Abstract
At the regional scale, although environmental factors are known to shape the distributions of belowground communities in terrestrial ecosystems, these environmental factors account for relatively low percentages of the variation in belowground communities. More of this variation might be explained by considering ecosystem stable isotopic values, which can provide insight into environmental conditions. Here, we investigated ecosystem (plant and soil) δ13C and δ15N values and belowground communities (microbes and nematodes) as well as environmental factors (climates, soils, and plants) across the Mongolian Plateau. The regression analyses showed that plant isotopic values were more closely associated with belowground communities than soil isotopic values, while ecosystem δ13C values were more closely associated with the belowground communities than ecosystem δ15N values. We also found isotopic values were more closely associated with nematode communities than microbial communities. Variation partioning analyses indicated that environmental variables together explained 16–45% of total variation in belowground communities. After isotopic variables were added as predictors to the variation partition analyses, the explanation of the variance was improved by14–24% for microbial communities and was improved by 23–44% for nematode communities. These findings indicate that isotopic values could be used to predict the properties of belowground communities at a regional scale.
Similar content being viewed by others
Introduction
Determinants of the spatial distribution of aboveground and belowground communities is a relevance issue of community ecology1. Although the spatial distributions of both aboveground2, 3 and belowground communities4, 5 have been well documented at regional scales, we still lack comprehensive understanding about the determinants of belowground communities across a broad range of environmental gradients6. This critical knowledge gap limit our understanding of the importance of these communities in regulating interactions in soil food webs and ecosystem process6, 18. A growing number of observations have demonstrated that climate4, 5, 7, soil variables7, 8, and plant characteristics9, 10 help shape the structure and composition of belowground communities (mostly microbial communities) at regional scale in terrestrial ecosystems. However, these environmental factors still have relatively low ability to predict the variation in belowground communities at regional scale5, 9, 10. In addition, these studies have not yet detected how different trophic levels of soil micro-food webs (e.g., microbes and nematodes) are regulated by these environmental variables, and this is especially true for the semi-arid grasslands on the Mongolian Plateau, which have contrasting climatic, plant, and soil conditions5.
Terrestrial carbon (C) and nitrogen (N) cycling is central to predicting future patterns of many ecosystem processes and functions (e.g., primary productivity, C and N sequestration, nutrient fluxes)11, 12. Previous studies suggested that measurement of ecosystem (plant and soil) stable isotopic compositions (δ13C and δ15N) can provide insight into soil C and N cycling13, 14. For example, Garten et al.15 and Craine et al.16 documented that plant δ15N values increased with increasing N availability and potential N mineralization across natural N supply or N availability gradients. Furthermore, it has been well documented that soil C and N cycling are also greatly affected by belowground communities through respiration and N mineralization17, 18, and understanding the belowground communities that drive these processes has long been a goal of soil scientists19, 20. For example, a recent regional-scale study showed that microbial biomass alone predicted 61% of the variance in C mineralization rates at 0–5 cm depth in mineral soils21. Overall, both the ecosystem stable isotopic compositions and belowground communities have great effects on the soil C and N cycling hinted that there properly exist an inherent correlation between them, and therefore our understanding of belowground communities could be increased by considering ecosystem stable isotopic compositions. Stable isotope information can provide insight into climate conditions and changes in the plant community composition, water-use efficiency, nitrogen status, and terrestrial ecosystem functions along environmental gradients13, 14, 22. Information on isotopic compositions could be useful in evaluating spatial patterns of belowground communities and their feedback to global change ecology15, 23. For example, one report has provided direct evidence regarding the relationship between isotopic values and belowground communities24; that report documented a positive correlation between the δ15N values and abundance of ammonia oxidizing archaea at four sites in Arizona and in Hawai’i. However, it is still unclear whether ecosystem δ13C and δ15N values can be used to predict the spatial patterns in belowground communities along environmental gradients at a regional scale.
The natural semi-arid grassland ecosystem on the Mongolia Plateau covers about 10% of the global land surface, and even though this plateau stores substantial quantities of C and N25, its belowground communities have seldom been studied5, 26. In the present study, we assessed the relationships between ecosystem (plant and soil) isotopic values (δ13C and δ15N) and changes in belowground communities along environmental gradients (climate, soils, and plants) on two perpendicular transects on the Mongolia Plateau (Fig. 1). We attempted to answer three questions. First, how do the ecosystem isotopic values relate to the soil microbial and nematode communities at the regional scale? Second, after controlling for environmental variables, how much of the variance in the functional groups of the belowground microbial and nematode communities is explained by isotopic values? Third, what explains the relationships between the ecosystem isotopic values and soil microbial and nematode communities on the Mongolia Plateau?
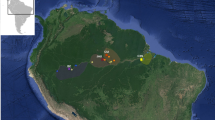
Locations of the study sites along the east–west (Inner Mongolia) and north–south (Mongolia) transects across the Mongolian Plateau5, 26. The map was created by the Google Maps module in R (https://cran.r-project.org/package=ggmap).
Specifically, we tested three hypotheses. H1: We hypothesize that belowground communities on the Mongolia Plateau will be more closely associated with plant isotopic values than with soil isotopic values and will be more closely associated with ecosystem δ15C values than with ecosystem δ13N values. Because photosynthates released by roots are a major source of available C and N for belowground communities27, 28. In addition, the availability of C is a better predictor of belowground communities than the availability of N7, 29. H2: We hypothesize that the isotopic values will be more closely associated with soil microbes than with soil nematodes along the environmental gradients. Because bottom-up effects derived from C and N substrates are often depend on trophic levels in micro-food web, with stronger effects on lower trophic levels (e.g., soil microbes) than on higher trophic levels (e.g., soil nematodes)30,31,32. H3: We hypothesize that, after considering ecosystem stable isotopic values, the explanation of the variance in belowground communities will be greatly improved than the situations which only consider environmental variables. Because stable isotope information can provide insight not only into environmental variables but also into water-use efficiency and terrestrial ecosystem functions along environmental gradients13, 14, 22.
Results
Relationships between isotopic values and belowground communities
From desert to meadow steppe, we found that MAP, SOC, TSN, TSP, plant C content increased while MAT and plant N content decreased (Table S1). Soil δ13C decreased from −22.2 to −25.3‰, and plant δ13C decreased from −18.8 to −25.9‰ (Table 1). Similarly, soil δ15N decreased from 6.62 to 4.77‰, and plant δ15N decreased from 3.19 to 0.26‰ (Table 1). For microbes, total soil microbial biomass, biomass of all microbial groups, B/F ratio increased while microbial community structure decreased from desert to meadow steppe (Table 1). For nematodes, total nematode abundance, abundance of four nematode trophic groups, nematode taxon richness, and nematode community structure increased from desert to meadow steppe (Table 1).
Regression analyses showed that ecosystem isotopic values were closely associated with most variables in belowground communities (Figs 2–4). Ecosystem δ13C values (plant and soil) were negatively related to total microbial biomass, the biomass of all four microbial groups, and B/F ratios, and were positively related to microbial community structure across the Mongolian Plateau (Figs 2A and 4A). Plant δ15N values were negatively related to total microbial biomass, the biomass of all four microbial groups, and B/F ratios while were positively related to microbial community structure. Soil δ15N values, however, were not related to any of the microbial variables (Figs 2B and 4B). Ecosystem δ13C or 15N values were negatively associated with total nematode abundance, the abundance of all nematode trophic groups, nematode taxon richness, and nematode community structure (Figs 3 and 4C). Soil nematode variables were more closely associated with ecosystem δ13C values than with ecosystem 15N values (Figs 3 and 4D).
Relationships between microbial community variables and (A) δ13C isotopic values and (B) δ15N isotopic values of plants (pink symbols) and soils (green symbols) at the regional scale in the Mongolian grassland. Abbreviations are explained in Table 1. Regression analysis is indicated by r 2 and significance level (NS, P > 0.05; **P < 0.01; ***P < 0.001).
Relationships between nematode community variables and (A) δ13C isotopic values and (B) δ15N isotopic values of plants (pink symbols) and soils (green symbols) at the regional scale in the Mongolian grassland. Abbreviations are explained in Table 1. Regression analysis is indicated by r 2 and significance level (**P < 0.01; ***P < 0.001).
Relationships between additional microbial and nematode community variables and (A and C) δ13C isotopic compositions and (B and D) δ15N isotopic compositions of plants (pink symbols) and soils (green symbols) at the regional scale in the Mongolian grassland. Abbreviations are explained in Table 1. Regression analysis is indicated by r 2 and significance level (NS, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001).
Relative effects of isotopic values and environmental variables on belowground communities
Variation partition analyses showed that environmental variables (climate, plants, and soils) together explained 16–45% of total variation in in microbial communities and 20–32% of total variation in in nematode communities (Fig. 5). When considering the ecosystem isotopic variables, we found that ecosystem δ13C values explained 5–16% of the total variation in microbial communities and 9–29% of the total variation in nematode communities, while ecosystem δ15N values explained 5–10% of the total variation in microbial communities and 8–15% of the total variation in nematode communities (Fig. 5). Plant isotopic values explained 11–16% of the total variation in microbial communities and 11–23% of the total variation in nematode communities, while soil isotopic values explained only 3–7% of the total variation in microbial communities and 5–24% of the total variation in nematode communities (Fig. 5). Overall, our results indicated that, after ecosystem isotopic variables were added as predictors to the variation partition analyses, the explanation of the variance was improved by14–24% for microbial communities and was improved by 23–44% for nematode communities (Fig. 5). Partial regressions further indicated that, after environmental variables were controlled for, most variables in nematodes significantly related with ecosystem isotopic values. For microbes, most variables significantly related with plant δ13C values while did not relate with soil δ15N values; part of microbial variables related with soil δ13C values or plant δ15N values (Table 2).
Percentages of variation in soil microbial and nematode properties explained by ecosystem isotopic values (δ13C and δ15N) at the regional scale in grasslands on the Mongolian Plateau. Abbreviations are explained in Table 1.
Discussion
Linkages of ecosystem isotopic values to belowground communities
Our regional-scale study revealed that belowground communities were more closely associated with plant isotopic values than with soil isotopic values, which was consistent with our first hypothesis. As primary producers and sources of resources/energy for soil organisms in the decomposer food web, plant-related variables can highly associated with composition and structure of belowground communities and functioning they regulated27, 28, 33. Therefore, the isotopic values of plants will greatly affect the isotopic values of belowground communities28, 34. Consistent with our first hypothesis, belowground communities were more closely associated with ecosystem δ13C values than with ecosystem δ15N values. The higher percentages of variation in belowground communities explained by ecosystem δ13C values than ecosystem δ15N values has several explanations. First, C availability is a better predictor of belowground communities than N availability because C provides the energy required by most soil organisms7, 29. Second, plant δ13C values reflect plant intrinsic characteristics, plant genetic types and life forms by controlling photosynthetic capacity35, 36. Third, in contrast to δ13C isotope discrimination, N isotope discrimination is far from complete because the isotopic composition of N leaving the system (e.g., volatilization, mineralization and leaching, and plant uptake) is the most relevant determinant of ecosystem δ15N values23, 37.
Inconsistent with our second hypothesis, ecosystem isotopic values explained less of the variation in microbes than in nematodes. Bottom-up effects on lower trophic levels (e.g., soil microbes) are generally stronger than on higher trophic levels (e.g., soil nematodes)30, 31. In the present study, however, ecosystem isotopic values explained more of the variation in nematode communities than in microbial communities. This unexpected finding might be explained by that nematode survival and therefore abundance is very dependent on soil moisture than microbial communities5. Previous regional-scale reports documented that ecosystem isotopic values are strongly associated with climate and especially with precipitation37,38,39. Consistent with these previous reports, our results indicated strong negative relationships between ecosystem δ13C and δ15N values and MAP on the Mongolia Plateau. The close relationship between ecosystem isotopic values and precipitation could help explain why nematode communities were more sensitive to ecosystem isotopic values than microbial communities5, 40.
Potential mechanisms explaining the linkages between isotopic values and belowground communities
Our results showed that ecosystem isotopic values were strongly associated with environmental factors (climate, soil, and plant) (Table S4). Many previous studies have documented that the variation in ecosystem isotopic values is closely associated with climate, soils, and plants37,38,39. Regional-scale studies have indicated that climate and especially precipitation is predominant in determining ecosystem δ13C or δ15N values37, 39. Also, our finding that ecosystem δ13C or δ15N values increased with increasing MAT is consistent with finding from Männel et al.41, which documented a clear and positive relationship between plant δ13C values and MAT in arid and semiarid grasslands. Ecosystem δ13C or δ15N values were related to plant characteristics in the current study, which again can be explained by the effects of these characteristics on photosynthetic capacity35, 36. In addition, the negative relationships between soil factors and soil δ13C or δ15N values in the current study (Table S4) were consistent with a previous study in alpine grasslands42, which showed that soil clay content and soil C/N explained much of the variation in soil δ15N values. Overall, the results indicate that regional-scale isotopic composition can be a good predictor of climate, plants, and soils.
Consistent with our third hypothesis, our variation partitioning analyses showed that, besides environmental variables, ecosystem isotopic values independently explained much of the spatial variation in belowground communities across the Mongolia Plateau. Specifically, after isotopic variables were added as predictors to the variation partition analyses, the explanation of the variance was improved by14–24% for microbial communities and was improved by 23–44% for nematode communities. Our study, to our knowledge, is the first one to provide direct information concerning the linkages between ecosystem isotopic values and more than one trophic level of belowground communities at a regional scale. Our findings suggest that ecosystem isotopic values could be used to predict the characteristics of belowground communities at a regional scale. Because ecosystem δ13C and δ15N values are related to C assimilation and to N status as influenced by water stress and cycling of elements, ecosystem isotopic values are closely coupled to belowground communities43, 44. The relationships between isotopic composition and below-ground communities have seldom been separated from environmental factors in previous studies, which may be one reason why isotopic composition explained only low percentages of the total variation in belowground communities in these studies5, 9, 10. To assess the potential of ecosystem soils to sequester C and N, researchers must be able to readily evaluate belowground communities6, 18. Interestingly, the ecosystem isotopic composition explained similar percentage of the total variation in specific variable in microbial or nematode communities. This could be due to the fact that their similar responses to changes in ecosystem isotopic values across the Mongolia Plateau. Although we were not able to identify detailed mechanisms that how ecosystem isotopic values shaped the belowground communities in detail, our results from the Mongolian Plateau indicate that ecosystem isotopic values help to predict the spatial variation in belowground communities for semi-arid grasslands.
Implications of ecosystem isotopic values for semi-arid grasslands
Numerous studies have concluded that stable C and N isotopic signatures provide insight into the biotic and abiotic factors controlling ecosystem functions (e.g., the cycling of C and N)13, 45. Because the community-averaged foliar δ13C value is related to C assimilation and to the diffusion of CO2 as influenced by water stress and cycling of elements, and adaptations of plants to local climate therefore lead to plant δ13C values that are closely coupled to N and C cycling43, 46. As part of the largest contiguous grassland in the world, the Mongolian Plateau is an important terrestrial ecosystem that greatly affects global C and N storage and cycling47. Therefore, understanding the mechanisms governing belowground communities is crucial for evaluating ecosystem C and N balance in the Mongolia Plateau and its feedbacks to climate change. In recent decades, the semi-arid grassland ecosystems on the Mongolia Plateau have been affected by multiple anthropogenic stressors (e.g., global climate change and land-use change). A significant consequence of these anthropogenic stressors is the widespread changes in both belowground communities and ecosystem functions48. To rapidly assess the potential of ecosystem soils to sequester C and N, researchers must be able to readily evaluate belowground communities13, 14. The assessment of ecosystem stable isotopes of C and N allows researchers to rapidly and precisely measure belowground communities and hence estimate C or N storage and cycling. Our results from the Mongolian Plateau indicate that predictive models of C and N sequestration regulated by belowground communities should include assessment of ecosystem isotopic values.
Materials and Methods
Study area and sampling design
The research location as well as sampling design were explained thoroughly by Chen et al.5, 26. In brief, we developed two vertical regional-scale transects on the Mongolian Plateau in the Eurasian steppe in August 2010 and 2011, respectively (Fig. 1). Both transects went across four major vegetation types, consisting of meadow steppe, typical steppe, desert steppe, and desert. From desert to meadow steppe, the soil type of each vegetation type was dominated by light brown, calcic brown, typical chestnut, and dark chestnut, respectively. About 10–12 sites were selected for each vegetation type (A total of 44 sites); these sites were not foraged obviously by large mammals. For each site, the mean annual precipitation (MAP) and mean annual temperature (MAT) during 1970–2000 were obtained from the WorldClim database (http://www.worldclim.org/current)49.
Sampling and measurements
At each site, aboveground net primary productivity (ANPP) was determined in five 1 × 1 m herbaceous subplots or 5 × 5 m shrub subplots situated randomly within a 100 × 100 m site. All plant materials in each subplot were oven-dried at 65 °C for 48 h and weighed as ANPP. We classified all plant species into five plant functional groups based on life forms50: perennial rhizome grasses, perennial bunchgrasses, perennial forbs, shrubs and semi-shrubs, and annuals. Principal component analysis (PCA), based on the relative biomass proportion (%) of the five plant functional groups, was performed, and the first ordination axes (PC1) was used as indicator of plant community structure (Table S2). Then, all of the aboveground live plant materials from each subplot were ground with a ball mill. These ground plant samples were used to estimate plant C and N contents and δ13C and δ15N values. Soils from 0 to 20 cm depth were randomly collected by taking three soil cores (7-cm-diameter) in each subplots. The soils from the three cores were mixed in situ to form one composite soil sample. After passed through 2-mm-mesh sieves, the soil was separated into two parts. One part was maintained fresh for determination of soil moisture, soil microbes, and soil nematodes. The other part, which was air-dried, was used to determine soil δ13C and δ15N values, soil pH, soil organic C content (SOC), total soil N content (TSN), and total soil phosphorus (TSP). All results are expressed on a dry weight basis.
Plant and soil δ13C and δ15N values
The δ13C and δ15N values of plant and soil samples were determined with an isotope ratio mass spectrometer (Delta Plus XP, Thermo Finnigan, Berlin, Germany) coupled with an elemental analyser (Euro EA 3000, EuroVector, Milan, Italy). The C or N isotope data were specified as δ13C or δ15N relative to the Vienna Pee Dee Belemnite standard: δ13C or δ15N (‰) = (R sample/R standard − 1) × 1000, where R sample and R standard are the ratios of 13C/12C or 15N/14N in the sample and standard.
Soil microbial and nematode communities
Composition and structure of soil microbial communities were analyzed by phospholipid fatty acid (PLFA) technique51. The separated fatty acid were detected by an Agilent 6890 gas chromatograph (Agilent Technologies, Palo Alto, CA) and equipped with a MIDI Sherlock Microbial Identification System (MIDI Inc., Newark, DE, USA). The biomass of each specific FAs for each soil sample was expressed as nmol of fatty acid g−1 dry soil. FAs specific to bacteria, fungi, actinobacteria, and arbuscular mycorrhizal fungi were used to determine the biomass of these microbial groups5, 26 and to calculate fungi/bacteria ratios (F/B)52. Like plant community structure, microbial community structure was indicated using the first ordination axes of the relative abundances of four microbial groups (mol%) (Table S2).
Soil nematodes from 50 g of moist soil per soil sample were extracted using Baermann funnel method for 48 h. After fixation in a 4% formalin solution, the nematodes were counted with an inverted microscope. Based on morphology, the first 100 individuals were identified to genus and assigned to five trophic groups53: plant-feeding, bacterial-feeding, fungal-feeding, omnivorous, and carnivorous. The results of nematode abundance are expressed on 100 g dry weight basis. The number of genera was used as an indicator of taxon richness (NTR). Nematode community structure was indicated using the first ordination axes of the relative abundances of four nematode trophic groups (%) (Table S2).
Statistical analyses
All statistical analyses were done with R 3.3.2 (R Development Core Team 2016). First, one-way ANOVAs with Tukey’s multiple range tests was performed across all variables to compare the means among vegetation types. To satisfy assumption of ANOVAs, the data for some variables were transformed to natural logarithms. Second, the relationships between ecosystem (plant and soil) isotopic values (δ13C and δ15N) and belowground communities (microbes and nematodes) were tested using linear regressions. Third, hierarchical variation partitioning analyses (with the calc.relimp function by the LMG methods in the “relaimpo” package) were used to determine the relative importance of ecosystem isotopic values and environmental factors (climate, plants, and soils) on belowground community variables54. LMG assigns each variable the average increase in R2 when it is added to a regression model containing a subset of other variables. Finally, partial regressions were used to test the relationships of partial residuals between ecosystem isotopic values and belowground community variables after the three environmental variables were controlled for. To facilitate our interpretations, we classified the environmental factors into three groups5, 26: (1) climate (MAP and MAT); (2) soils (pH, SOC, TSN, and TSP), and plants (ANPP, plant species richness, plant C and N contents, and plant community structure). We conducted PCA on the multiple variables for each environmental group and used the PC1 scores as indicators of each environmental group26. The first ordination axes (PC1) explained 54–90% of the total variance for each environmental group and was used in our analysis and interpretations (Table S3).
References
Levin, S. A. The problem of pattern and scale in ecology: the Robert H. MacArthur award lecture. Ecology 73, 1943–1967 (1992).
Cleland, E. E. et al. Sensitivity of grassland plant community composition to spatial vs. temporal variation in precipitation. Ecology 94, 1687–1696 (2013).
Armas, C. & Pugnaire, F. I. Plant interactions govern population dynamics in a semi-arid plant community. Journal of Ecology 93, 978–989 (2005).
Ettema, C. H. & Wardle, D. A. Spatial soil ecology. Trends in Ecology and Evolution 17, 177–183 (2002).
Chen, D. et al. Regional-scale patterns of soil microbes and nematodes across grasslands on the Mongolian plateau: relationships with climate, soil, and plants. Ecography 38, 622–631 (2015).
Bardgett, R. D. & Wardle, D. A. Aboveground-belowground linkages: biotic interactions, ecosystem processes, and global change. Oxford Series in Ecology and Evolution, Oxford University Press, Oxford, UK (2010).
Fierer, N., Strickland, M. S., Liptzin, D., Bradford, M. A. & Cleveland, C. C. Global patterns in belowground communities. Ecology Letters 12, 1238–1249 (2009).
Rousk, J. et al. Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME Journal 4, 1340–1351 (2010).
Orwin, K. H. et al. Linkages of plant traits to soil properties and the functioning of temperate grassland. Journal of Ecology 98, 1074–1083 (2010).
de Vries, F. T. et al. Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecology Letters 15, 1230–1239 (2012).
Maestre, F. T. et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 335, 214–218 (2012).
Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 304, 1623–1627 (2004).
Staddon, P. L. Carbon isotopes in functional soil ecology. Trends in Ecology and Evolution 19, 148–154 (2004).
Nadelhoffer, K. & Fry, B. Nitrogen isotope studies in forest ecosystems. Stable isotopes in ecology and environmental science. Blackwell, Oxford 316 (1994).
Garten, C. T., Hanson, P. J., Todd, D. E., Lu, B. B. & Brice, D. J. Natural 15N- and 13C-abundance as indicators of forest nitrogen status and soil carbon dynamics. Stable Isotopes in Ecology and Environmental Science, 61 (2008).
Craine, J. M. et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytologist 183, 980–992 (2009).
Ingham, R. E., Trofymow, J., Ingham, E. R. & Coleman, D. C. Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecological Monographs 55, 119–140 (1985).
Wardle, D. A. et al. Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633 (2004).
Schmidt, M. W. I. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011).
Schimel, J. P. & Schaeffer, S. M. Microbial control over carbon cycling in soil. Front Microbiol 3, 348 (2012).
Dangal, S. R. et al. Synergistic effects of climate change and grazing on net primary production of Mongolian grasslands. Ecosphere 7 (2016).
Luo, T. et al. Correlations between net primary productivity and foliar carbon isotope ratio across a Tibetan ecosystem transect. Ecography 32, 526–538 (2009).
Handley, L. et al. The 15N natural abundance (δ15N) of ecosystem samples reflects measures of water availability. Functional Plant Biology 26, 185–199 (1999).
Zolda, P. & Hanel, L. Soil nematodes inhabiting an original dry meadow and an abandoned vineyard in the National Park Seewinkel, Eastern Austria. Helminthologia 44, 112–117 (2007).
Robin, W. S. M. & Rohweder, M. Pilot analysis of global ecosystem: grassland ecosystems. Washington, DC: World Resource Institute 49 (2000).
Chen, D. et al. Effect of diversity on biomass across grasslands on the Mongolian Plateau: contrasting effects between plants and soil nematodes. Journal of Biogeography 43, 955–966 (2016).
Rajaniemi, T. & Allison, V. Abiotic conditions and plant cover differentially affect microbial biomass and community composition on dune gradients. Soil Biology and Biochemistry 41, 102–109 (2009).
Hobbie, E. A. & Högberg, P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytologist 196, 367–382 (2012).
Lilleskov, E. A., Hobbie, E. A. & Fahey, T. J. Ectomycorrhizal fungal taxa differing in response to nitrogen deposition also differ in pure culture organic nitrogen use and natural abundance of nitrogen isotopes. New Phytologist 154, 219–231 (2002).
Eilers, K. G., Lauber, C. L., Knight, R. & Fierer, N. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biology and Biochemistry 42, 896–903 (2010).
Meier, C. L., Rapp, J., Bowers, R. M., Silman, M. & Fierer, N. Fungal growth on a common wood substrate across a tropical elevation gradient: Temperature sensitivity, community composition, and potential for above-ground decomposition. Soil Biology and Biochemistry 42, 1083–1090 (2010).
Li, Q. et al. Nitrogen addition and warming independently influence the belowground micro-food web in a temperate steppe. PloS One 8, e60441 (2013).
Eisenhauer, N. et al. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 91, 485–496 (2010).
Hobbie, E. A., Macko, S. A. & Shugart, H. H. Insights into nitrogen and carbon dynamics of ectomycorrhizal and saprotrophic fungi from isotopic evidence. Oecologia 118, 353–360 (1999).
Smedley, M. P. et al. Seasonal carbon isotope discrimination in a grassland community. Oecologia 85, 314–320 (1991).
Balesdent, J., Mariotti, A., Boutton, T. & Yamasaki, S. Measurement of soil organic matter turnover using 13C natural abundance. Mass Spectrometry of Soils., 83–111 (1996).
Peri, P. L. et al. Carbon (δ13C) and nitrogen (δ15N) stable isotope composition in plant and soil in Southern Patagonia’s native forests. Global Change Biology 18, 311–321 (2012).
Murphy, B. P. & Bowman, D. M. J. S. The carbon and nitrogen isotope composition of Australian grasses in relation to climate. Functional Ecology 23, 1040–1049 (2009).
Swap, R., Aranibar, J., Dowty, P., Gilhooly, W. & Macko, S. A. Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: patterns and implications. Global Change Biology 10, 350–358 (2004).
Todd, T. C., Blair, J. M. & Milliken, G. A. Effects of altered soil-water availability on a tallgrass prairie nematode community. Applied Soil Ecology 13, 45–55 (1999).
Männel, T. T., Auerswald, K. & Schnyder, H. Altitudinal gradients of grassland carbon and nitrogen isotope composition are recorded in the hair of grazers. Global Ecology and Biogeography 16, 583–592 (2007).
Yang, Y. et al. Vegetation and soil 15N natural abundance in Alpine grasslands on the Tibetan Plateau: patterns and implications. Ecosystems 16, 1013–1024 (2013).
Ehleringer, J. R., Buchmann, N. & Flanagan, L. B. Carbon isotope ratios in belowground carbon cycle processes. Ecological Applications 10, 412–422 (2000).
Ladd, B. et al. Carbon isotopic signatures of soil organic matter correlate with leaf area index across woody biomes. Journal of Ecology 102, 1606–1611 (2014).
Evans, R. D. Soil nitrogen isotope composition. Stable Isotopes in Ecology and Environmental Science 2, 83–98 (2007).
Craine, J. M. et al. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant and Soil 396, 1–26 (2015).
Zhou, Z. et al. Soil carbon and nitrogen stores and storage potential as affected by land-use in an agro-pastoral ecotone of northern China. Biogeochemistry 82, 127–138 (2007).
Jiang, G., Han, X. & Wu, J. Restoration and management of the Inner Mongolia grassland require a sustainable strategy. AMBIO: A Journal of the Human Environment 35, 269–270 (2006).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25, 1965–1978 (2005).
Bai, Y. F., Han, X. G., Wu, J. G., Chen, Z. Z. & Li, L. H. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431, 181–184 (2004).
Bossio, D. A., Scow, K. M., Gunapala, N. & Graham, K. J. Determinants of soil microbial communities: Effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microbial Ecology 36, 1–12 (1998).
Frostegård, Å., Tunlid, A. & Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biology and Biochemistry 43, 1621–1625 (2011).
Bongers, T. The maturity index - an ecological measure of environmental disturbance based on nematode species composition. Oecologia 83, 14–19 (1990).
Groemping, U. & Matthias, L. Package ‘relaimpo’ (2013).
Acknowledgements
We thank students from the Inner Mongolia Agriculture University for their help with the field work. This study was supported by the Chinese National Key Development Program for Basic Research (2016YFC0500804 and 2015CB954201), the Natural Science Foundation of China (31570450 and 31320103916), and the Youth Innovation Promotion Association CAS (2015061).
Author information
Authors and Affiliations
Contributions
D.C. designed the research. B.W. and Y.W. wrote the main manuscript text. B.W., Y.W., and D.C. performed statistical analyses. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, B., Wu, Y. & Chen, D. Stable isotopes of carbon and nitrogen help to predict the belowground communities at a regional scale. Sci Rep 7, 7276 (2017). https://doi.org/10.1038/s41598-017-07517-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07517-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.