth factor), Kdr (kinse insert domain protein receptor also known as Vegfr2), Muc1 (mucin 1), Prl8a2 (prolactin family 8, subfamily a, member 2), Ptgs2 (prostaglandin-endoperoxide synthase 2), Thbs1 (thrombospondin 1), vascular endothelial growth factors (Vegfa, Vegfb, and Vegfc), Wnt4 and Wnt5a (wingless-type MMTV integration site family, member 4 and 5a, respectively) are as followed (5′ → 3′): Adm-F: CATCCAGCAGCTACCCTACG, Adm-R: TTCGCTCTGATTGCTGGCTT, Antpt2-F: TCGCTGGTGAAGAGTCCAAC, Antpt2-R: GTCAAACCACCAGCCTCCTG, Bmp2-F: GACTGCGGTCTCCTAAAGGTC G, Bmp2-R: CTGGGGAAGCAGCAACACTA, Ccnb1-F: TTGTGTGCCCAAGAAGATGCT, Ccnb1-R: GTACATCTCCTCATATTTGCTTGCA, Cdc2a-F: GGACGAGAACGGCTTGGAT, Cdc2a-R: GGCCATTTTGCCAGAGATTC, Clca3-F: AACAACGGCTATGAGGGCAT, Clca3-R: TGAGTCACCATGTCCTTTATGTGT, Egfr-F: GCATCATGGGAGAGAACAACA, Egfr-R: CTGCCATTGAACGTACCCAGA, Fkbp5-F: CCTCGCAGCCTTCCTGAAC, Fkbp5-R: CACTCCACGGCTTTGTTGTACTC, Flt1-F: GTGTCTATAGGTGCCGAGCC, Flt1-R: CGGAAGAAGACCGCTTCAGT, Flt3-F: CCGCAAGTGCATTCACAGAG, Flt3-R: TCGGACATAGTCGGGGTCTT, Gja-F: AGTGAAAGAGAGGTGCCCAGA, Gja-R: AATGAAGAGCACCGACAGCC, Hbegf-F: TTCTGGCCGCAGTGTTGTC, Hbegf-R: CTGAGCACGATCACCTCCC, Kdr-F: GCATACCGCCTCTGTGACTT, Kdr-R: AAATCGCCAGGCAAACCCAC, Muc1-F: CCCCTATGAGGAGGTTTCGG, Muc1-R: CAGATCAGAGTGCAGGGGTC, Ptgs2-F: AAGGCTCAAATATGATGTTTGCATT, Prl8a2-F: AAACACTTGTTTCACGCATGTATAG, Prl8a2-R: AGGAGTGATCCATGCACCCA, Ptgs2-R: CCCAGGTCCTCGCTTATGATC, Wnt4-F: AGTGACAAGGGCATGCAGC, Thbs1-F: CCTCCCCTCTGCTTTCACAA, Thbs1-R: TAACCGAGTTCTGGCAGTGAC, Vegfa-F: TATTCAGCGGACTCACCAGC, Vegfa-R: AACCAACCTCCTCAAACCGT, Vegfb-F: AGCTGACATCATCCATCCCAC, Vegfb-R: CAGCTTGTCACTTTCGCGG, Vegfc-F: GTGCTTCTTGTCTCTGGCGT, Vegfc-R: TTCAAAAGCCTTGACCTCGC, and Wnt4-R: CATCCTGACCACTGGAAGCC, Wnt5a-F: CGTGGTGTGAATGAACTGGG, Wnt5a-R: CCAAATGTGGGCGTGATTGT.
Immunohistochemical (IHC) analysis
Formalin-fixed tissues were cross-sectioned (5 microns). The tissues were stained with mouse primary antibodies against ESR1 (ImmunoTech, Beckman Coulter, Pasadena, CA, #1545), PGR (ImmunoTech #1546), and Ki67 (BD Pharmingen, BD Biosciences, San Jose, CA, #550609) as indicated previously22. CEBPB (SC150, Santa Cruz Biotechnology, Dallas, TX) was stained at a concentration of 1:100 antibody diluted in blocking reagent containing 1% non-fat milk (Santa Cruz Biotechnology), 1% bovine serum albumin (Sigma), and 10% normal goat serum (Jackson ImmunoResearch, West Grove, PA) in automation buffer (BioCare Medical, Concord, CA). Detection of EdU incorporation for DNA synthesis in the uteri was performed as described previously49.
Quantification analysis for Ki67- and PGR-positive cells
The images were taken using bright field microscope (DMi8, Leica Microsystems, Buffalo Grove, IL) with 10x and 100x objective lenses using Leica Application Suite (Leica Microsystems). Ki67- (10x) and PGR- (100x) positive cells were quantified using ImageJ software with Cell Counter Plugins. The Ki67-positive epithelial cells were counted and calculated as a percentage of total epithelial cells. The glandular epithelial cells were excluded as the majority of the glands are embedded within the stroma of the anti-mesometrial pole. As a result, the proliferation rate of glandular epithelial cells may vary based on the degree of ESR1 deletion in each animal. The total epithelial cell count ranged from 45–324 cells per microscopic field from a total of 3–8 animals per genotype. To eliminate bias from selecting the compartment that does or does not express ESR1, we instead calculated the Ki67-positive luminal epithelial cells of the entire mesometrial pole (top 50% of the uterus) and anti-mesometrial pole (bottom 50% of the uterus), regardless of ESR1 expression status (dotted lines indicated in Supplementary Fig. S2A).
The stromal cells with brown staining (PGR-positive) and blue staining (hematoxylin or PGR-negative) were counted per microscopic field by two different individuals. The number of brown and blue cells were summed as total stromal cells per microscopic field. The PGR-positive cells were calculated as a percentage of the total stromal cells. The total cells counted ranged from 131–285 cells per microscopic field from a total of 3 animals/genotype/treatment.
Measurement of relative signal intensity of PGR in nuclear and cytosolic compartments
All images were taken with a 100x objective lens with similar and software settings. To quantify the light intensities of the nuclear and cytoplasmic compartment, the RGB images were converted into grayscale 8-bit images using ImageJ software and then were inverted. The darkest areas were converted to light white areas, and vice versa (color values from black to white = 0–255). The value of grayscale value was measured in the nuclear or cytoplasmic compartments of the epithelial cells using the wand tool to select an area of 1 × 1 pixel for each compartment within the microscopic field. The relative intensities were calculated as a percentage relative to 255-white signal (255 white signal = 100% relative intensity). The total cells counted ranged from 15–67 cells per image from a total of 3 animals/genotype/treatment.
Statistical analysis
All graphs represent mean ± SEM. Statistical analysis was performed using GraphPad Prism version 6.0 h for Mac OS X (GraphPad Software, Inc., La Jolla, CA). Statistical significance is considered when p < 0.05 using two-way ANOVA with Tukey post hoc test, unless otherwise indicated.
References
Couse, J. F. & Korach, K. S. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20, 358–417 (1999).
Hewitt, S. C., Winuthayanon, W. & Korach, K. S. What’s new in estrogen receptor action in the female reproductive tract. J Mol Endocrinol 56, R55–71 (2016).
Couse, J. F., Lindzey, J., Grandien, K., Gustafsson, J. A. & Korach, K. S. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 138, 4613–4621 (1997).
Quarmby, V. E. & Korach, K. S. The influence of 17 beta-estradiol on patterns of cell division in the uterus. Endocrinology 114, 694–702 (1984).
Martin, L. & Finn, C. A. Hormonal regulation of cell division in epithelial and connective tissues of the mouse uterus. J Endocrinol 41, 363–371 (1968).
Martin, L., Finn, C. A. & Trinder, G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol 56, 133–144 (1973).
Cooke, P. S. et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA 94, 6535–6540 (1997).
Winuthayanon, W., Hewitt, S. C., Orvis, G. D., Behringer, R. R. & Korach, K. S. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA 107, 19272–19277 (2010).
Zhu, L. & Pollard, J. W. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci USA 104, 15847–15851 (2007).
Murphy, L. J. & Ghahary, A. Uterine insulin-like growth factor-1: regulation of expression and its role in estrogen-induced uterine proliferation. Endocr Rev 11, 443–453 (1990).
Nelson, K. G. et al. Transforming growth factor-alpha is a potential mediator of estrogen action in the mouse uterus. Endocrinology 131, 1657–1664 (1992).
Alden, R. H. Implantation of the rat egg; experimental alteration of uterine polarity. J Exp Zool 100, 229–235 (1945).
Stewart, C. L. et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79 (1992).
Ramathal, C. Y., Bagchi, I. C., Taylor, R. N. & Bagchi, M. K. Endometrial decidualization: of mice and men. Semin Reprod Med 28, 17–26 (2010).
Curtis Hewitt, S., Goulding, E. H., Eddy, E. M. & Korach, K. S. Studies using the estrogen receptor alpha knockout uterus demonstrate that implantation but not decidualization-associated signaling is estrogen dependent. Biology of reproduction 67, 1268–1277 (2002).
Lydon, J. P. et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9, 2266–2278 (1995).
Pawar, S., Laws, M. J., Bagchi, I. C. & Bagchi, M. K. Uterine Epithelial Estrogen Receptor-alpha Controls Decidualization via a Paracrine Mechanism. Molecular endocrinology 29, 1362–1374 (2015).
Arango, N. A. et al. A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev 75, 1154–1162 (2008).
Huang, C. C., Orvis, G. D., Wang, Y. & Behringer, R. R. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One 7, e44285 (2012).
Daikoku, T. et al. Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology 155, 2718–2724 (2014).
Hewitt, S. C. et al. Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. FASEB J 24, 4660–4667 (2010).
Winuthayanon, W., Hewitt, S. C. & Korach, K. S. Uterine Epithelial Cell Estrogen Receptor Alpha-Dependent and -Independent Genomic Profiles That Underlie Estrogen Responses in Mice. Biology of reproduction 91, 1–10 (2014).
Mantena, S. R. et al. C/EBPbeta is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci USA 103, 1870–1875 (2006).
Ray, S. & Pollard, J. W. KLF15 negatively regulates estrogen-induced epithelial cell proliferation by inhibition of DNA replication licensing. Proc Natl Acad Sci USA 109, E1334–1343 (2012).
Winuthayanon, W. et al. Oviductal estrogen receptor alpha signaling prevents protease-mediated embryo death. Elife 4, e10453 (2015).
Chen, J. R. et al. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology 141, 4365–4372 (2000).
Lee, K. et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 38, 1204–1209 (2006).
Lee, D. K. et al. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Molecular endocrinology 24, 930–940 (2010).
Jeong, J. W., Lee, K. Y., Lydon, J. P. & DeMayo, F. J. Steroid hormone regulation of Clca3 expression in the murine uterus. J Endocrinol 189, 473–484 (2006).
Cha, J. et al. Appropriate crypt formation in the uterus for embryo homing and implantation requires Wnt5a-ROR signaling. Cell Rep 8, 382–392 (2014).
Das, S. K. et al. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 120, 1071–1083 (1994).
Daikoku, T. et al. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell 21, 1014–1025 (2011).
Kashiwagi, A. et al. The postimplantation embryo differentially regulates endometrial gene expression and decidualization. Endocrinology 148, 4173–4184 (2007).
Kim, M. et al. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol Med 5, 1415–1430 (2013).
Edwards, A. K. et al. Expression of angiogenic basic fibroblast growth factor, platelet derived growth factor, thrombospondin-1 and their receptors at the porcine maternal-fetal interface. Reprod Biol Endocrinol 9, 5 (2011).
Laws, M. J. et al. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development 135, 2659–2668 (2008).
Buchanan, D. L. et al. Tissue compartment-specific estrogen receptor-alpha participation in the mouse uterine epithelial secretory response. Endocrinology 140, 484–491 (1999).
Nelson, S. & Ascoli, M. Epidermal growth factor, a phorbol ester, and 3′,5′-cyclic adenosine monophosphate decrease the transcription of the luteinizing hormone/chorionic gonadotropin receptor gene in MA-10 Leydig tumor cells. Endocrinology 131, 615–620 (1992).
Bruchim, I., Sarfstein, R. & Werner, H. The IGF Hormonal Network in Endometrial Cancer: Functions, Regulation, and Targeting Approaches. Front Endocrinol 5, 76 (2014).
Ghazal, S. et al. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol Med 7, 996–1003 (2015).
Couse, J. F. et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Molecular endocrinology 9, 1441–1454 (1995).
Curtis, S. W., Clark, J., Myers, P. & Korach, K. S. Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor alpha knockout mouse uterus. Proc Natl Acad Sci USA 96, 3646–3651 (1999).
Paria, B. C., Tan, J., Lubahn, D. B., Dey, S. K. & Das, S. K. Uterine decidual response occurs in estrogen receptor-alpha-deficient mice. Endocrinology 140, 2704–2710 (1999).
Tan, J., Paria, B. C., Dey, S. K. & Das, S. K. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 140, 5310–5321 (1999).
Kaya Okur, H. S., Das, A., Taylor, R. N., Bagchi, I. C. & Bagchi, M. K. Roles of Estrogen Receptor-alpha and the Coactivator MED1 During Human Endometrial Decidualization. Molecular endocrinology 30, 302–313 (2016).
Jamin, S. P., Arango, N. A., Mishina, Y., Hanks, M. C. & Behringer, R. R. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 32, 408–410 (2002).
Pan, H., Zhu, L., Deng, Y. & Pollard, J. W. Microarray analysis of uterine epithelial gene expression during the implantation window in the mouse. Endocrinology 147, 4904–4916 (2006).
Nagy, A., Gertsenstein, M., Vintersten, K. & Behringer, R. Isolating Postimplantation Embryos: Prestreak-Stage. Cold Spring Harb Protoc 2006, pdb.prot4362 (2006).
Winuthayanon, W. et al. The natural estrogenic compound diarylheptanoid (D3): in vitro mechanisms of action and in vivo uterine responses via estrogen receptor alpha. Environ Health Perspect 121(433–439), 439e431–435 (2013).
Acknowledgements
We thank Dr. Richard Behringer (University of Texas MD Anderson) for Amhr2 Cre/+ animals, Drs. Yukitomo Arao and Margeaux Wetendorf for critical reading of this manuscript, Jeff Tucker (NIEHS Fluorescent Microscope Imaging Center) for technical assistance, NIEHS Histology Core for some of the tissue processing, NIEHS Comparative Medicine Branch for animal care and surgery. Supported by WSU, College of Veterinary Medicine start-up fund (W.W.) and the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (K.S.K., 1Z1ES070065).
Author information
Authors and Affiliations
Contributions
W.W., S.C.H., and K.S.K. wrote the manuscript, W.W., S.L.L., and L.J.D. collected the data and prepared the figures. K.C.D. and S.R.S. performed quantitation analysis for data in Figures 2 and 4. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Winuthayanon, W., Lierz, S.L., Delarosa, K.C. et al. Juxtacrine Activity of Estrogen Receptor α in Uterine Stromal Cells is Necessary for Estrogen-Induced Epithelial Cell Proliferation. Sci Rep 7, 8377 (2017). https://doi.org/10.1038/s41598-017-07728-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07728-1
This article is cited by
-
The role of mesenchymal estrogen receptor 1 in mouse uterus in response to estrogen
Scientific Reports (2023)
-
Endometrial cancer
Nature Reviews Disease Primers (2021)
-
Pluripotent Stem (VSELs) and Progenitor (EnSCs) Cells Exist in Adult Mouse Uterus and Show Cyclic Changes Across Estrus Cycle
Reproductive Sciences (2021)
-
Hashimoto’s thyroiditis impairs embryo implantation by compromising endometrial morphology and receptivity markers in euthyroid mice
Reproductive Biology and Endocrinology (2019)
-
Estrogen Action in the Epithelial Cells of the Mouse Vagina Regulates Neutrophil Infiltration and Vaginal Tissue Integrity
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.