Abstract
Divergent synthesis of antimalarial troponoids, including naturally occurring compounds, some of which were identified and isolated by our group, has been achieved utilizing the total synthetic route of puberulic acid. Structure-activity relationships of natural products and simple troponoids inspired us to explore more detailed properties of this class of compounds. Access to new derivatives was facilitated through intermediate compounds generated during the total synthesis of puberulic acid by a stepwise oxidation-aromatization sequence to provide 7-hydroxytropolones and bromination for conversion of the carboxylic acid moiety. The first total synthesis of viticolin A, as well as the synthesis of different methyl-substituted derivatives, has also been achieved. In vitro antimalarial activity and cytotoxicity of novel derivatives were evaluated and fundamental information to facilitate the discovery of more promising antimalarials was obtained.
Similar content being viewed by others
Introduction
As one of the most critical infectious diseases in the world, particularly in sub-Saharan Africa, malaria remains a serious global health problem. The World Health Organization (WHO) estimated that there were 212 million clinical cases of malaria and 429 000 deaths in 2015 and has been warning that it puts 3.2 billion people, about half of the world’s population, at risk1. In spite of various traditional antimalarial drugs such as quinine2, chloroquine3 and sulfadoxine/pyrimethamine4 having been developed for malaria, plus current artemisinin-based combination therapy (ACT)5, drug-resistant parasites and multi-drug resistance against ACT have been rapidly and continually emerging6. Development of antimalarial drugs with novel structures and new modes of action is, therefore, incessantly and urgently required.
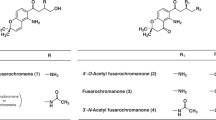
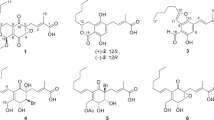
Puberulic acid (1)7, stipitatic acid (2)8 and viticolins A and B (3, 4) as novel natural products have been isolated from a culture broth of Penicillium viticola 9 FKI-4410 through our screening system and found to have promising antimalarial activity (Fig. 1)10, 11. In these highly-oxygenated 7-membered aromatic compounds, 1 shows the most potent antimalarial activity in vitro against the Plasmodium falciparum K1 (chloroquine-resistant) parasite strain (IC50 = 0.050 µM), as well as in vivo efficacy with 69% inhibition for a dose of 2 mg/kg × 4 through subcutaneous (s.c.) administration in 4-day suppressive test using a P. berghei-infected mouse model12. However, 1 exhibits toxicity in vivo, four out of five mice dying by day 3, after a s.c. dose of 5 mg/kg × 2 (day 0 and 1). While structually simple compounds such as tropone (5), tropolone (6) and hinokitiol (8), and natural 2 and 3 showed weaker activity than that of 1, 7-hydroxytropolone (7)13 was much more potent, exhibiting a >18-fold stronger IC50 value of 6.44 µM than that of 5. This observation suggested that the presence of more than three contiguous oxygen atoms in a compound might significantly affect antimalarial activity. These results stimulated us to undertake a structure-activity relationship (SAR) study based on the establishment of a total synthetic route, aiming to create new antimalarial candidates which retained potency but which were non-toxic. Furthermore, we expected that these compounds’ properties, especially low molecular weight and simple planar structures, could be invaluable for antimalarial drug leads with respect to ease of supply14, enabled by efficient and practical synthesis. Herein, we report the divergent synthesis of several related troponoids, including natural products, via utilization of the established total synthetic route of 1 15, and biological evaluation of their in vitro antimalarial activity and cytotoxicity.
Results and Discussion
Synthetic strategy
In contrast with synthetic strategy for puberulic acid (1)16, 17, we proposed an original divergent synthetic route to efficiently produce various analogues and novel derivatives to help clarify the SAR (Fig. 2). A critical point in the synthesis of this class of compounds is fabrication of the 7-membered aromatic ring18. We envisaged that the unique highly-oxygenated tropolone framework of 1 could be constructed by multi-oxidation of the 7-membered aliphatic polyalcohol 9 via simultaneous tautomerization and aromatization. Stepwise oxidation of the 7-membered compound 10, followed by subsequent aromatization, might also allow access to naturally occurring analogues and/or non-natural type derivatives through functionalizations facilitated by the enone 11. The cyclic compound 10 could be synthesized by functionally-tolerated ring-closing metathesis of the diene 12, which could be obtained from D-(+)-galactose (13) containing the C-C and C-O bonds in the backbone of the target compound by a mild Barbier type addition of allyl chloride19. With an efficient supply of compounds established, we overlapped the characteristic highly-oxygenated structure of 1, with a sugar as one of the cheapest and unlimited natural sources, and chose 13 as a starting material. Although sugars are generally used in syntheses of complex molecules in the chiral pool method from 3-dimensional information20, 21, in this synthesis we focused on structural information to utilize the sugar as a “framework source”. This should lead to the target compound using minimal bond-forming reactions22, 23.
Synthesis and oxidation of diol 17 and tetraol 18
Manipulation of protection of the hydroxyl groups on the 6-membered ring of 13 and Appel reaction of the resulting primary alcohol, afforded the iodide 14 24. The diene 16 was made possible by the Barbier type addition of the allylchloride 15 25 with 14, in the presence of zinc dust, providing the desired compound in good yield. Subsequently, ring-closing metathesis of 16, using Grubbs 2nd catalyst (10 mol%) under high dilution condition (0.01 M), afforded the cyclic compound 17 in excellent yield. Using the major diastereomer (6S)-17, deprotection of the acetonide group under an acidic condition produced the desired tetraol 18 (Fig. 3a). With respect to investigations of multi-oxidation, we assumed that the PMB group of 18 might be removed under acidic oxidation conditions (e.g. Jones oxidation) or undergo oxidative cleavage (e.g. DDQ or CAN), directly providing 1 or the corresponding aldehyde. However, efforts were unsuccessful (see Supplementary Table S2 in detail) owing to difficulties of monitoring and handling. Consequently, general DMSO-mediated oxidation conditions (e.g. Swern or Parikh-Doering oxidation) or hyper valent iodine reagents (e.g. IBX, DMP or IBS26, 27) were attempted with expectations that the PMB ether moiety would remain. In some of these reaction conditions, the loss of two molecules of the hydroxyl group from the desired compound 19 was observed, as determined by mass spectrometry analysis, suggesting that aromatization by β-elimination of oxygen functions occurred when the allylic hydroxyl group on the 7-membered ring was oxidized. Although we considered that stepwise oxidation of diol 17 might be beneficial to obtain clues about multi-oxidation, this transformation was unsuccessful under any conditions (see Supplementary Table S3), probably due to problematic isomerization when the homoallyl alcohol was oxidized and β-elimination of oxygenated functions (Fig. 3b).
Total synthesis of puberulic acid and viticolin A
As a result of our initial investigations, we turned our attention to the exo-cyclic oxygen atom and envisaged that in multi-oxidation of the triol 21, the allylic primary alcohol would be oxidized first, providing the α,β-unsaturated aldehyde A as a stable intermediate, thus avoiding problematic isomerization and β-elimination. The diol moiety of the resultant A might then be further oxidized to generate the triketone B, which could undergo tautomerization to produce the enol C. Aromatization of C would immediately occur by protonation, affording the tropolone 22 (Fig. 4a).
Although removal of the PMB group of the diol 17 was troublesome due to the resulting triol 21 being water-soluble and the condition using DDQ sometimes causing decomposition, it was found that Birch reduction with an unusual work-up procedure reproducibly afforded the desired compound in good yield. With the desired 21 in hand, several oxidation conditions were tried (see Supplementary Table S4). Consequently, we found that treatment of 21 under the Parikh-Doering condition caused multi-tandem oxidation, including additional reaction of 22 to the corresponding aldehyde 23, which allowed us a fewer step synthesis of the target compound. Finally, Pinnick oxidation of 23 and subsequent removal of the acetonide group afforded puberulic acid (1) in 8 steps, with 52% overall yield (Fig. 4b), enabling a practical and scalable total synthesis of 1 15.
We synthesized compounds 25 and 26 during the total synthesis of 1 to detect the generation of the carboxylic acid 24 which was unable to be purified by silica gel due to chelate ability with metals28. Although all compounds constructing the tropolone framework (but not the tropone framework) could not be monitored and purified by silica gel, it was found that these compounds could be monitored using LC-UV analysis and purified by reverse-phase chromatography, enabling reliable supply of this class of compounds. We envisioned that the methylated compounds would be valuable to clarify more detailed SAR induced by the information from natural products 3 and 4, and exploited them for further derivatization. Hydrolysis of the methyl ester in both 25 and 26 afforded two methoxy carboxylic acids 27 and 28, respectively. Interestingly, removal of the acetonide group of 28 proceeded with 80% TFA at 120 °C in a sealed tube to provide iso-viticolin A (29) in 94% yield over 2 steps. However, we failed to obtain the natural product, viticolin A (3), because the methyl group of its regioisomer 27 was removed faster than the acetonide group under any acidic conditions. We therefore decided to produce natural products 3 and 4 from puberulic acid (1) as the starting point. Treatment of 1 with MeOH in the presence of TsOH, followed by careful methylation with TMSCHN2, afforded the mono-methylated methylester 30 in 47% yield as a single regioisomer, along with the recovered starting material. We supposed that the inner hydroxyl group of 1 is partly anionic and thus more nucleophilic than the two outer hydroxyl groups due to delocalization of the tropolone framework. It, hence, selectively underwent the methylation allowing access to the natural product. Finally, total synthesis of 3 was accomplished by hydrolysis of 30 in 73% yield. On the other hand, direct methylation of 1 using two equivalents of TMSCHN2 gave a complex mixture of the starting material, mono-, di-, and possibly tri-methylated products, as well as stepwise methylation of 30. In these reactions, viticolin B was detected by LC-UV analysis and1H NMR but no selectivity was observed, and thus the given mixture was unable to be separated even by HPLC.
With respect to bioactivity, we first intended to explore the SAR of hydroxyl groups on the tropolone ring which might possibly intensify previously reported bioactivity of the natural compounds (1, 2) and simple troponoids (5–8)10, 11. Since the SAR of 1, 2, 6 and 7 indicated that more than three contiguous oxygen atoms on the tropolone ring seem to increase antimalarial activity, we planned to synthesize 7-hydroxytropolone-type derivatives and envisioned that stepwise oxidation of a total synthetic intermediate might allow access to different substituted-type derivatives from 2.
Synthesis of 7-hydroxytropolones
With the observation of stepwise oxidation of the total synthetic intermediate, diol 17, we envisaged that 7-hydroxytropolones could be synthesized by aromatization through β-elimination of an alkoxide (Fig. 5). As aforementioned, because diol oxidation was unsuccessful under any conditions, the protective group of (6S)-17 was converted from the acetonide group to the methyl groups in a 2-step manipulation, methylation of the hydroxyl groups and removal of the acetonide group under an acidic condition, providing (6S)-31 in 99% yield. Finally, oxidation of the vicinal diol with IBX proceeded smoothly to afford the diketone (6S)-32, which was then treated with DBU occurring aromatization by β-elimination to provide the 7-methoxytropolone 33. For purification, the hydroxyl group of 33 was protected with a methyl group by TMSCHN2 to give tropones 34 and 35 as a 1:1.4 separable mixture of the regioisomers in 94% yield over 3 steps. The structure of these compounds was determined by NOE analysis. Using the minor isomer 34, simultaneous removal of the methyl groups and bromination of the PMB-ether moiety was found to occur following treatment with 33% HBr/AcOH in the presence of H2O, providing the bromide 36, which was then converted to the corresponding alcohol 37 in 94% yield, or methyl ether 38 in 80% yield, by working-up with H2O or MeOH, respectively. Conversely, when the major isomer 35 was treated with DDQ, concurrent deprotection of the PMB group and oxidation occurred to provide the aldehyde 39 in a single step. Subsequent oxidation to the carboxylic acid under the Pinnick condition, followed by removal of the methyl groups with HBr/AcOH, afforded the desired iso-stipitatic acid (40) in a quantitative yield over 2 steps. In this way, we successfully prepared three non-natural 7-hydroxytropolones.
Bioactivity of tropolone derivatives
Synthesized derivatives, including intermediate compounds, were evaluated for in vitro antimalarial activity against the Plasmodium falciparum K1 (chloroquine-resistant) parasite strain and for cytotoxicity against a human lung fibroblast cell line MRC-5 (Table 1). We found that synthetic puberulic acid (1) showed a similar IC50 value (0.044 µM) to that of natural 1. All intermediates possessing the acetonide group, such as 23–28, did not show any antiparasitic activity, whereas 7-hydroxytropolones, such as 37, 38, and 40, were active (IC50 = 4.33, 2.09 and 2.12 µM, respectively), indicating that free hydroxyl groups on the 7-membered ring seem to bestow potency. Although introduction of the methylene hydroxyl group to the C-4 position of 7 did not affect the activity, IC50 values of compounds 38 and 40 were approximately 2-fold better than those of 7 and 37. However, compounds 37 and 38 possessing the methyleneoxy group showed strong cytotoxicity (IC50 values of 3.39 and 1.32 nM, respectively), suggesting that substituents at the C-4 position might critically affect cytotoxicity. Remarkably, comparing 40 with stipitatic acid (2), the substitution pattern on the tropolone ring seems to be very important. In addition, from the SAR of 7-hydroxytropolones 37, 38 and 40, it was found that the carboxyl group is not indispensable for activity. Iso-viticolin A (29) showed approximately 100-fold stronger activity than viticolin A (3). The SAR among natural products 3, 4 and 29 suggests that three contiguous oxygen atoms with no substitutions increase antiparasitic activity.
Since the SAR information suggested that tetra-substituted tropones with at least three consecutive free-hydroxyl groups and a carbonyl group might have more potent antiparasitic activity, in addition to the convertible carboxylic acid moiety, we next planned to synthesize 6,7-dihydroxytropolones via conversion of the carboxyl group.
Synthesis of 6,7-dihydroxytropolones
Varying the carboxyl group of 1, we envisioned that the same method to synthesize derivatives 30 and 31 could be applicable for transformation of the carboxylic acid moiety (Fig. 6). The total synthetic intermediate, triol 21, was subjected to multi-tandem oxidation and a subsequent reduction condition using NaBH4 to afford the alcohol 22 in 73% yield over 2 steps. Then, treatment of 22 with 33% HBr/AcOH in the presence of H2O caused simultaneous removal of the acetonide group and bromination to provide the bromide 41. This intermediate was converted to the alcohol 42, in 83% yield, or methyl ether 43, in 38% yield, by working-up with H2O or MeOH, respectively. Furthermore, 41 was found to undergo azidation by treatment with NaN3 to afford the azide 44 in 38% yield over 4 steps from triol 21. This compound would be useful for further derivatization using Click chemistry29.
Bioactivity of 6,7-dihydroxytropolones
In vitro antimalarial activity and cytotoxicity of synthesized 6,7-dihydroxytropolones 42–44 were evaluated, as shown in Table 2. The alcohol 42 and the methyl ether 43 showed the same order of activity as natural 1. Although the azide 44 exhibited weaker activity compared with 42 and 43, all these derivatives were approximately 10-fold more potent than the 7-hydroxytropolones, suggesting that contiguous four oxygen atoms on the tropolone ring are important for the antiparasitic property. In addition, all these compounds showed cytotoxicity. Our result indicated that this class of compounds, with potent antimalarial activity, tends to show strong cytotoxicity in human cells as well.
These results will be useful in helping to explore more promising drug leads for malaria, and more detailed bioactivity evaluation of synthesized derivatives in a mouse model are now ongoing.
Conclusions
Inspired by the SAR information of natural products isolated by our group and the apparent antiparasitic activity of simple troponoids, we divergently synthesized troponoids using intermediates obtained during the established method for synthesizing puberulic acid. Synthesis of the 7-hydroxytropolones was accomplished by stepwise oxidation-aromatization sequence via β-elimination, which allowed access to non-natural derivatives. Conversion of the carboxylic acid moiety in the natural products was accomplished via brominated key intermediates generated by treatment with HBr/AcOH. This method was applicable to convert the bromide to nucleophiles, which allowed divergent production of derivatives. In vitro antimalarial activity and cytotoxicity of synthesized derivatives were evaluated and several derivatives were found with the same order of antiparasitic activity as the potent natural product, puberulic acid. The SAR information we obtained will be invaluable for exploration of more promising candidates for antimalarial drugs and further derivatization of this class of compounds, and further analysis of the bioactivity of these chemicals, is currently in progress.
Methods
General information
Unless otherwise noted, reagents and solvents were purchased at the highest commercial quality and used without further purification. LC-UV analysis was carried out with Agilent 1100 system (Agilent Technology, Inc.) under the following condition; column, Symmetry C18 (Waters Co., Ltd., 2.1 φ × 150 mm); UV detection, 210 nm; flow rate, 0.2 mL/min; mobile phase, MeCN-H2O with 0.05% H3PO4, (5–100% linear gradient over 20 min). ODS column chromatography was carried out with Sep-Pak® Plus C18 Short Cartridge (Waters Co., Ltd.) or CHROMATOREX® (Fuji Silysia Chemical, Ltd.). 1H NMR spectra were recorded on JEOL JNM-ECA-500 (500 MHz) and 13C NMR spectra were recorded on JEOL JNM-ECA-500 (125 MHz). Chemical shifts are expressed in ppm downfield from the internal solvent peaks for CD3OD (1H; δ = 3.31 ppm,13C; δ = 49.0 ppm) and J values are given in Hertz. The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, and br = broad. High- and Low-resolution mass spectra were measured on JEOL JMS-AX505 HA, JEOL JMS-700 MStation and JEOL JMS-T100LP.
Viticolin A (3)
To a solution of 30 (7.0 mg, 30.97 µmol) in THF (1.55 mL) and H2O (1.55 mL) was added K2CO3 (21.4 mg, 0.15 mmol) at room temperature. After being stirred at 50 °C for 1 d, H2O (20 mL) was added to the reaction mixture. The resulting mixture was extracted with CHCl3 (20 mL × 2). The aqueous layer was acidified by 1 M HCl, and extracted with CHCl3:i-PrOH = 10:1 (40 mL × 4). The combined organic layer was dried over sodium sulfate, and concentrated under reduced pressure. The residue was purified by Sep-pak® Plus C18 Short Cartridge to afford 3 (4.8 mg, 73%) as a yellow solid.
1H NMR (500 MHz, CD3OD) δ7.82 (d, J = 1.2 Hz, 1 H), 7.72 (d, J = 1.2 Hz, 1 H), 3.95 (s, 3 H); 13C NMR (125 MHz, CD3OD) δ172.0, 168.7, 163.3, 160.6, 150.0, 134.4, 123.4, 112.0, 59.6; HRMS-ESI (m/z) [M–H]− calcd for C9H7O6 211.0243, found 211.0234; mp 227 °C.
Iso-viticolin A (29)
To a solution of 26 (10.7 mg, 40.19 µmol) in MeOH (0.40 mL), H2O (0.40 mL), and THF (0.40 mL) was added K2CO3 (27.8 mg, 0.20 mmol) at room temperature. After being stirred at room temperature for 5 h, H2O (20 mL) was added to the reaction mixture. The resulting mixture was extracted with CHCl3 (20 mL) The aqueous layer was acidified by 1 M HCl, and extracted with CHCl3:i-PrOH = 10:1 (40 mL × 3). The combined organic layer was dried over sodium sulfate, and concentrated under reduced pressure to yield the crude product as a yellow solid. This crude product was used in the next reaction without further purification.
To the crude product in a sealed tube was added 80% TFA aq. (0.40 mL) at room temperature. After being stirred at 120 °C for 6 h, the reaction mixture was concentrated under reduced pressure. The residue was purified by Sep-pak® Plus C18 Short Cartridge to afford 29 (8.0 mg, 94% over 2 steps) as a yellow solid.
1H NMR (500 MHz, CD3OD) δ8.01 (s, 1H), 7.96 (s, 1H), 4.03 (s, 3H); 13C NMR (125 MHz, CD3OD) δ169.6, 162.7, 160.7, 157.4, 156.7, 128.8, 121.6, 116.4, 57.3; HRMS-ESI (m/z) [M–H]− calcd for C9H7O6 211.0243, found 211.0240; mp 183 °C (decomp.).
4-Hydroxymethyl-7-hydroxytropolone (37)
To 34 (6.0 mg, 18.97 µmol) in a sealed tube was added a 4:1 mixture of 33% HBr/AcOH and H2O (0.19 mL) at room temperature. After being stirred at 120 °C for 2 h, the reaction mixture was concentrated under reduced pressure. The residue was purified by Sep-pak® Plus C18 Short Cartridge using acetone/H2O to afford 37 (3.0 mg, 94%) as a yellow solid.
1H NMR (500 MHz, CD3OD) δ7.73 (s, 1H), 7.60 (d, J = 10.9 Hz, 1H), 7.42 (d, J = 10.9 Hz, 1H), 4.64 (s, 2H); 13C NMR (125 MHz, CD3OD) δ168.9, 161.5, 160.8, 144.5, 127.9, 122.0, 121.2, 67.2; HRMS-EI (m/z) [M+] calcd for C8H8O4 168.0423, found 168.0426; mp 144 °C (decomp.).
4-Methoxymethyl-7-hydroxytropolone (38)
To 34 (5.0 mg, 15.81 µmol) in a sealed tube was added a 4:1 mixture of 33% HBr/AcOH and H2O (0.16 mL) at room temperature. After being stirred at 120 °C for 2 h, the reaction mixture was concentrated under reduced pressure. The residue was purified by Sep-pak® Plus C18 Short Cartridge using MeOH/H2O to afford 38 (2.3 mg, 80%) as a yellow oil.
1H NMR (500 MHz, CD3OD) δ7.48 (br-s, 1H), 7.41 (d, J = 10.9 Hz, 1H), 7.18 (br-d, J = 10.9 Hz, 1H), 4.42 (s, 2H), 3.40 (s, 3H); 13C NMR (125 MHz, CD3OD) δ169.5, 161.4, 161.2, 141.1, 129.0, 121.73, 121.68, 77.7, 58.5; HRMS-EI (m/z) [M+] calcd for C9H10O4 182.0579, found 182.0572.
Iso-stipitatic acid (40)
To a solution of 39 (4.0 mg, 20.60 µmol) and 2-methyl-2-butene (43.77 µL, 0.41 mmol) in THF (0.21 mL) and t-BuOH (0.21 mL) was added the mixture of NaClO2 (2.8 mg, 30.90 µmol) and NaH2PO4 (9.6 mg, 61.80 µmol) in H2O (0.21 mL) dropwise by a Pasteur pipette at room temperature. After being stirred at room temperature for 30 min, the reaction mixture was directly filtrated by silica gel, eluted with CHCl3/MeOH = 10:1, and concentrated under reduced pressure to afford the crude product as a yellow solid. This crude product was used in the next reaction without further purification.
To the crude product in a sealed tube was added a 4:1 mixture of 33% HBr/AcOH and H2O (0.42 mL) at room temperature. After being stirred at 120 °C for 2 h, the reaction mixture was concentrated under reduced pressure. The residue was purified by Sep-pak® Plus C18 Short Cartridge to afford 40 (3.8 mg, quant. over 2 steps) as a yellow solid.
1H NMR (500 MHz, CD3OD) δ8.15 (s, 1H), 8.06 (d, J = 10.9 Hz, 1H), 7.45 (d, J = 10.9 Hz, 1H); 13C NMR (125 MHz, CD3OD) δ171.7, 169.2, 164.3, 159.9, 132.3, 130.3, 120.5, 119.5; HRMS-ESI (m/z) [M–H]− calcd for C8H5O5 181.0137, found 181.0128; mp 170 °C (decomp.).
4-Hydroxymethyl-6,7-dihydroxytropolone (42)
With the same procedure for the synthesis of 37, 42 (9.8 mg, 83%) was obtained from 22 (14.4 mg, 64.23 µmol) as a yellow solid.
1H NMR (500 MHz, CD3OD) δ 7.15 (s, 2H), 4.52 (s, 2H); 13C NMR (125 MHz, CD3OD) δ 158.4, 157.2, 143.8, 117.2, 67.3; HRMS-EI (m/z) [M+] calcd for C8H8O5 184.0372, found 184.0388; mp 162 °C.
4-Methoxymethyl-6,7-dihydroxytropolone (43)
With the same procedure for the synthesis of 38, 43 (1.7 mg, 38%) was obtained from 22 (5.1 mg, 22.75 µmol) as a yellow solid.
1H NMR (500 MHz, CD3OD) δ7.10 (s, 2H), 4.38 (s, 2H), 3.40 (s, 3H); 13C NMR (125 MHz, CD3OD) δ158.4, 157.2, 140.1, 117.8, 77.6, 58.3; HRMS-ESI (m/z) [M–H]− calcd for C9H9O5 197.0450, found 197.0450; mp 120 °C.
4-Azidomethyl-6,7-dihydroxytropolone (44)
With the same procedure for the synthesis of 42 without purification, 41 was obtained as a intermediate from (6S)-17 (21.4 mg, 92.93 µmol) as a dark brown amorphous. This crude product was used in the next reaction without further purification.
To a solution of the crude 41 in DMF (0.93 mL) was added NaN3 (18.1 mg, 0.28 mmol) at room temperature. After being stirred at room temperature for 2.5 h, the reaction mixture was quenched with H2O (10 mL). The organic layer was separated, and the aqueous layer was extracted with EtOAc (10 mL × 3). The combined organic layer was dried over sodium sulfate, and concentrated under reduced pressure. The residue was purified by Sep-pak® Plus C18 Short Cartridge to afford 44 (7.4 mg, 38% over 4 steps) as a yellow solid.
1H NMR (500 MHz, CD3OD) δ7.09 (s, 2H), 4.35 (s, 2H); 13C NMR (125 MHz, CD3OD) δ158.0, 157.6, 137.3, 118.8, 58.8; HRMS-ESI (m/z) [M–H]− calcd for C8H6O4N3 208.0358, found 208.0358; mp 159 °C.
*Experimental details of other new compounds and additional information are given in the Supplementary Information.
In vitro cultivation of Plasmodium falciparum and the antimalarial assay
In vitro cultivation and antimalarial activity against the Plasmodium falciparum K1 (chloroquine-resistant) parasite strain were measured using the method described previously30. Briefly, the P. falciparum K1 strain was cultured in human erythrocytes in RPMI medium supplemented with 10% human plasma at 37 °C, under 93% N2, 4% CO2, and 3% O2. Asynchronous parasites (2% hematocrit and 0.5 or 1% parasitaemia) were seeded in a 96-well microtiter plate and serially diluted test compounds were added. Positive controls, such as chloroquine and artemisinin, were added in a similar fashion. After 72-hours incubation, parasite lactate dehydrogenase (p-LDH) was assayed using a slight modification of the procedure reported by Makler et al.31 and Vivas et al.32. The 50% inhibitory concentration (IC50) value was calculated from a dose response curve. This study was approved by “Kitasato Institute Hospital Research Ethics Committee (No12102)” using human erythrocytes donated by volunteers.
Cytotoxic assay against MRC-5 cells
Measurement of cytotoxicity against human fetal lung fibroblast MRC-5 cells was carried out as described previously33.
References
World Health Organization, World Malaria Report (2016).
Achan, J. et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar. J. 10, 144–155 (2011).
Cooper, R. G. & Magwere, T. Chloroquine: novel uses & manifestations. Indian J. Med. Res. 127, 305–316 (2008).
Peters, P. J., Thigpen, M. C., Parise, M. E. & Newman, R. D. Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf. 30, 481–501 (2007).
World Health Organization, Antimalarial drug combination therapy (2001).
Sinha, S., Medhi, B. & Sehgal, R. Challenges of drug-resistant malaria. Parasite 21, 61–74 (2014).
Birkinshaw, J. H. & Raistrick, H. Studies in the biochemistry of micro-organisms, XXIII. Puberulic acid C8H6O6 and an acid C8H4O6, new products of the metabolism of glucose by Penicllium puberulum Bainler and Penicillium aurantio-virens Biourge. With an appendix on certain dihydroxybenzenecarboxylic acids. Biochem. J. 26, 441–453 (1932).
Birkinshaw, J. H., Chambers, A. R. & Raistrich, H. Studies in the biochemistry of micro-organisms. Stipitatic acid, C8H6O5, a metabolic product of Penicillium stipitatum Thom. Biochem. J. 36, 242–251 (1942).
Nonaka, K. et al. Penicillium viticola, a new species isolated from a grape in Japan. Mycoscience 52, 338–343 (2011).
Iwatsuki, M. et al. In vitro and in vivo antimalarial activity of puberulic acid and its new analogs, viticolins A–C, produced by Penicillium sp. FKI-4410. J. Antibiot. 64, 183–188 (2011).
Ishiyama, A. et al. Antimalarial tropones and their Plasmodium falciparum glyoxalase I (pfGLOI) inhibitory activity. J. Antibiot. 67, 545–547 (2014).
Peters, W., Portus, J. H. & Robinson, B. L. The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizonticidal activity. Ann. Trop. Med. Parasitol. 69, 155–171 (1975).
Meck, C., D’Erasmo, M. P., Hirsch, D. R. & Murelli, R. P. The biology and synthesis of α-hydroxytropolones. Med. Chem. Commun. 5, 842–852 (2014).
Katsuno, K. et al. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 14, 751–758 (2015).
Sennari, G., Hirose, T., Iwatsuki, M., Ōmura, S. & Sunazuka, T. A concise total synthesis of puberulic acid, a potent antimalarial agent. Chem. Commun. 50, 8715–8718 (2014).
Johns, R. B., Johnson, A. W. & Murray, J. Synthetic experiments in the cycloheptatrienone series. Part III. Syntheses of puberulic acid and isostipitatic acid. J. Chem. Soc. 198–202 (1954).
Banwell, M. G., Collis, M. P., Mackay, M. F. & Richards, S. L. cis-Dihydrocatechols as precursors to highly oxygenated troponoids. Part 2. Regiocontrolled syntheses of stipitatic and puberulic acids. J. Chem. Soc. Perkin Trans. 1, 1913–1920 (1993).
Liu, N., Song, W., Schienebeck, C. M., Zhang, M. & Tang, W. Synthesis of naturally occurring tropones and tropolones. Tetrahedron 70, 9281–9305 (2014).
Hanna, I. & Richard, L. From galactose to highly functionalized seven- and eight-membered carbocyclic rings by ring-closing metathesis. Org. Lett. 17, 2651–2654 (2000).
Hanessian, S. The enterprise of synthesis: from concept to practice. J. Org. Chem. 77, 6657–6688 (2012).
Rouf, A. & Taneja, S. C. Synthesis of single-enantiomer bioactive molecules: a brief overview. Chirality 26, 63–78 (2014).
Wilson, R. M. & Danishefsky, S. J. Pattern recognition in retrosynthetic analysis: snapshots in total synthesis. J. Org. Chem. 72, 4293–4305 (2007).
Willot, M. et al. Total Synthesis and Absolute Configuration of the Guaiane Sesquiterpene Englerin A. Angew. Chem. Int. Ed. 48, 9105–9108 (2009).
Yu, Z., Ya-Peng, L. & Li, Z. Synthesis of per-acetyl D-fucopyranosyl bromide and its use in preparation of diphyllin D-fucopyranosyl glycoside. J. Carbohydr. Chem. 27, 113–119 (2008).
Kałuża, Z. et al. A new synthetic approach to 5-dethia-4-methyl-5-oxacephems. Tetrahedron 59, 5893–5903 (2003).
Uyanik, M., Akakura, M. & Ishihara, K. 2-Iodoxybenzenesulfonic acid as an extremely active catalyst for the selective oxidation of alcohols to aldehydes, ketones, carboxylic acids, and enones with oxone. J. Am. Chem. Soc. 131, 251–262 (2009).
Uyanik, M. & Ishihara, K. 2-Iodoxy-5-methylbenzenesulfonic acid-catalyzed selective oxidation of 4-bromobenzyl alcohol to 4-bromobenzaldehyde or 4-bromobenzoic acid with oxone. Org. Synth. 89, 105–114 (2012).
Pittre, S. R., Ganzhorn, A., Hoflack, J., Islam, K. & Hornsperger, J.-M. α-Hydroxytropolones: a new class of potent inhibitors of inositol monophosphatase and other bimetallic enzymes. J. Am. Chem. Soc. 119, 3201–3204 (1997).
Hirose, T. et al. Rapid ‘SAR’ via Click chemistry: an alkyne-bearing spiramycin is fused with diverse azides to yield new triazole-antibacterial candidates. Heterocycles 69, 55–61 (2006).
Otoguro, K. et al. Potent antimalarial activities of polyether antibiotic, X-206. J. Antibiot. 54, 658–663 (2001).
Makler, M. T. et al. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Med. Hyg. 48, 739–741 (1993).
Vivas, L. et al. Plasmodium falciparum: Stage specific effects of a selective inhibitor of lactate dehydrogenase. Exper. Parasitol. 111, 105–114 (2005).
Otoguro, K. et al. In vitro antimalarial activities of the microbial metabolites. J. Antibiot. 56, 322–324 (2003).
Acknowledgements
We thank Dr K. Nagai and Ms N. Sato (School of Pharmacy, Kitasato University) for various instrumental analyses. This work was supported by a Kitasato University Research Grant for Young Researchers (to M. Iwatsuki).
Author information
Authors and Affiliations
Contributions
T.H., S.Ō. and T.S. designed research. G.S. and R.S. synthesized compounds. M.I., A.I., R.H. and K.O. performed biological evaluation. G.S. wrote the main manuscript text and the Supplementary Information, and prepared the figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sennari, G., Saito, R., Hirose, T. et al. Antimalarial troponoids, puberulic acid and viticolins; divergent synthesis and structure-activity relationship studies. Sci Rep 7, 7259 (2017). https://doi.org/10.1038/s41598-017-07718-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07718-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.