Abstract
Marine calcifying organisms, such as stony corals, are under threat by rapid ocean acidification (OA) arising from the oceanic uptake of anthropogenic CO2. To better understand how organisms and ecosystems will adapt to or be damaged by the resulting environmental changes, field observations are crucial. Here, we show clear evidence, based on boron isotopic ratio (δ11B) measurements, that OA is affecting the pH of the calcification fluid (pHCF) in Porites corals within the western North Pacific Subtropical Gyre at two separate locations, Chichijima Island (Ogasawara Archipelago) and Kikaijima Island. Corals from each location have displayed a rapid decline in δ11B since 1960. A comparison with the pH of the ambient seawater (pHSW) near these islands, estimated from a large number of shipboard measurements of seawater CO2 chemistry and atmospheric CO2, indicates that pHCF is sensitive to changes in pHSW. This suggests that the calcification fluid of corals will become less supersaturated with respect to aragonite by the middle of this century (pHCF = ~8.3 when pHSW = ~8.0 in 2050), earlier than previously expected, despite the pHCF-upregulating mechanism of corals.
Similar content being viewed by others
Introduction
The pH of the surface seawater (pHSW) is considered to have declined by ~0.1 since the beginning of the industrial era, and an additional decline of ~0.3 is projected by the end of this century1,2,3. It has been suggested that coral reef ecosystems are susceptible to reductions in the pH and aragonite saturation state of seawater (ΩarSW)4,5,6, although the critical threshold below which reef growth will be hampered is still contested4, 7,8,9. In the surface layers of tropical–subtropical oceans, ΩarSW is projected to decrease to as low as 3 by 2050, when atmospheric CO2 will reach ~500 μatm1, 4.
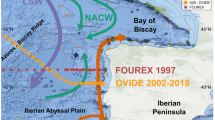
To better understand the response of corals to ocean acidification, we reconstructed past pH changes using skeletal δ11B (Methods; Fig. 1b) as an indicator of the pH of the calcification fluid (pHCF) in long-living massive Porites corals obtained from the islands of Chichijima (27.1°N, 142.2°E)10 and Kikaijima (28.3°N, 130.0°E)11. These corals have experienced OA since the beginning of the industrial revolution (ca. 1750), and particularly during the past 50 years, following the post-1960s increase in anthropogenic CO2 emissions. An advantage of field-based observations over culture experiments is that the latter often subject corals to excessively acidic water (e.g., pH < 7.8), which are not expected to occur this century, even under the “business as usual” CO2 emissions scenario (e.g., refs 12 and 13).
Location of Chichijima and Kikaijima in the North Pacific Ocean. (a) Contours indicate climatological mean sea-surface dynamic height (in units of metres relative to the 1000 m level), with arrows showing the major surface ocean currents in the western North Pacific. Data were downloaded and plotted with the ODV software, version 4.6.2 (ref. 43, http://odv.awi.de). MLO, Mauna Loa Observatory. (b,c) Bathymetric maps around Chichijima and Kikaijima. Depth contours are at 50 m intervals. The GMT software, version 4.5.8 (ref. 44), was used to map the bathymetric data, ETOPO1 (ref. 45, https://www.ngdc.noaa.gov/mgg/global/global.html). The corals were collected at a location with good open ocean seawater circulation (black dot) (Supplementary Figs S7 and S8)10, 11.
Measurements of the seawater CO2 chemistry have been made in the vicinity of these two islands for the last three decades (Methods, Fig. 2). The ocean surrounding the islands is oligotrophic, with limited vertical mixing and low biological productivity14. Seasonality dominates the temporal variability in pHSW at these locations, driving changes in the partial CO2 pressure of the seawater (pCO2SW) (Fig. 2a,c), primarily through variability in the sea-surface temperature (SST; ~20 °C in winter and ~29 °C in summer), and changes in the dissolved inorganic carbon (DIC) concentration (~1960 µmol kg−1 in summer and ~1990 µmol kg−1 in winter in 2010 when normalized to a salinity of 35)15. The increasing trend in pCO2SW (and the decreasing trend in pHSW) in the northern subtropical zone of the western North Pacific follows the rate of increase in atmospheric CO2 (Fig. 2c)14, 16. Because the air–sea CO2 equilibrium has remained unchanged since preindustrial times, the estimated pHSW can be extended back to the preindustrial era (Fig. 2d) using atmospheric CO2 records from the Mauna Loa Observatory (MLO, Fig. 1a)17 and the Antarctic ice sheet18.
pHSW and pCO2 variability. (a–c) Time series (lines) and discrete data (open symbols) for pHSW, ΩarSW, and pCO2 in the western North Pacific near Chichijima and Kikaijima since 1980 at monthly resolution. Monthly atmospheric pCO2 records measured at the MLO17 are also shown in (c). (d) Variability in pHSW (red) and pCO2 (blue, seawater; black, atmosphere) since the preindustrial era. Atmospheric pCO2 in 1959–2013 was measured at the MLO17 and atmospheric pCO2 before 1959 was reconstructed from trapped air in the Antarctic ice sheet18. pHSW calculated from Global Data Analysis Project DIC and TA for the years 1994 and 1750 (ref. 3) are indicated by the green diamonds in (a and d).
Results and Discussion
Statistically insignificant variations in δ11B were recorded for the period before 1960, whereas a rapid decline in δ11B occurred after 1960 (−0.18 ± 0.04‰/decade for the Chichijima coral, p < 0.001; −0.29 ± 0.07‰/decade for the Kikaijima coral, p < 0.01). This trend correlates with the trend in pHSW evaluated from the time series record of the atmospheric CO2 concentration, and the correlation is derived from a long-term decreasing trend, not inter-annual variability (Fig. 3a,b). Kubota et al.19 demonstrated that the coral skeleton δ11B from Chichijima Island follows the trend in ocean acidification during the 20th century, and we confirmed their initial finding by measuring δ11B in a second massive Porites coral skeleton collected from nearby Kikaijima Island. The stable carbon isotopic ratios of the corals (δ13Ccoral) behave in a similar way (Fig. 3c), decreasing slightly or remaining steady until 1960 (−0.11 ± 0.02‰/decade for the Chichijima coral, p < 0.001, N = 49; −0.04 ± 0.06‰/decade for the Kikaijima coral, p = 0.53, N = 6), and declining sharply thereafter (−0.25 ± 0.04‰/decade for the Chichijima coral, p < 0.01, N = 35; −0.34 ± 0.08‰/decade for the Kikaijima coral, p < 0.01, N = 8). These patterns in δ13Ccoral are consistent with the previously reported records of stable carbon isotopic ratios for atmospheric CO2 (δ13Catm), corresponding to −0.04 ± 0.01‰/decade (p < 0.01) before 1960 and −0.24 ± 0.01‰/decade (p < 0.01) after 1960 (ref. 18) (Fig. 3c,d). Such recent reductions in the δ13C of the carbon reservoir in the Earth surface system is called the ‘13C Suess effect’20, and are caused by the anthropogenic addition of 12C-rich carbon, derived from fossil fuel burning and deforestation, to the Earth surface system. The recent reductions in δ13Ccoral in the Kikaijima and Chichijima corals are consistent with the expected reductions attributable to the13C Suess effect, which has been observed directly in atmospheric CO2 (ref. 18) and the δ13CDIC of the surface seawater in the subtropical North Pacific21, 22. Other potential drivers of δ13Ccoral and δ11B are the growth rate of the skeleton23 and the photosynthetic activity of algal symbionts24. We argue these effects on δ13Ccoral are minor compared with the13C Suess effect, for the following reasons. If the decline in δ13Ccoral is solely explained by changes in the growth rate, it would require a substantial increase in the growth rate (e.g., from 5 mm/yr to 10 mm/yr). However, it is inconsistent with the observation that both the Chichijima coral and Kikaijima coral show no increase in their growth rates10, 11. We infer that the correlation between the annual δ13C variation and the linear extension rate of the Chichijima coral reported by Felis et al.10 can be interpreted as the growth rate effect superimposed on the13C Suess effect. During photosynthesis, symbiotic algae preferentially utilize isotopically lighter carbon and leave isotopically heavier carbon in the carbon pool from which the corals precipitate their skeletons13, 24. The recent declines in δ13Ccoral have been interpreted as reflecting substantial reductions in light intensity or photosynthetic activity. However, a previous culture experiment with Porites coral24 indicated that this would require a reduction in light intensity of >50%, which is unlikely to occur in the shallow waters where corals live. We also observed significant positive correlations between δ11B (and pHCF) and δ13Ccoral (r = 0.63, p < 0.03 for the Chichijima coral; r = 0.85, p < 0.001 for the Kikaijima coral; Supplementary Fig. S2). Therefore, we conclude that the coherent declines in δ11B and δ13C in the Chichijima and Kikaijima corals resulted from reductions in pHSW and δ13CDIC, respectively. This suggests that the declines in both δ11B and δ13Ccoral are anthropogenic in origin, and that fingerprints of anthropogenic CO2 uptake by the ocean (OA and the13C Suess effect, respectively) are recorded in the coral skeleton. The probability that the reductions in both δ11B and δ13C are caused by local factors is very small, for the following reasons: no rivers bring organic matter from the land at these sites, which would cause local acidification and lower the δ13CDIC when it is degraded; and there is no coastal upwelling around these islands that would acidify the subsurface water and lower its δ13CDIC. We directly compared the geochemical record of the corals with the open ocean CO2 chemistry, because they were collected from locations that receive good open ocean seawater circulation (Methods). However, the seawater CO2 chemistry may be locally modified by the net community calcification/respiration of the coral reef ecosystems, and we did not confirm this by measuring the variables of the seawater CO2 system. Even if these are local signals, and are not related to the CO2 chemistry of the open ocean seawater, the community calcification/respiration in these coral reef ecosystems has still changed. If so, this intriguing observation gives many clues to the local ecosystem. However, as stated above, we infer that the reductions in both δ11B and δ13C are related to changes in the CO2 chemistry of the open ocean, based on geographic observations at the study sites.
Coral δ11B and δ13C records, pHSW, and δ13C of atmospheric CO2. (a) δ11B records of the corals from Chichijima (green diamonds) and Kikaijima (blue squares). Each point is a 3-year average. Error bar is 2σ of the analytical uncertainty of JCp-1. (b) As in Fig. 2d, but for years 1900–2013. (c) As in (a) but for δ13Ccoral at Chichijima10 and Kikaijima. δ13Ccoral decreased at the same rate as δ13Catm after 1960 (black regression line). (d) δ13Catm in 1981–2012 was measured at the MLO and the values before 1981 were reconstructed from trapped air in the Antarctic ice sheet18.
We observed large differences between pHSW (8.12–8.18), determined from measurements of the CO2 chemistry, and pHCF (8.43–8.53), derived from the δ11B of the corals and a theoretical curve for δ11B of the borate ion25 (Fig. 4a,b and Supplementary Table 1). One plausible explanation for the fact that pHCF is higher than pHSW is the proposed “pH upregulation mechanism” (refs 6, 26,27,28). The (extracellular) calcification fluid occurs between the coral polyp and the underlying skeleton, and so is semi-isolated from the ambient seawater. Corals use Ca2+-ATPase to pump H+ from and Ca2+ into the calcification fluid, which in turn increases pHCF and Ωar (ΩarCF), thus promoting calcification26,27,28. In situ pHCF measurements using micro-pH electrodes and pH-sensitive dyes support the idea of pH upregulation6, 26, 27. pHCF has been estimated in Stylophora pistilata with pH-sensitive dyes and δ11B measurements, which were in excellent agreement assuming three times faster calcification in the light than that in the dark29. Because light-enhanced calcification probably creates a certain bias in the time of maximum calcification (i.e., day time in summer), the δ11B of the Porites corals in this study represents a mean state of pHCF, unless the time of maximum calcification changes. The periodic patterns in the seasonality of geochemical proxies, such as the Sr/Ca ratio, in the Chichijima and Kikaijima coral skeletons do not support any marked changes in the growth season10, 11. Therefore, as in previous studies26, 28, 29, we regard the δ11B of the Porites corals as representative of pHCF on average, at a given pHSW. It has been suggested that Porites corals, as well as other scleractinian coral genera, can use their pH-upregulating mechanism to maintain their pHCF under ongoing OA26,27,28. Therefore, corals are able to increase ΔpH to maintain pHCF as high as possible, perhaps by maintaining the homeostasis of the calcification fluid (Fig. 4a).
Relationships between pHSW and pHCF. (a) δ11B values for long-living and cultured corals (green diamonds, Chichijima Porites sp.; blue squares, Kikaijima Porites sp.; yellow triangles, cultured Porites sp., ref. 12; red triangles, cultured Porites cylindrica, ref. 13) with the theoretical curve for δ11B of the borate ion in seawater25. With the pH-upregulating mechanism, δ11B records not pHSW but pHCF, and ΔpH represents pHCF minus pHSW. (b) A cross-plot of pHCF versus pHSW. Regression lines are shown for each coral record. Dashed horizontal lines indicate pH values when Ωar becomes 3, 2, and 1, which were calculated using present SST, SSS, and TA. (c) As in (b) but for pHCF versus ΔpH.
The relationship between pHSW and pHCF in two cultured Porites corals12, 13 predicts reductions of only 0.03 and 0.05 in pHCF per reduction of 0.1 in pHSW (Fig. 4b), which suggests that coral δ11B may not be sensitive enough to detect anthropogenic OA. However, we detected a clear indication of a rapid decline in pHCF in both the Chichijima and Kikaijima corals with the decline in pHSW (p < 0.03), which was not observed in the cultured corals (Fig. 4b,c). The observation of a one-to-one relationship between pHCF and pHSW strengthens the reliability of the δ11B of Porites coral as a proxy for pHSW, although in situ calibration is crucial19, 30. Although the ΔpH values are similar (0.3–0.5) among the corals in the field and in the culture environments in the present pHSW range of 8.09–8.17 (Fig. 4c), the rates of ΔpH for both cases differ significantly (p < 0.01) (Fig. 4c). We also found that the changes in pHCF were more sensitive to OA in the Chichijima and Kikaijima corals than in any other previously cultured scleractinian corals reported to date (e.g., Acropora, Stylophora, and Cladocora; p < 0.03), and their pHCF was estimated from δ11B (ref. 26). A recent study found that the pHCF of the branching coral Porites cylindrica cultured on Heron Island, on the Great Barrier Reef, was unaffected by a reduction in pHSW 28, which contradicts our observations on Chichijima and Kikaijima Islands. That study inferred that the highly variable conditions of the seawater CO2 chemistry at that location caused the pHCF of the corals to be more resilient to OA. If so, the more sensitive declines in pHCF seen in the Chichijima and Kikaijima corals may be attributable to seawater in which the CO2 chemistry is less variable than that affecting large coral reefs such as the Great Barrier Reef. This suggests that colonies in similar environments may be more susceptible to OA.
Because corals expend energy when upregulating pHCF, maintaining a constant ΔpH (Fig. 4c) seems reasonable from the perspective of biological adaptation5, 6, 26. However, lower pHCF under OA leads to the slower calcification of corals26, increasing their susceptibility to bio-erosion by grazers and burrowers, and perhaps creating a competitive disadvantage relative to organisms such as macroalgae31. Because the Ca2+ concentration in the calcification fluid is almost the same as that in seawater (<10% change)6, 29, an sensitive decline in pHCF will lead to a faster decline in ΩarCF, because pHCF regulates ΩarCF (Supplementary Section 1 and Supplementary Fig. S3 and Supplementary Table S2). Therefore, it is reasonable to infer that OA overwhelms or disables the pHCF homeostasis of the coral, rather than that the coral spontaneously regulates pHCF. Although we observed no long-term changes in the linear extension rate of the Chichijima and Kikaijima coral skeletons during the last 100 years (Supplementary Fig. S1; refs 10 and 11), this may be attributable to the stretch modulation of corals because some stony corals maintain a linear skeletal extension rate in a stressed environment, at the expense of skeletal density (e.g., ref. 8).
As well as pHSW and ΩarSW, temperature is also an important factor for corals because coral–dinoflagellate symbioses are strongly temperature dependent. Global warming and the resultant coral bleaching are major threats to corals globally, but we speculate that ongoing OA is another potential stressor (Fig. 4b). One modelling study predicted that in the worst-case scenario, living coral communities will disappear from the coastal regions of Japan before the mid-21st century in response to the simultaneous degradation of their living conditions at both higher and lower latitudes, with acidification in the north and warming in the south32. Many coral reefs, including those at Chichijima and Kikaijima, receive good circulation from open ocean seawater. Open oceans will acidify more rapidly than inner-reef environments, which are characterized by the long residence time of the seawater, and are buffered by the dissolution of reef sediments, mitigating OA5. If the sensitivity of the Chichijima and Kikaijima corals to OA can be regarded as representative of the sensitivity of other corals, many coral reefs surrounding oceanic islands in the subtropics may be in more danger than originally thought. However, we note that there are large uncertainties in predicting the thresholds for each coral colony and each coral reef community on both the global and regional scales. This is because global and local stressors (e.g., OA, global warming, destructive fishing, sediment influx) interact in a highly complex ways, and the coral response can vary within and among coral species4, 31. For example, massive Porites coral in Guam33 display high sensitivity to the 13C Suess effect (with a large reduction in δ13Ccoral), but little sensitivity to OA (with a slight reduction in δ11B). This suggests that corals living in natural environments respond differently to OA, depending on the environmental factors affecting individual reefs. Therefore, more field-based studies with the boron technique are required to understand how corals have adapted to or are threatened by OA, before corals disappear under the impact of physical, chemical, and biological erosion4, 31.
Methods
Seawater CO2 chemistry estimation around Chichijima and Kikaijima
To describe the seawater CO2 chemistry, two of four measurable CO2 parameters must be determined. In this work, we used the fugacity of CO2 (fCO2) data stored in the Surface Ocean CO2 Atlas (SOCAT v.2)34. Total alkalinity (TA) was calculated from sea-surface salinity (SSS) using the relationship TA = (SSS/35) × 2295 μmol kg−1 (ref. 16). We used the fCO2 data obtained in a specific area (26.5–27.5°N, 125–145°E) and averaged it for each month for 1983–2011 (ref. 16). These discrete data were used to calculate the monthly DIC, ΩarSW, and pHSW. The values calculated for normalized DIC at a salinity of 35 (nDIC) were fitted to an empirical function for the time of the measurement, SST, and SSS with multi-parameter regression (Supplementary Section 2).
We also simulated a time series of seawater CO2 chemistry parameters for the 27°N,137°E grid point by combining the empirical function of nDIC obtained, TA, the monthly (1° × 1°)-resolution SST, and the SSS records from the Multivariate Ocean Variational Estimation (MOVE) system developed by the Meteorological Research Institute35. The validity of this estimate was confirmed by a comparison of the pHSW and pCO2SW time series obtained with the time series for 0.5° latitude, with a 2.5° longitude grid centred on Chichijima and Kikaijima (Supplementary Figs S4 and S5, Supplementary Section 2).
We extended the annual pHSW estimate to before the industrial era using atmospheric pCO2 records from continuous observations at the MLO17 and the air trapped in the Antarctic ice sheet18. We determined the b value in the equation2, 19, 30:
we used two boundary conditions, pHSW and atmospheric pCO2, for 1994 (8.125 and 358.8 μatm, respectively) and the preindustrial era (8.202 and 277.6 μatm, respectively)3, 17, 18 to determine the constant b (Fig. 2d). We used an increase in anthropogenic DIC of 50 μmol kg−1 for the subtropical North Pacific since the preindustrial era3 for the calculation, and determined b to be 0.7. We calculated this while keeping SST and SSS constant for all the annual pHSW and pCO2SW calculations before the industrial era, because we confirmed that their effects on the pHSW estimated for the years 1911–1994 were negligibly small (Supplementary Section 3 and Supplementary Fig. S6).
Geographic and ecological features of the study areas
Massive Porites coral was found at a water depth of 5.6 m in Miyanohama inlet, located on the north coast of Chichijima Island (Supplementary Fig. S7)10. The coral reef of Ogasawara Archipelago, including Chichijima, is categorized as an apron reef, i.e., the coastal area is limited and lacks a reef-flat system. Miyanohama inlet is located in the west of Anijima Strait and receives good water circulation from the open ocean. There is no river flowing into Miyanohama. The inclination is gentle inside the inlet, but becomes steep at its mouth. The corals in this region grow on volcanic basement rock or dead corals, with a moderate coral cover (~50%) and high diversity, including massive Merulinidae corals (e.g., Platygyra daedalea, Leptoria phrygia, Goniastrea pectinata), branching/encrusting Acroporidae corals (e.g., Acropora florida, Acropora hyacinthus, Acropora gemmifera), massive Poritidae corals (e.g., Porites lutea), and massive/encrusting Oculinidae corals (e.g., Galaxea fascicularis)36.
Another massive Porites coral was found at a water depth of 3.5 m, offshore from Arakizaki point, located on the south west coast of Kikaijima Island (Supplementary Fig. S7)11. The coral reef of Kikaijima is a small reef-flat system, almost all of which dries up on the ebb tide37, 38. As a result, the coral colony has developed in the limited area of reef slopes surrounding the island, with moderate coral cover (5–50%)39. There is no river on the surface of Kikaijima Island because the bedrock is composed of highly permeable calcium carbonate. The living coral reef assemblage offshore from Arakizaki has not been described, but according to observations at water depths of 1–5 m on the northeast side of the island (Shidooke)38, it is highly diverse, including branching Pocilloporidae corals (e.g., Pocillopora verrucosa), branching/tabulate/encrusting Acroporidae corals (e.g., Acropora palifera, Acropora gemmifera, Acropora monticulosa, Acropora digitifera), massive Poritidae corals (e.g., Porites lobata), and massive Merulinidae corals (e.g., Favites abdita, Favites pentagona, Goniastrea retiformis). These assemblages are also seen on the exposed terrace of Holocene reefs near Arakizaki37. We monitored the variability of the water temperature at this location by attaching a temperature logger to the appropriate massive Porites sp. from summer 2009 to summer 2011. This showed good agreement with the variation in the open ocean SST (Supplementary Fig. S8), confirming that the study site receives good circulation of open ocean seawater.
δ11B and δ13C analyses
For the geochemical analysis of coral skeletons, we used a massive Porites sp. collected at Chichijima in October 2002 (ref. 10) and another sample collected at Kikaijima in June 2009 (ref. 11) (Fig. 1). The methodology used to prepare the 3-year-resolution subsamples of the Chichijima coral is described by Kubota et al.19. Briefly, we drilled a coral skeleton slab along the major growth direction and obtained single-year-resolution subsamples for the years 1873–2002. We discarded the subsamples for the most recent 4 years because the Sr/Ca and U/Ca values showed anomalously high values10. We also discarded subsamples from before 1910 because there was a climatic regime shift in 1905–1910 (ref. 10). We mixed equal amounts of powdered subsample for each single year and prepared 3-year-resolution subsamples to measure δ11B for 1910–1998. To prepare the 3-year-resolution subsamples of the Kikaijima coral to make the δ11B measurements for 1910–2009, we drilled the coral skeleton along the major growth direction and homogenized it. Typically, we used 3–6 mg of carbonate for the δ11B measurements. After removing the organic matter with 30% H2O2, we purified the boron using cation- and anion-exchange resin columns40 and dissolved the samples with a mixed acid composed of 0.15 M HNO3, 0.05 M HF, and 0.1% mannitol to obtain a solution of 75 ppb boron. We measured δ11B in both the Chichijima coral and Kikaijima coral with a multi-collector inductively coupled plasma mass spectrometer (MC-ICPMS; Thermo Finnigan NEPTUNE) installed at the Kochi Core Center (KCC), Japan, against the isotopic reference NIST-SRM 951, with a standard-sample bracketing technique under wet plasma conditions. We used the method of Foster41 to optimize the operating conditions for MC-ICPMS. All the δ11B values reported here are the averages of duplicate analyses (Supplementary Table 1). We compared the newly obtained δ11B data for the Chichijima coral with those measured with thermal ionization mass spectrometry (TIMS; Thermo Finnigan Triton) at KCC that were reported previously by Kubota et al.19, confirming the good reproducibility of the two different methods (MC-ICPMS and TIMS) (Supplementary Fig. S9 and Supplementary Table 1). The δ11B value of the international carbonate standard JCp-1, a Porites coral skeleton collected at Ishigakijima Island, determined with MC-ICPMS, was 24.44 ± 0.24‰ (2σ, n = 91), which was consistent with the previously reported value of 24.28 ± 0.14‰ (2σ, n = 14) determined with TIMS (Supplementary Fig. S9)19.
To determine the δ13C of the coral skeleton, the sub-monthly δ13C data for the Chichijima coral for 1910–1998, reported by Felis et al.1, were used and new measurements were made for the Kikaijima coral with an isotope ratio mass spectrometer (Thermo Fisher Scientific; Delta V plus) installed at the Atmosphere and Ocean Research Institute, Japan. All the isotope values are reported with respect to Pee Dee Belemnite (PDB) based on an NBS-19 value of 1.9‰. All the reported δ13C values are the averages of duplicate analyses (Supplementary Table 1). The repeated analysis of an in-house standard yielded an external reproducibility for the δ13C measurements of better than 0.13‰ (1σ, N = 123).
ΔpH calculation from pHCF
We used a previously reported δ11B-pHCF equation26, 28, 29 to determine the relationship between coral calcification and OA.
Here, pHCF and pHSW are the pH of the calcification fluid and of seawater, respectively; δ11BSW is the global average δ11B of seawater (39.61‰; ref. 42); and α3–4 is the fractionation factor (1.0272; ref. 25). The dissociation constant for boric acid, pK B, is 8.60 at 24.6 °C (24.5 °C) and the salinity at Chichijima (Kikaijima) is 34.8 (34.5). We confirmed that the past changes in SST and SSS had negligible effects on the estimation of pHCF (Supplementary Section 3 and Supplementary Fig. S6).
References
Feely, R. A., Doney, S. C. & Cooley, S. R. Ocean Acidification: Present Conditions and Future Changes in a High-CO2 World. Oceanography 22, 36–47 (2009).
Tans, P. An Accounting of the Observed Increase in Oceanic and Atmospheric CO2 and an Outlook for the Future. Oceanography 22, 26–35 (2009).
Key, R. M. et al. A global ocean carbon climatology: Results from Global Data Analysis Project (GLODAP). Glob. Biogeochem. Cycle 18, doi:10.1029/2004GB002247 (2004).
Hoegh-Guldberg, O. et al. Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science 318, 173–175 (2007).
Kleypas, J. A. & Yates, K. K. Coral Reefs and Ocean Acidification. Oceanography 22, 108–117 (2009).
Cohen, A. L. & Holcomb, M. Why Corals Care About Ocean Acidification Uncovering the Mechanism. Oceanography 22, 118–127 (2009).
Barkley, H. C., Cohen, A. L., McCorkle, D. C. & Golbuu, Y. Mechanisms and thresholds for pH tolerance in Palau corals. J. Exp. Mar. Biol. Ecol. 489, 7–14 (2017).
Crook, E. D., Cohen, A. L., Rebolledo-Vieyra, M., Hernandez, L. & Paytan, A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl Acad. Sci. USA 110, 11044–11049 (2013).
Kleypas, J. A., McManus, J. W. & Meñez, L. A. B. Environmental limits to coral reef development: Where do we draw the line? A m. Zool. 39, 146–159 (1999).
Felis, T. et al. Subtropical coral reveals abrupt early-twentieth-century freshening in the western North Pacific Ocean. Geology 37, 527–530 (2009).
Kawakubo, Y. et al. Precise determination of Sr/Ca by laser ablation ICP-MS compared to ICP-AES and application to multi-century temperate corals. Geochem. J. 48, 145–152 (2014).
Krief, S. et al. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim. Cosmochim. Acta 74, 4988–5001 (2010).
Hönisch, B. et al. Assessing scleractinian corals as recorders for paleo-pH: Empirical calibration and vital effects. Geochim. Cosmochim. Acta 68, 3675–3685 (2004).
Ishii, M. et al. Ocean acidification off the south coast of Japan: A result from time series observations of CO2 parameters from 1994 to 2008. J. Geophys. Res. 116, JC006831 (2011).
Japanese Meteorological Agency. Data of Oceanographic and Marine Meteorological Observation. http://www.data.jma.go.jp/gmd/kaiyou/db/vessel_obs/data-report/html/index_e.html Date of access:20/07/2014 (2014).
Midorikawa, T. et al. Decreasing pH trend estimated from 25-yr time series of carbonate parameters in the western North Pacific. Tellus 62B, 649–659 (2010).
Tans, P. & Keeling, D. Trends in Atmospheric Carbon Dioxide. http://www.esrl.noaa.gov/gmd/ccgg/trends/ Date of access:04/12/2015 (2015).
Rubino, M. et al. A revised 1000 year atmospheric δ13C-CO2 record from Law Dome and South Pole, Antarctica. J. Geophys. Res. (Atmos.) 118, 8482–8499 (2013).
Kubota, K., Yokoyama, Y., Ishikawa, T. & Suzuki, A. A new method for calibrating a boron isotope paleo-pH proxy using massive Porites corals. Geochem. Geophys. Geosyst. 16, doi:10.1002/2015GC005975 (2015).
Suess, H. E. Radiocarbon concentration in modern wood. Science 122, 415–417 (1955).
Keeling, C. D., Brix, H. & Gruber, N. Seasonal and long-term dynamics of the upper ocean carbon cycle at Station ALOHA near Hawaii. Glob. Biogeochem. Cycle 18, doi:10.1029/2004GB002227 (2004).
Quay, P., Sonnerup, R., Westby, T., Stutsman, J. & McNichol, A. Changes in the 13C/12C of dissolved inorganic carbon in the ocean as a tracer of anthropogenic CO2 uptake. Glob. Biogeochem. Cycles 17, doi:10.1029/2001GB001817 (2003).
McConnaughey, T. A. 13C and 18O isotopic disequilibrium in biological carbonates: I. Patterns. Geochim. Cosmochim. Acta 53, 151–162 (1989).
Grottoli, A. G. Effect of light and brine shrimp on skeletal δ13C in the Hawaiian coral Porites compressa: A tank experiment. Geochim. Cosmochim. Acta 66, 1955–1967 (2002).
Klochko, K. et al. Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 248, 276–285 (2006).
McCulloch, M., Falter, J., Trotter, J. & Montagna, P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Change 2, 623–627 (2012).
Venn, A. A. et al. Impact of seawater acidification on pH at the tissue–skeleton interface and calcification in reef corals. Proc. Natl Acad. Sci. USA 110, 1634–1639 (2013).
Georgiou, L. et al. pH homeostasis during coral calcification in a free ocean CO2 enrichment (FOCE) experiment, Heron Island reef flat, Great Barrier Reef. Proc. Natl Acad. Sci. USA 112, 13219–13224 (2015).
Allison, N. et al. Corals concentrate dissolved inorganic carbon to facilitate calcification. Nat. Commun. 5, 5741, doi:10.1038/ncomms6741 (2014).
Kubota, K., Yokoyama, Y., Ishikawa, T., Obrochta, S. & Suzuki, A. Larger CO2 source at the equatorial Pacific during the last deglaciation. Sci. Rep. 4, doi:10.1038/srep05261 (2014).
Pandolfi, J. M., Connolly, S. R., Marshall, D. J. & Cohen, A. L. Projecting Coral Reef Futures Under Global Warming and Ocean Acidification. Science 333, 418–422 (2011).
Yara, Y. et al. Ocean acidification limits temperature-induced poleward expansion of coral habitats around Japan. Biogeosciences 9, 4955–4968 (2012).
Shinjo, R., Asami, R., Huang, K.-F., You, C.-F. & Iryu, Y. Ocean acidification trend in the tropical North Pacific since the mid-20th century reconstructed from a coral archive. Mar. Geol. 342, 58–64 (2013).
Bakker, D. C. E. et al. An update to the Surface Ocean CO2 Atlas (SOCAT version 2). Earth Syst. Sci. Data 6, 69–90 (2014).
Usui, N. et al. Meteorological Research Institute multivariate ocean variational estimation (MOVE) system: Some early results. Adv. Space Res. 37, 806–822 (2006).
Inaba, M. Ecological feature and status of reef-building corals in the Bonin Islands, Japan. Midoriishi 14, 20–23 (2003).
Webster, J. M., Davies, P. J. & Konishi, K. Model of fringing reef development in response to progressive sea level fall over the last 7000 years – (Kikai-jima, Ryukyu Islands, Japan). Coral Reefs 17, 289–308 (1998).
Sugihara, K., Nakamori, T., Iryu, Y., Sasaki, K. & Blanchon, P. Holocene sea-level change and tectonic uplift deduced from raised reef terraces, Kikai-jima, Ryukyu Islands, Japan. Sediment. Geol. 159, 5–25 (2003).
Uchida, H. & Fujimura, H. In The Report of the Marine Biotic Environment Survey in the 4th National Survey on the Natural Environment: Vol. 3: Coral reefs. (Nature Conservation Bureau of the Environment Agency & Marine Parks Center of Japan, 1994).
Ishikawa, T. & Nagaishi, K. High-precision isotopic analysis of boron by positive thermal ionization mass spectrometry with sample preheating. J. Anal. At. Spectrom. 26, 359–365 (2011).
Foster, G. L. Seawater pH, pCO2 and [CO3 2−] variations in the Caribbean Sea over the last 130 kyr: A boron isotope and B/Ca study of planktic foraminifera. Earth Planet. Sci. Lett. 271, 254–266 (2008).
Foster, G. L., Pogge von Strandmann, P. A. E. & Rae, J. W. B. Boron and magnesium isotopic composition of seawater. Geochem. Geophys. Geosyst. 11, GC003201 (2010).
Schlitzer, R. Ocean Data View. http://odv.awi.de Date of access:29/01/2016 (2014).
Wessel, P. & Smith, W. H. F. New improved version of the Generic Mapping Tools released. EOS. Transactions of the American Geophysical Union 79, 579 (1998).
Amante, C. & Eakins, B. ETOPO 1. https://www.ngdc.noaa.gov/mgg/global/global.html Date of access:22/05/2015 (2015).
Acknowledgements
We thank J. Matsuoka, K. Nagaishi, T. Kawai, and M. Tanimizu for technical support with the δ11B measurements; S. Tsukamoto, T. Sato, and Y. Yoshinaga for coral sample preparation; N. Izumoto for the δ13C measurements; and K. Tsuzuki, A. Okada, and K. Machida for laboratory assistance. We thank the local government of Kikai Island for logistical support, particularly Y. Ijichi. We also thank K. Tanaka, T. Higuchi, and K. Shirai for fruitful discussions and S.P. Obrochta and H. Kan for helpful comments. This study was partly supported by the Japan Society for the Promotion of Science NEXT Program GR031 and grants to Y.Y. (26247085, JP15KK0151, 15H02813 and 17H01168), T.I. (24340127), and A.S. (24244090) and a Research Fellowship for Young Scientists to K.K.
Author information
Authors and Affiliations
Contributions
K.K., Y.Y., and T.I. designed the study and measured the coral boron isotope ratios. K.K., Y.Y., and A.S. collected the coral from Kikaijima. K.K. and A.S. prepared the coral subsamples. M.I. measured and offered recent CO2 chemistry data. K.K., Y.Y., T.I., A.S., and M.I. contributed to the interpretation and the preparation of the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubota, K., Yokoyama, Y., Ishikawa, T. et al. Rapid decline in pH of coral calcification fluid due to incorporation of anthropogenic CO2 . Sci Rep 7, 7694 (2017). https://doi.org/10.1038/s41598-017-07680-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07680-0
This article is cited by
-
Differences in carbonate chemistry up-regulation of long-lived reef-building corals
Scientific Reports (2023)
-
Recent ocean acidification trends from boron isotope (δ11B) records of coral: Role of oceanographic processes and anthropogenic CO2 forcing
Journal of Earth System Science (2022)
-
Diurnal cycles of coral calcifying fluid aragonite saturation state
Marine Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.