Abstract
Biological control using predators of key pest species is an attractive option in integrated pest management (IPM). Molecular gut analysis can provide an estimation of predator efficiency on a given prey. Here we use a combination of various experimental approaches, both in field and lab, to identify a potential biological control species of the common pest of commercially grown tea, Empoasca vitis (Göthe) (Hemiptera), in a Chinese plantation. We collected 2655 spiders from plantations and established relative abundances of spider species and their temporal overlap with the pest species in tea canopy. We analyzed DNA from 1363 individuals of the most common spider species using targeted RQ-PCR to quantify the potential efficiency of spiders as a predator on E. vitis. The results showed that, in the field, the jumping spider Evarcha albaria was the most abundant, had the closest temporal overlap with the pest, and frequently fed on it. Therefore, this spider may play a key role in pest suppression. The present study demonstrates the potential of our experimental approach to study predator-prey relationships in taxa that do not lend themselves to morphological identification of gut contents, such as spiders.
Similar content being viewed by others
Introduction
Tea, as one of the world’s most popular beverages and is an important cash crop that is cultivated worldwide1,2,3,4, with China, as the largest current producer4. Its popularity, in part, stems from its richness in polyphenols that may have various health benefits such as reduce risk of cancer2, 5. The yield and quality of tea from plantations is negatively impacted by several tea pest species, putting a premium on effective means of control6. Direct applications of pesticides applied to tea is the most widely used and effective pest control6, however, this method has a series of negative side effects, including pesticide residues, pesticide resistance in important pest species, damage to natural pest enemies, and others7,8,9. Hence, emphasis on biological control is increasing, focusing on the preservation and introduction of natural enemies like predators, parasitoids and pathogens for suppression of pests to commercially tolerable levels6, 10.
Spiders are among the most abundant predators of insects in terrestrial ecosystems11. Nyffeler & Birkhofer12 have estimated that the annual prey kill of the global spider community is in the range of 400–800 million metric tons. In tea plantations, spiders, ladybird beetles, assassin bugs, lacewings, and praying mantises, are the predominant predators on pest species13. Spiders, in particular, play key functional role. They are the most abundant predators with dominance ranging from 65.0% to 97.8% in Chinese tea plantations14. Spiders have been shown to play an important role in biological control through analyses of population dynamics between the spiders and pests in the field15,16,17,18,19,20. However, field evidence is often lacking for if, and how effectively, spiders prey on the pest of concern. Detailed studies of the actual prey-predator interactions between the spiders and the pests and efforts to quantify predator efficiency are thus important to better understand the roles spiders might play in biological pests control21, 22. The dominance of predators, temporal and spatial overlap between predators and pests, predation rates, and prey numbers consumed per predatory individual, are often used as indicators of which predators control pests23,24,25,26,27,28. One of the challenges here is to identify prey post-predation. Spiders liquefy their food externally before ingestion, ruling out morphological identification of gut contents. PCR methodology has been shown to be useful in detecting the presence prey species in spider guts29, however, this does not allow good estimation of predator efficiency on a given prey. Real-time quantitative PCR (RQ-PCR) offers additional information on the abundance of target DNA of prey tissue digested in predators’ gut and thus can yield an estimate of relative importance of predators29.
As discussed in King et al.29, RQ-PCR can quantify single target prey species DNA copies in predator guts, using a single primer. The predation rates and prey numbers consumed per predatory individual are then estimated by the positive rates and copy numbers of target fragment of prey remains in the gut of predators, respectively, offers a qualitative assessment of predation in the field. To date, TaqMan RQ-PCR has already been used to amplify and quantify target DNA of the prey remains in predators’ gut25, 28, 30, 31. Using a pair of primers and a fluorogenic oligodeoxy-nucleotide probe, which is specific for the target prey, the target DNA of the prey remains in predators’ gut can be quantified. Short prey amplicons (usually <300 bp) are optimal to target the already fragmented DNA ‘post digestion’ in the predator32, 33. The mitochondrial cytochrome oxidase subunit I (mtCOI) has proven useful as a marker due to the abundance of copies of this gene, species specificity, and richness of data available in public databases29.
Empoasca vitis (Göthe) (Hemiptera) is one of the most damaging tea pests in China that can seriously decrease tea yield by sucking juices out of the tender tea-leaf directly34,35,36. Gao et al.37 studied the predation of Evarcha albaria (L. Koch) (Araneae) on E. vitis under laboratory conditions. The results showed that both male and female spiders preyed on the nymph and adult of E. vitis. However, the role of spiders as predator on E. vitis in the field is unknown. Spiders are highly variable in hunting strategies, habitat preferences, and activity periods, which combine to make the group potentially highly efficient in controlling certain groups of pests16. In tea plantations, many spider species hunt prey in the canopies of tea38, indicating relatively high spatial-sympatry with the tea pests. Here we aim to screen the main predators of E. vitis in the canopies of tea, testing the spider species as potential biological control agents of E. vitis. Therefore, the present study focus mainly on three aspects: (1) establishing the relative abundances of spider species; (2) determining the temporal overlap between dominant spider species and the pest species in tea canopy; (3) providing an approximately quantitative assessment of relative efficiency of spiders as a predator on E. vitis using RQ-PCR.
Results
The abundances, population fluctuations, and temporal overlap of dominant spider species and E. vitis in the field
In total 2655 (including 885 adults and 1770 juveniles) individual spiders were collected belonging to 50 species. Among them, Evarcha albaria (L. Koch) (Salticidae), Xysticus ephippiatus Simon (Thomisidae), Meotipa pulcherrima (Mello-Leitão) (Theridiidae) and Coleosoma octomaculatum (Bösenberg & Strand) (Theridiidae) (Fig. 1) were the most dominant in the canopies of tea with relative adult abundances of 43.2%, 10.7%, 8.5% and 8.4%, respectively (Table S1). We focus on these four spider species in further analyses. Abundances of dominant spider species (E. albaria, X. ephippiatus, M. pulcherrima and C. octomaculatum) and the pest E. vitis are shown in Fig. 2 and Table S2. Abundances show seasonal fluctuation with E. vitis being most abundant from May to September, E. albaria from May to October, X. ephippiatus from June to October, M. pulcherrima from May to September and C. octomaculatum from March to May. The temporal overlap values between spider species and E. vitis was 1.28, 1.25, 1.23 and 1.02, respectively. The largest overlap values are between E. albaria and E. vitis.
Population dynamics of E. vitis and four dominant spider species in the canopies of tea. Values presented as mean ± SD (N = 3). Temporal overlap values (TOV) between spider species and E. vitis was estimated by following measure, developed by Hurlbert61: \({Lij}={S}{\sum }_{h=1}^{S}{P}_{ih}{P}_{jh}\); where, L ij is the temporal and spatial overlap measure of species i on species j, S is the unit number of resource sequence, P ih is the proportion that resource h is of the total resource that species i utilizes and P jh is the proportion that resource h is of the total resource that species j utilizes. E. vitis: EV; E. albaria: EA; X. ephippiatus: XE; M. pulcherrima: MP; C. octomaculatum: CO.
Specificity of primers
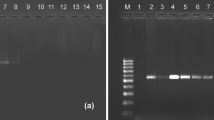
The primers (ZJ-F and ZJ-R), using TaqMan RQ-PCR, successfully amplified E. vitis DNA (146 bp), but did not amplify predators, 16 other potential prey species, or tea-leaf (Table 1).
Standard curve, sensitivity and reproducibility of TaqMan RQ-PCR
The sensitivity of the assay was evaluated using 10-fold serial dilution of all stock plasmids ranging from 1.92 × 109 to 1.92 × 10° copies/µL. The result showed that the test could detect as few as 192 copies of the target-fragment (Table 2) and there was a strong linear relationship between the Ct values and the log10 of the input number of copies (R 2 = 0.9992). The slope and intercept of standard curve was −3.34 and 45.37, respectively. Amplification efficiency was 97.6% (Fig. S1). Evaluating reproducibility of the assay between three sub-samples, the coefficients of variation were <5% (0.20–1.39%), which indicated that the assay was highly reliable and reproducible (Table 2).
Efficiency of molecular marker in detecting E. vitis remains in spider guts
TaqMan RQ-PCR was employed to qualitatively and quantitatively detect the genomic DNA of four adult spider species at various time periods after consuming one adult E. vitis. The results show that all spiders that consumed prey were positive for E. vitis DNA at t = 0 h, decreasing to 60% (E. albaria), 0 (X. ephippiatus), 60% (M. pulcherrima) and 40% (C. octomaculatum) after 60 h of digestion. After 72 h of digestion, the positive rate decreased to 0 (Table 3). The CNTF (copy numbers of target fragment) of 0 hour digestion was 1.23 × 107 ± 9.88 × 105 (E. albaria), 9.77 × 106 ± 2.55 × 106 (X. ephippiatus), 1.49 × 107 ± 7.01 × 106 (M. pulcherrima) and 4.50 × 107 ± 3.07 × 106 (C. octomaculatum).
Qualitative and quantitative evaluation of predation in the field
Genomic DNA of all spiders (including juvenile and adult) were detected by TaqMan RQ-PCR. The results show that the PRTF (positive rates of target fragment) in spiders gut was 54.2% (E. albaria, N = 943, male: 160, female: 222, juvenile: 561), 30.1% (X. ephippiatus, N = 176, male: 25, female: 70, juvenile: 81), 17.5% (M. pulcherrima, N = 114, male: 24, female: 50, juvenile: 40) and 4.6% (C. octomaculatum, N = 130, male: 28, female: 47, juvenile: 55), and it showed significant differences between those of E. albaria and any other species (Table 4). Correspondingly, the average of residual minimum number of E. vitis in individual spider gut was 0.63 ± 0.05, 0.05 ± 0.02, 0.04 ± 0.02 and 0.02 ± 0.01, respectively, and it also showed significant differences between those of E. albaria and any other species (Table 4).
As shown in Figs 3 and 4, the PRTF in E. albaria guts was significantly greater than those of any other predators, during the months when E. vitis was most abundant in, May, June, July and September. The residual minimum number of E. vitis in E. albaria guts was also greater than those of any other predator, significantly so in May, June, and July.
Discussion
Tea is an important commercial crop in China impacted by a variety of pest species, with one of the most common being the true bug E. vitis (Hemiptera). Effective control of this pest has long been achieved via chemical pest control6, 9. However, the negative side effects of chemical use put a premium on establishing viable alternative pest control approaches. Biological control using natural enemies of pest species is an attractive option but relies on identifying the interactions between the pest and the potential control agent. Previous studies have indicated a jumping spider (E. albaria, Salticidae) as a dominant spider in tea plantations of China39,40,41,42, and laboratory experiments have demonstrated that it feeds on E. vitis 37. However, data on field predation efficiency are more challenging to obtain, not the least in predator species that predigest prey outside the body, such as spiders. Thus, we lack an understanding of these species interactions in the more complex food web in the field, and therefore the fundamental basis for establishing the potential of E. albaria as a biological control agent.
We performed analyses of gut contents from field-collected spiders using a combination of various experimental approaches both in field and lab based on 2655 spiders from plantations, and established relative abundances of spider species and their temporal overlap with the pest species. DNA analyzed from 1363 individuals of the most common spider species using targeted RQ-PCR clearly show that the jumping spider E. albaria—found to be the most abundant spider in the field and the one with the closest temporal overlap with the pest in tea canopy—was the most effective predator on E. vitis. Pest DNA was amplified from 54.2% of these spiders with a minimum of 0.63 ± 0.05 E. vitis individuals per E. albaria gut from the field, despite limits of detection success beyond 72 hrs after feeding. Relatively high feeding rates combined with high spider abundance, and temporal overlap and spatial-sympatry between the predatory natural enemies and the prey all are indicators of a potentially effective biological control species27, 43, 44. Therefore, we conclude that E. albaria is a key predator of E. vitis and that it has potential as a biological control agent of E. vitis. To evaluate the potential effectiveness of E. albaria as a component in an IPM strategy, however, further experimental research is necessary. Most urgently needed are experiments with selective exclusion—or population enhancements—of the predator species.
To date, there are many methods (including direct observation45, gut dissection46, radioisotope labeling47, monoclonal antibodies48, 49 and molecular gut analysis29) that can be employed to identify the interactions between predators and prey in the field. Among them, molecular gut analysis and monoclonal antibodies are especially practical for studying predation of some relatively small arthropod predators in the field. Our approach can qualitatively assess predation in the field, however, like monoclonal antibodies, our method cannot accurately estimate the number of the prey consumed by the predator per time unit29, 50, and is therefore not directly quantitative.
Our estimate of predator efficiency is limited by this difficulty of quantifying the number of prey consumed per predator, as well as by the decay of prey DNA in predator gut (0 detection rate after 72 hours). However, our approach allows at least a relative estimate of prey numbers per spider species. The more prey consumed, the more CNTF remains in the gut of the spiders25. We quantitatively detected the target fragment of E. vitis remains in the gut of four dominant spider species in tea plantation, and compared the residual minimum number of E. vitis in individual spider guts. We found that the average of residual minimum number of E. vitis in E. albaria gut (0.63 ± 0.05) was significantly greater than those of any other common spider species. The number of prey DNA copies in spider guts result from an interplay of ambient temperature, prey number, prey size, and time since ingestion51 and thus these estimates are approximate. However, while our methods do not establish the number of consumed prey, they do allow a meaningful comparison of the relative importance of various predators by comparing the residual minimum number of E. vitis in the guts of individual predators. We find clear statistical differences in the average of residual minimum number of E. vitis in individual spider guts, indicating differences in number of prey, and these numbers are highest for our targeted biological control species. Clearly then, the consumption of this prey is a frequent and likely important part of the E. albaria daily diet. In turn, frequent predation on the pest by majority of prey individuals suggests that the spider is a significant predator of E. vitis meriting further research of its potential as a part of an IPM52.
PCR is a sensitive method for gene amplification, even capable of amplification from a single target molecule53. It is, however, susceptible to many interference factors, such as PCR inhibitors, that can lead to amplification-failure29. Design of modern DNA extraction and purification kits have greatly reduced this problem and furthermore, simply diluting the concentration of the DNA template has proven to be an easy and effective approach to overcoming PCR inhibition29. Our experiments have also demonstrated that diluting the concentration of the DNA template could effectively overcome the occurrence of the false-negatives (Fig. S2). Wang et al.28 had detected the effect of different background DNA of predators by using target prey DNA diluted in water and in the genomic DNA mixture of starved predator species. The result indicated that background DNA does not affect amplification. Our experiments have also demonstrated that different background DNA of four dominant spider species does not affect amplification of target fragment of E. vitis (Table S3). This suggests the current RQ-PCR protocol may be very effective in target identification of gut contents. Additionally, in order to ensure the fidelity and reliability of the assay, each DNA sample should assay in triplicate54, and a non-template control (without any nucleic acid), a negative control (without target DNA), and a positive control (including target DNA), should be contained in each PCR run. In this study, three sub-samples yielded the consistent results, indicating that the assay is highly reliable and reproducible.
Determining the relationship between predator and prey and the potential role a predator may play in pests control in the field is important55, 56. It is also significant studying the trophic niche of predators as they can be feeding on multiple types of prey. Molecular approaches provide useful tools for determining the dietary breadth of such predators. Petráková et al.57 has studied on the trophic niche of Ammoxenus amphalodes (Araneae, Ammoxenidae) using Next Generation Sequencing (NGS), and revealing that A. amphalodes is a specialist termite-eating spider, only capturing Hodotermes mossambicus (Isoptera, Hodotermitidae) as its food. Such approaches using NGS would be the natural next step in determining the dietary breadth of the main predators identified in Chinese crop fields.
Methods
Collection of samples
The study was carried out in Wang Dazhen tea plantation which is located near Xianning city, Hubei province, China (114.352°E, 29.953°N). E. vitis is a dominant species of tea pests, in this area. The total area of the studied tea plantation is about 6.5 ha. with parallel rows of tea plants about 100 m long and 1 m apart. During the sampling period, no insecticide was applied in the tea plantation.
The spiders and pest were collected on dry days, and each sampling period lasted at least seven days (three times a month), with the same person in tea plantation from September 2012 to August 2013. A total of 30 transects, separated by at least 10 m were sampled every time, with three sampling sites randomly chosen at each transect separated by at least 15 m. A total of 90 sites were randomly collected each sampling period using an insect net (diameter: 40 cm) under tea canopies, and beating the canopies ten times with a stick. The number of E. vitis was counted, and all spiders were individually put in 1.5 mL microcentrifuge tubes with 100% ethanol, kept on ice until returning to the laboratory and later stored at −80 °C.
Spiders were identified from the reference keys and catalogues provided by Yin et al.58 and World Spider Catalog59.
DNA extraction
The genomic DNA of spiders was subsequently extracted and purified individually using an animal genomic DNA extraction kit (Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) according to the manufacturer’s instructions. Finally, The DNA was resuspended in 100 µL of manufacturer’s elution buffer and stored at −80 °C.
Design of primers and TaqMan minor groove binder (MGB) probe
We followed the rules on species-specific primer design outlined by King et al.34. Ideal primers should be efficient at high annealing temperatures (if possible, Ta > 55 °C), thus reducing the risk of nonspecific amplification. In addition, shorter fragments <300 bp should be targeted wherever possible as the DNA molecules are broken into smaller fragments during digestion in the predator guts. We used a fragment of the COI gene of E. vitis from GeneBank (named ZJ5 isolate, 773 bp, GeneBank Accession no. KC172507.1) to design the primer pair ZJ-F: 5′-AGGTGCTGTATTTGCTAT-3′ and ZJ-R: 5′-CTAAGAAATGTTGAGGGA-3′, which amplify a 146 bp fragments, using Gene tool software.
The TaqMan MGB probe for the E. vitis target DNA quantification was designed by using the Primer Express v3.0 software. The probe (5′-TTACCCCAAAGAATATCAC-3′), hybridized within the region amplified by the PCR primers from base 507 to base 525 of ZJ5 isolate, and synthesized by Shanghai Bioligo Biotechnology Ltd. (Shanghai, China), at the 5′-end there is a reporter [FAM (6-carboxy-fluorescein)] and at the 3′-end a minor groove binding (dehydrocyclopyrroindole tripetide, DPI3).
The specificity of primers
Our goal was to detect the specific DNA fragments of E. vitis remains in the gut of generalist-predators spiders. Therefore, to confirm that the developed primers (ZJ-F and ZJ-R) can specifically amplify DNA fragments of E. vitis remains in the gut of spiders, we performed cross-reactivity assays with the primers (ZJ-F and ZJ-R) using the various prey species. Species specificity of the primers was tested using genomic DNA from target prey, predator and potential prey species and tea leaf. The potential prey species of spiders and tea leaf were collected in tea plantation. The genomic DNA of individual target prey, predator and potential prey species and tea leaf (50 mg) were amplified simultaneously by the primers (ZJ-F and ZJ-R).
The standard curve, sensitivity and reproducibility of TaqMan RQ-PCR
The purified target-fragment of E. vitis were ligated and cloned into PUC57 vector (Shanghai Bioligo Biotechnology Co., Ltd., Shanghai, China), propagated in DH5a competent cells (Beijing TransGen Biotechnology Co., Ltd., Beijing, China). The recombinant plasmids DNA were isolated from the obtained white colonies and inoculated in liquid medium (LB/Amp). The recombinant plasmids DNA were purified by using an AxyPrep Plasmid Miniprep Kit (Axygen Biosciences, USA) according to the manufacturer’s instructions. The DNA was eluted in 50 μL eluent and stored at −80 °C. To confirm whether the inserted DNA is consistent with the target DNA, the recombinant plasmids DNA was sequenced by Wuhan TsingKe Biological Technology Co., Ltd. (Wuhan, China). Its concentration (ng/µL) was determined by spectrophotometric measurement (NanoDrop 2000c, Thermo Fisher Scientific Inc, USA) and the copy numbers of target fragment (CNTF) were calculated using the expression: CNTF = [DNA mass (g)/recombinant plasmid molarmass] × 6.02 × 1023.
The recombinant plasmid was used as standards for calibration of copy number. The number of transcripts was calculated per 1 µL, which was the volume used as template in each TaqMan RQ-PCR assay. Tenfold serial dilutions of the transcripts were prepared from 1.92 × 109/µL–1.92 × 100/µL, aliquoted and stored at −80 °C until use. Dilutions from 1.92 × 106/µL–1.92 × 102/µL were employed to generate the standard curve and used for the TaqMan RQ-PCR assay. The sensitivity of RQ-PCR assay was determined by testing tenfold serial dilution from 1.92 × 109/µL–1.92 × 100/µL of the recombinant plasmid25.
TaqMan RQ-PCR was carried out in Multiplate Low-Profile 96-Well Unskirted PCR Plates (Bio-Rad, USA), closed with Microseal ‘B’ Adhesive Seals (Bio-Rad, USA). Amplification and detection were performed using a CFX ConnectTM Real-Time PCR detection system (Bio-Rad, USA) and accompanying software (Bio-Rad CFX Manager 3.1) according to the manufacturer’s instructions. The TaqMan RQ-PCR protocol was performed in a final volume of 20 μL. Each tube contained: 1 μL forward primer (10 μM), 1 μL reverse primer (10 μM), 0.4 μL fluorogenic probe (10 μM), 10 μL TransStart ® Probe qPCR SuperMix (Beijing TransGen Biotechnology Co., Ltd., Beijing, China), 1 μL sample DNA and 6.6 μL ultra-pure water. Each DNA sample was assayed in triplicate. Three wells containing 1 μL ultra-pure water each were used for a nontemplate control, without any nucleic acid. Amplification and detection were performed by using the manufacturer’s standard protocols. The PCR protocol consisted of an initial step of 30 s at 94 °C, followed by 40 cycles of 5 s at 94 °C and 30 s at 55 °C. All standards (obtained from purified plasmid DNA), controls and unknown samples were run simultaneously. Data acquisition and analysis were performed with the Bio-Rad CFX Manager 3.1 software. The CNTF were determined from the Ct value (real-time PCR cycle number where fluorescence signal curve crosses certain threshold line60). For generation of standard quantization curves, the Ct values were plotted proportionally against the log10 of the number of input copies. The Ct values were subsequently used to calculate the amount of DNA in unknown samples by the equations of standard curves.
Detection efficiency of molecular diagnostic marker in spiders feeding trials
To determine whether the DNA of E. vitis could be detected in the gut of the predator, separate feeding trials were performed using adult E. albaria, X. ephippiatus, M. pulcherrima and C. octomaculatum that were collected in the tea plantation. The spiders were reared individually in 20 × 100 mm glass tube with moistened sponge in the bottom to ensure high humidity. All the spiders (including males and females) for the experiment were starved for 5–7 d under greenhouse conditions at 25 ± 1 °C, 80–85% RH and L12: D12 h photoperiod prior to the start of the experiment. After starvation, individual spiders were provided one adult E. vitis and allowed to feed for 1 h. Spiders that were not observed to feed were excluded from the experiment. After feeding, we tested the detection efficiency for four spider species at post-feeding intervals of 0, 12, 24, 36, 48, 60 and 72 h. Each post-feeding interval was provided five individual spiders. After each feeding interval, spiders were placed individually in 1.5 mL microcentrifuge tubes with 100% ethanol, and stored at −80 °C, and later submitted to the DNA extraction procedure as described above. After DNA extraction, the DNA samples from each feeding interval were amplified by TaqMan RQ-PCR using the primers (ZJ-F and ZJ-R). PCR conditions and thermocycling program as described above. Each run contained a nontemplate control (without any nucleic acid), a negative control (adult E. albaria, starved for 5–7 d) and a positive control (adult E. albaria fed on one adult E. vitis). Each sample was assayed in triplicate25.
Qualitative and quantitative evaluation of predation in the field
We qualitatively and quantitatively detected the DNA samples from field-caught spiders using TaqMan RQ-PCR. PCR conditions and thermocycling program as described above. Each run contained a nontemplate control (without any nucleic acid), a negative control (adult E. albaria, starved for 5–7 d) and a positive control (adult E. albaria fed on one adult E. vitis). Each sample was assayed in triplicate. The CNTF remain in the gut of spiders from field-collected samples were calculated by interpolation from the DNA standard curve amplified in the same PCR run. The positive rates of target fragment (PRTF) was calculated using the following equations: PRTF = (Number of positive spiders/Total spiders tested) × 100%. Residual minimum number of E. vitis in individual spider guts was calculated by N/M (N is the CNTF of field-collected individual spiders; M is the CNTF of individual spiders which feeding one adult E. vitis after 0 hour digestion).
Data analysis
The temporal overlap between predators and pests was estimated by following measure, developed by Hurlbert61: \({Lij}={S}{\sum }_{h=1}^{S}{P}_{ih}{P}_{jh}\); where, L ij is the temporal and spatial overlap measure of species i on species j, S is the unit number of resource sequence, P ih is the proportion that resource h is of the total resource that species i utilizes and P jh is the proportion that resource h is of the total resource that species j utilizes. Difference test of percentage was used to compare the PRTF of E. vitis per dominant spider species. One-way ANOVA was used to compare the residual minimum number of E. vitis per dominant spider species.
References
Ali, L. The regulation of trade in tea. J. World Trade Law 4, 565–85 (1970).
Weisburger, J. H. Tea and health: a historical perspective. Cancer Lett. 114, 315–317 (1997).
Karmakar, K. G. & Banerjee, G. D. The tea industry in India: a survey. (National Bank for Agriculture and Rural Development, Mumbai, 2005).
Chen, Z. M. & Chen, L. Delicious and healthy tea: an overview In Global tea breeding (eds Chen, L., Apostolides, Z. & Chen, Z. M.) 1–11 (Springer Berlin Heidelberg, Berlin, 2012).
Yang, C. S. et al. Cancer prevention by tocopherols and tea polyphenols. Cancer let. 334, 79–85 (2013).
Mamun, M. S. A., Ahmed, M. & Paul, S. K. Integrated approaches in tea pest management for sustainable tea production In Proceedings of the Workshop on Tea Production Technology Updated 18–32 (held on 24 December, 2014, organized by Bangladesh Tea Research Institute, Srimangal, Moulvibazar and Krishi Gobeshona Foundation, BARC campus, Dhaka, 2014).
Tan, J. C., Deng, X. & Yuan, Z. M. Community structure of insects and spiders in different types of tea plantation. Acta. Ecol. Sin. 18, 289–294 (1998).
Wang, G. H., Liang, Y. F., Tian, Y. H., Wang, J. L. & Zhou, G. L. Effect of chemical insecticides on the diversity of arthropods in tea garden. J. Tea Sci. 24, 99–104 (2004).
Hazarika, L. K., Bhuyan, M. & Hazarika, B. N. Insect pests of tea and their management. Annu. Rev. Entomol. 54, 267–284 (2009).
Ye, G. Y. et al. Tea: biological control of insect and mite pests in China. Biol. Control 68, 73–91 (2014).
Nyffeler, M. & Benz, G. Spiders in natural pest control: A review. J. Appl. Entomol. 103, 321–339 (1987).
Nyffeler, M. & Birkhofer, K. An estimated 400–800 million tons of prey are annually killed by the global spider community. Sci. Nat. 104, 1–12 (2017).
Das, S., Roy, S. & Mukhopadhyay, A. Diversity of arthropod natural enemies in the tea plantations of North Bengal with emphasis on their association with tea pests. Curr. Sci. India 99, 1457–1463 (2010).
Chen, Y. F., Chen, Z. H., Song, C. Q. & Xu, H. Z. Review on the investigation and protection measurement of spiders in Chinese tea gardens. Acta Arachnol. Sin. 13, 125–128 (2004).
Murdoch, W. W., Chesson, J. & Chesson, P. L. Biological control in theory and practice. Am. Nat. 125, 344–366 (1985).
Marc, P., Canard, A. & Ysnel, F. Spiders (Araneae) useful for pest limitation and bioindication. Agr. Ecosyst. Environ. 74, 229–273 (1999).
Sunderland, K. D., Greenstone, M. H. & Symondson, B. Spiders for pest control. Pest. Outl. 10, 82–85 (1999).
Chen, B. G. The researches of using spiders to control insects in tea field. Acta Arachnol. Sin. 12, 125–127 (2003).
Wen, Z. M. et al. Studies on the varieties of pest and natural enemy and the dynamic state of community in organic tea garden. Chin. Agr. Sci. Bull. 25, 272–276 (2009).
Yang, L. et al. Spatial relationships among Empoasca vitis (Gothe) and Toxoptera aurantii (Boyer) and natural enemies in tea gardens of autumn-winter season in Hefei suburban. Acta Ecol. Sin. 32, 4215–4227 (2012).
Greenstone, M. H. Spider predation: how and why we study it. J. Arachnol. 27, 333–342 (1999).
Ma, J., Li, D., Keller, M., Schmidt, O. & Feng, X. A DNA marker to identify predation of Plutella xylostella (Lep., Plutellidae) by Nabis kinbergii (Hem., Nabidae) and Lycosa sp. (Aranaea, Lycosidae). J. Appl. Entomol. 129, 330–335 (2005).
Xu, H. F., Mu, S. M., Xu, Y. Y., Mu, J. Y. & Dong, C. X. On the community structure of major insect pests and natural enemies in summer corn field inlaid in cotton area. Acta Phyt. Sin. 27, 199–204 (1999).
Zhang, G. F., Lü, Z. C. & Wan, F. H. Detection of Bemisia tabaci remains in predator guts using a sequence-characterized amplified region marker. Entomol. Exp. Appl. 123, 81–90 (2007).
Zhang, G. F., Lü, Z. C., Wan, F. H. & Lövei, G. L. Real-time PCR quantification of Bemisia tabaci (Homoptera: Aleyrodidae) B-biotype remains in predator guts. Mol. Ecol. Not. 7, 947–954 (2007).
Wang, Y. N. et al. Structure, dynamics and niche of dominant population of insect community in peach orchards of Beijing. Sci. Silv. Sin. 45, 88–94 (2009).
Afzal, G., Mushtaq, S., Rana, S. A. & Sheikh, M. A. Trophic niche breadth and niche overlap among different guilds of spider species in wheat agro-ecosystem. Pak. J. Life Soc. Sci. 11, 107–111 (2013).
Wang, G. H., Wang, X. Q., Qiao, F., Zhu, Z. R. & Cheng, J. A. Development and preliminary application of a triplex real-time polymerase chain reaction assay for evaluating predation on three planthoppers in a rice ecosystem. Mol. Ecol. Resour. 13, 811–819 (2013).
King, R. A., Read, D. S., Traugott, M. & Symondson, W. O. C. Molecular analysis of predation: a review of best practice for DNA-based approaches. Mol. Ecol. 17, 947–963 (2008).
Albaina, A. et al. A TaqMan real-time PCR based assay targeting plaice (Pleuronectes platessa L.) DNA to detect predation by the brown shrimp (Crangon crangon L.) and the shore crab (Carcinus maenas L.)-assay development and validation. J. Exp. Mar. Biol. Ecol. 391, 178–189 (2010).
Brandl, S. et al. Ten real-time PCR assays for detection of fish predation at the community level in the San Francisco Estuary-Delta. Mol. Ecol. Resour. 15, 278–284 (2015).
Zaidi, R. H., Jaal, Z., Hawkes, N. J., Hemingway, J. & Symondson, W. O. C. Can multiple-copy sequences of prey DNA be detected amongst the gut contents of invertebrate predators? Mol. Ecol. 8, 2081–2087 (1999).
Symondson, W. O. C. Molecular identification of prey in predator diets. Mol. Ecol. 11, 627–641 (2002).
Han, B. Y., Cui, L. & Dong, W. X. The effect of farming methods in organic, safety, and common tea gardens on the composition of arthropod communities and the abundances of main pests. Acta. Ecol. Sin. 26, 1438–1443 (2006).
Fu, J. Y., Han, B. Y. & Xiao, Q. Mitochondrial COI and 16sRNA evidence for a single species hypothesis of E. vitis, J. formosana and E. onukii in East Asia. PloS one 9, e115259 (2014).
Su, H. F. et al. The effect of mixed planting on tea production, main predators and insect pests in tea plantations. J. Tea Sci. 34, 122–128 (2014).
Gao, J. L., Zhao, D. X. & Chen, Z. M. Predatory function of Evarcha albaria upon Empoasca vitis. Chin. J. Trop. Crops 25, 72–74 (2003).
Ye, H. X., Cui, L., He, X. M. & Han, B. Y. Effect of intercropping tea with citrus, waxberry, or snake gourd on population density and spatial distribution of the tea green leafhopper and araneids. Acta. Ecol. Sin. 30, 6019–6026 (2010).
Li, J. L. et al. Effects on arthropod communities under multiple intercropping cultivation model in tea garden. Guangdong Agr. Sci. 9, 129–131 (2010).
Cui, Q. M. et al. Comparative analysis of survey methods of spiders of tea garden in Enshi. Heilongjiang Agr. Sci. 1, 55–57 (2012).
Cui, Q. M. et al. Survey on spider species in tea garden in Enshi. Hubei Agr. Sci. 51, 2735–2737 (2012).
Cui, Q. M. et al. Investigation on species of spiders in tea plantations in Enshi region of China. Plant Dis. Pests 4, 15–17 (2013).
Liu, W. X. et al. Evaluation on role of predators in Helicoverpa armigera control. Chin. J. Biol. Contr. 16, 97–101 (1999).
Huang, B. H., Zou, Y. D., Bi, S. D., Li, H. G. & Zhu, Q. L. Characteristics, dynamics and niche of insect community in plum orchard. Chin. J. Appl. Ecol. 16, 307–312 (2005).
Heimpel, G. E., Rosenheim, J. A. & Mangel, M. Predation on adult Aphytis parasitoids in the field. Oecologia 110, 346–352 (1997).
Triltsch, H. Gut contents in field sampled adults of Coccinella septempunctata (Col.: Coccinellidae). Entomophaga 42, 125–131 (1997).
Crossley, D. A. Radioisotope measurement of food consumption by a leaf beetle species, Chrysomela knabi Brown. Ecology 47, 1–8 (1966).
Symondson, W. O. C. & Liddell, J. E. The detection of predation by Abax parallelepipedus and Pterostichus madidus (Coleoptera: Carabidae) on Mollusca using a quantitative ELISA. B. Entomol. Res. 83, 641–647 (1993).
Hagler, J. R. et al. Development of a monoclonal antibody to detect predation of the sweetpotato whitefly. Bemisia tabaci. Entomol. Exp. Appl. 68, 231–236 (1993).
Sopp, P. I., Sunderland, K. D., Fenlon, J. S. & Wratten, S. D. An improved quantitative method for estimating invertebrate predation in the field using an enzyme-linked immunosorbent assay (ELISA). J. Appl. Ecol. 29, 295–302 (1992).
Hagler, J. R. & Naranjo, S. E. Measuring the sensitivity of an indirect predator gut content ELISA: detectability of prey remains in relation to predator species, temperature, time, and meal size. Biol. Control 9, 112–119 (1997).
Symondson, W. O. C., Sunderland, K. D. & Greenstone, M. H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 47, 561–594 (2002).
Lo, Y. M. D. & Chan, K. C. A. Introduction to the polymerase chain reaction In Clinical applications of PCR (eds Lo, Y. M. D., Chiu, R. W. K. & Chan, K. C. A.) 1–10 (Humana Press Inc, USA, 2006).
Hagler, J. R., Blackmer, F. & Spurgeon, D. W. Accuracy of a prey-specific DNA assay and a generic prey-immunomarking assay for detecting predation. Methods Ecol. Evol. 6, 1426–1434 (2015).
Harwood, J. D. et al. Tracking the role of alternative prey in soybean aphid predation by Orius insidiosus: a molecular approach. Mol. Ecol. 16, 4390–4400 (2007).
Kuusk, A. K., Cassel-Lundhagen, A., Kvarnheden, A. & Ekbom, B. Tracking aphid predation by lycosid spiders in spring-sown cereals using PCR-based gut-content analysis. Basic Appl. Ecol. 9, 718–725 (2008).
Petráková, L. et al. Discovery of a monophagous true predator, a specialist termite-eating spider (Araneae: Ammoxenidae). Sci. Rep. 5, 1–10 (2015).
Yin, C. M. et al. Fauna Hunan: Araneae in Hunan, China (Hunan Science and Technology Press, Changsha, China, 2012).
World Spider Catalog. online at http://wsc.nmbe.ch/ (2014).
Bustin, S, Real-time PCR In Encyclopedia of Medical Genomics and Proteomics 1117–1125 (Taylor and Francis, New York, USA, 2005).
Hurlbert, S. H. The measurement of niche overlap and some relatives. Ecology 59, 67–77 (1978).
Acknowledgements
This work was mainly supported by the National Natural Science Foundation of China (31172113). We thank the staff of Wang Dazhen tea plantation kindly providing experimental base. We also thank the staff of the Centre for Behavioural Ecology and Evolution (CBEE, Hubei University) and of the Behavioural Ecology and Sociobiology Lab (DBS, NUS) for all their help and support throughout this study.
Author information
Authors and Affiliations
Contributions
J.C. designed the study; L.Y., Y.Z. and T.Y. collected material at the study site; L.Y. designed the primers; T.Y. performed molecular analyses and analyzed data supervised by J.C. and J.L.; T.Y., J.C., J.L., I.A. and D.L. contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Tb., Liu, J., Yuan, Ly. et al. Molecular identification of spiders preying on Empoasca vitis in a tea plantation. Sci Rep 7, 7784 (2017). https://doi.org/10.1038/s41598-017-07668-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07668-w
This article is cited by
-
Molecular quantification of spider preying on springtails in winter-spring fallow waterlogged paddy fields
Applied Entomology and Zoology (2021)
-
Identification of Indian Spiders through DNA barcoding: Cryptic species and species complex
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.