Abstract
Most bacteria release extracellular vesicles (EVs). Recent studies have found these vesicles are capable of gene delivery, however the consequences of vesicle-mediated transfer on the patterns and rates of gene flow within microbial communities remains unclear. Previous studies have not determined the impact of both the genetic cargo and the donor and recipient species on the rate of vesicle-mediated gene exchange. This report examines the potential for EVs as a mechanism of gene transfer within heterogeneous microbial populations. EVs were harvested from three species of Gram-negative microbes carrying different plasmids. The dynamics of gene transfer into recipient species was measured. This study demonstrates that vesicles enable gene exchange between five species of Gram-negative bacteria, and that the identity of the genetic cargo, donor strain, and recipient strain all influence gene transfer rates. Each species released and acquired vesicles containing genetic material to a variable degree, and the transfer rate did not correlate with the relatedness of the donor and recipient species. The results suggest that EVs may be a general mechanism to exchange non-specialized genetic cargo between bacterial species.
Similar content being viewed by others
Introduction
Microorganisms possess complex abilities to transfer genetic material through horizontal gene transfer (HGT), fundamentally shaping genetic landscapes and affecting biological functions1,2,3,4. The capacity for DNA exchange in bacteria and the plasticity of their genetic material has amplified the rate of adaptation and evolution across species and has allowed for stability and growth of complex microbial ecosystems in a multitude of environments5,6,7,8. The ability of diverse bacterial populations to share genes supports cooperation and survival9, 10, and, in specific cases, contributes to the emergence and spread of antibiotic resistance and pathogenicity11, 12. Within heterogeneous populations, DNA transfer occurs within and between different species13,14,15,16,17,18. Polz et al. describe continuous and widespread horizontal gene transfers established through local gene networks19. Known limitations of the standard gene transfer pathways of transduction, transformation, and conjugation, and the extent of interspecies gene exchange within wild populations suggests there maybe alternative mechanisms of gene transfer20, 21.
Our current understanding of HGT consists of three well-described mechanisms to exchange genetic material between bacteria: transformation, conjugation, and transduction2,3,4, 6, 22. Transformation is the uptake of extracellular DNA6, 23,24,25,26,27. Free DNA is common in nearly all environments as the result of active excretion by living cells and cell death and lysis, therefore, recipient uptake is the main barrier to gene transfer by transformation. To acquire free DNA, cells must be in a state of competence, which is both transient and affected by many factors. Natural competence is often tightly regulated, involving 20 or more proteins23, 28,29,30,31. The number of species capable of natural transformation appears to be small, with rough estimates of 1% of bacterial species. Conjugation is mediated by cell-to-cell contact during which a pore directs transfer of DNA. Conjugative transfer systems are associated with and depend on plasmids that code for the necessary proteins to facilitate DNA exchange14, 32,33,34,35,36,37,38. The conjugative apparatus also allows for the transfer of other genetic material, known as mobile genetic elements. In general, horizontal gene transfer through conjugation is specific to a small set of specialized gene products, although some broad range conjugative systems have been identified39,40,41. The third form of HGT relies on bacteriophages that transfer DNA through infection. Bacteriophages package their own and non-phage DNA, in some cases taking up to 100 kilobases of “extra” genes with them to infect new cells42. As described by Marks and Sharp, cell surface interactions with bacteriophages limit host specificity7, 42,43,44,45. Although these known mechanisms enable gene flow within microbial ecosystems, these three mechanisms of horizontal gene transfer present barriers to gene exchange, e.g. limited genetic cargo in the case of conjugation, limited recipients in the case of transformation, and limited donor-recipient pairs in the case of transduction20, 46. Here we explore the properties of an emerging gene transfer mechanism, vesicle-mediated gene exchange.
Recent studies have uncovered a newly identified mechanism for DNA transfer utilizing ubiquitously produced extracellular vesicles47. Nearly all observed bacteria, including both Gram-negative and Gram-positive bacteria, secrete spherical structures known as EVs48,49,50,51,52,53,54,55. These nanovesicles, 20–300 nm in diameter, are produced and released during growth. Bacterial extracellular vesicles contain both periplasmic and cytoplasmic components, however, many aspects of vesicle biogenesis and the regulation of vesicle composition remain unclear51, 56, 57. Bacterial extracellular vesicles have been shown to serve a variety of functions in intra- and interspecies microbial communities58,59,60,61,62, and participate in protein and signal exchange between microbes and hosts62,63,64,65,66. These vesicles coordinate many forms of intercellular communication and facilitate the exchange of small molecules, proteins, and nucleic acids, including RNA and DNA65,66,67,68.
Vesicle-mediated transfer has been identified as an additional form of gene exchange. Intraspecies vesicle-mediated gene transfer has been reported in Escherichia coli, Acinetobacter baumannii, Acinetobacter baylyi and Pseudomonas aeruginosa 69,70,71,72. Recent work by Fulsundar et al. demonstrated interspecies gene transfer from A. baylyi EVs to E. coli cells. The unknown specificity and limitations of vesicle-mediated gene transfer make it difficult to gauge its importance to gene exchange in the wild. Extracellular vesicles are found in all studied species and the loading of DNA has been shown to be wide-spread50, 57, 73. We currently lack an understanding of DNA loading specificity, whether or not it is regulated, and what parameters determine the rate of vesicle uptake. Because vesicle exchange has been observed between distantly related cells, this suggests it may enable pervasive, low-barrier gene exchange. Here we explore the hypothesis that vesicle-mediated gene transfer is able to package multiple types of genetic material and that the species involved determine the rate of interspecies gene exchange.
Results
Bacteria package multiple types of plasmids into extracellular vesicles
Incorporation of bacterial plasmids into EVs has been previously observed70,71,72,73, but it is unclear if plasmid characteristics influence packaging. To examine the effects of plasmid identity on vesicle loading, we harvested vesicles using ultracentrifugation of the cell-free supernatant from stationary phase liquid cultures of Escherichia coli (Fig. 1B) carrying plasmids with different replication origins: pLC291, pUC19 and pZS2501 (Table S1). Purified EVs were filter sterilized to remove all bacterial cells and subsequently treated with DNase I to remove free DNA. Both pLC291 and pUC19 are high-copy number plasmids, with origins RK2 and pMB1 respectively74, 75. The RK2 origin was originally isolated from Klebsiella aerogenes 76, and pMB1 was originally isolated form E. coli 77. pZS2501 is a low-copy plasmid with the pSC101 origin78, originally isolated from E. coli 79. All three plasmids were demonstrated to be loaded into EVs from E. coli, see Fig. S1.
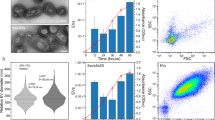
The impact of plasmid identity on the production and packaging of plasmid-containing vesicle. (A) Vesicle-mediated gene transfer. Donor cells load plasmid DNA into EV vesicles that can be acquired by a recipient cell. (B) Purification of EVs from liquid culture of bacterial cells through ultracentrifugation of cell-free supernatant. (C) 10% SDS-PAGE gel stained with Coomassie Blue showing concentration of outer membrane proteins, OmpA and OmpC/F, from EVs. (D) Distribution of EV diameters measured by dynamic light scattering. (E) Vesicle DNA content per pg of vesicle protein quantified by qPCR. P-value for all paired plasmid comparisons <0.001. Error bars signify standard deviation.
We characterized EVs isolated from cells carrying each of the three plasmids. The production of EVs was quantified, similar to previously used methods80, 81, by SDS-PAGE stained with Coomassie blue to measure outer membrane protein concentration (Figs 1C and S2). The total amount of EVs produced was similar for hosts containing each of the plasmids, vesicles contained roughly 0.05 mg of membrane protein. See Supplementary Materials for calculations to relate membrane protein content to vesicle number. Vesicle yield measured by protein concentration was confirmed using the BioRad Bradford assay (Fig. S3). Total protein was slightly higher when measured by Bradford which is likely caused by cellular debris proteins. We also compared vesicle production between plasmids using fluorescent lipophilic dye FM4–64 to measure relative EV production confirming nearly identical vesicle production by all three plasmids (Fig. S4). The size distribution of the vesicles was measured using dynamic light scattering23, 30, 81. Vesicle diameters were between ~100–300 nm for all three plasmids. Although vesicles harvested from hosts containing pZS2501 having a wider size distribution, each have average diameters of roughly 0.2 µm (Fig. 1D). DNA loading into vesicles was measured using quantitative PCR (Fig. 1E). Vesicles were lysed by boiling, and the amount of DNA in 0.001 μg of vesicles was quantified using primers designed around a ~200 bp region of each plasmid origin, see Table S3. Standard curves made using mini-prepped purified plasmids were used to calculate plasmid copy numbers (Fig. S5). The low copy number plasmid pZS2501 had the lowest loading density, with only 0.49 × 103 copies per pg of vesicle protein. pLC291 and pUC19 had 2.58 × 103 and 482.68 × 103 copies of plasmid per pg of vesicle protein, respectively. Taken together, our findings demonstrate that E. coli is able to load multiple types of plasmids into EVs and suggest that plasmid identity has an effect on vesicle size and gene loading.
Vesicle-mediated gene transfer is affected by plasmid identity
We next investigate the role of plasmid type on gene transfer rates. We isolated EVs from E. coli containing one of three plasmids, pLC291, pUC19 or pZS2501, as described above. Based on established protocols to measure gene transfer rate by transformation and transduction7, 25, we grew E. coli recipient cells to early log phase, OD600 0.2, and added 0.01 mg purified vesicles containing plasmid. Over time, 200 µL aliquots of the culture were transferred to LB agar with antibiotic to detect the transfer of the plasmid resistance marker to the recipient strain, enabling detection of resistant cells at densities above ~5 CFU/mL. As shown in Fig. 2A, after several hours antibiotic resistance was detected in the recipient strain. The inset to Fig. 2A shows that neither the addition of free DNA to the recipient culture nor purified vesicles from E. coli cultures without plasmids resulted in gain of antibiotic resistance (measurements taken for 60 h), demonstrating the essential role of EVs in gene transfer. Vesicle solutions were also treated with DNase I to remove genetic material outside of EVs. Resistant colonies were verified to have acquired plasmid DNA by re-streaking and running diagnostic colony PCR. We identify time to transfer based on the first appearance of antibiotic resistant colonies (Fig. 2B). Plasmid pLC291 transfers the fastest, after 4.5 hours, followed by pUC19 after 5.7 hours and pZS2501 after 8 hours. Transfer rates were not significantly (p = 0.62) influenced by choice of resistance marker, see Fig. S6. To address possible effects of plasmid size to transfer rates, we constructed plasmids with the same 3500 bp size varying only the origin of replication (Fig. S7). This data confirms the effects on transfer rates are dominated by plasmid identity regardless of plasmid size.
EVs facilitate HGT of multiple plasmids at a rate dependent on plasmid origin. (A) Purified EVs loaded with pLC291 were added to a recipient strain culture. Over time, the gain of antibiotic resistance was monitored by selective plating. The three curves show replicate experiments. The transfer time is the average time point at which resistant colonies were first observed. Inset shows recipient cells receiving either free plasmids (fp) or vesicles harvested from cells not containing the plasmid (p-) did not gain resistance by t = 60 h, whereas vesicle-mediated transfer (vm) occurred at 3.7 h. (B) Transfer time for EVs containing the plasmids pLC291, pUC19, and pZS2501. All pairs of transfer times have p-values < 0.05. (C) Gene transfer assay for three doses of vesicles containing pLC291. 1X corresponds to EV solution with 0.01 mg of characteristic outer-membrane protein (20% of total harvested). (D) Transfer rates for each plasmid were normalized by the number of plasmids per vesicle. Transfer rates have p-values < 0.001. Error bars signify standard deviation.
We next show, using EVs packed with pLC291, that the transfer time is proportional to the number of vesicles added at the beginning of the gene transfer measurement. We use E. coli EVs packed with pLC291 as our donor at 0.1 mg, 0.01 mg, and 0.001 mg concentrations into E. coli recipient cells and demonstrate that the transfer of pLC291 via EVs is dose dependent (Fig. 2C).
Even vesicle dosages of 0.001 mg generated measureable gene transfer within 12 h. As detailed in the Supplementary Materials, we estimate that vesicles containing 0.01 mg of Omp proteins contain 1.3 × 1010 individual vesicles. These calculations take into account the typical density of Omp proteins in the outer membrane, 6 × 10−9 µg OmpA/µm2 of membrane82, and the size of the vesicle. Stationary phase cultures of the donor contained 0.4 vesicles/cell. Vesicle uptake experiments were conducted at 0.8 vesicles/cell, similar to vesicle dosages found in stationary phase cultures. Figure 2C shows that transfer time for vesicles containing pLC291 were about 1.5 hours slower when vesicle dosage was reduced by a factor of 10. If this scaling holds for both reduction in vesicle number and recipient cell number, we estimate that a single vesicle transfer event would occur every 7.3 hours for 1 mL of stationary phase culture.
In Fig. 2D, we separate the effects of plasmid identity from plasmid copy number by normalizing rate of vesicle-mediated transfer by plasmid copy numbers reported in Fig. 1E. The transfer rate is defined as the inverse of the transfer time. Accounting for differences in plasmid loading, pZS2501 transfers the fastest followed by pLC291 and then pUC19, despite pUC19 having the highest plasmid copy number and plasmids per vesicle. Vesicle-mediated gene transfer rates are influenced by both the EV concentration and characteristics of the DNA cargo, including the plasmid origin as shown here.
Plasmid packaging did not depend on the donor strain
We next investigate the potential for EVs being released from different bacterial species to load plasmid DNA. Three bacterial species, Aeromonas veronii, Enterobacter cloacae and Escherichia coli are used as EV donors. The first two species, A. veronii and E. cloacae, are wild isolates while E. coli is the laboratory strain used in Figs 1 and 2. To look at EVs production and plasmid loading, broad-host range plasmid pLC291 with a kanamycin resistance selection marker was transformed into donor strains by electroporation. Extracellular vesicles from all three species carrying pLC291 were isolated from late-stationary phase liquid cultures as described above. The amount of outer membrane proteins in the harvested vesicles were quantified using SDS-PAGE (Fig. 3A). EVs are produced at relatively similar concentrations as conferred by membrane protein concentration. Each species produced about 0.05 mg of vesicle protein. The most abundant membrane proteins are seen at about 30, 60, and 45 kDa for A. veronii, E. cloacae and E. coli respectively83, 84. PCR targeting a 300 bp region of the plasmid was used to verify pLC291 loading into vesicles purified from all three species, (Fig. 3B). All three species showed a similar distribution of EVs diameters, (Fig. 3C). As in Fig. 1D, qPCR was used to quantify plasmid copy number per 1 pg of outer-membrane protein in purified donor vesicles. All three species package pLC291 into their EVs at similar concentrations ranging from 1350–2580 plasmids per pg of outer membrane protein (Fig. 3D).
Variation of EVs and plasmid packaging is less dependent on donor species. (A) The production of EVs from A. veronii (Av), E. cloacae (Ecl), and E. coli (Eco), are measured using SDS-PAGE to quantify protein concentration. EV production is similar across species. (B) Packaging of pLC291 is confirmed using PCR amplification from purified vesicles. (C) Size distributions of EVs measured by dynamic light scattering shows similar diameters of vesicles harvested from all three species. (D) Vesicle DNA content quantified by qPCR was used to calculate plasmid copy number per pg of vesicle protein. P-values ≤ 0.0002. Error bars signify standard deviation.
Rates of vesicle-mediated gene transfer are dependent on species but not relatedness
We next examine the potential for EVs to facilitate interspecies gene transfer. Interspecies gene transfer relies on the capacity of recipient species to take-up and maintain horizontally acquired DNA. Recipient cells actively try to degrade foreign DNA using restriction enzymes, and plasmid expression and replication are additional barriers of gene transfer22. These barriers of plasmid maintenance upon introduction to the recipient species contribute to the dependence of HGT on the relatedness of the donor and recipient species17, 85. To focus on how relatedness influences the uptake of EVs containing plasmids, for interspecies transfer experiments we use plasmid pLC291 with broad-host range origin RK2 which is part of the IncP-1 plasmid group from E. coli. The original RK2 plasmid was isolated from Klebsiella aerogenes infections of burn patients76, and this origin was later developed for use in Methylobacterium extorquens 75. In control experiments all recipient strains were capable of maintaining the plasmid. All recipient strains gained resistance within 20 min of electroporation (Fig. S8), demonstrating the time needed to express resistance genes did not strongly influence the measured differences in vesicle-mediated gene transfer times.
Vesicles were isolated from A. veronii, E. cloacae and E. coli containing the plasmid pLC291. We selected 5 recipient species that range in their relatedness based on 16S rRNA sequencing (Fig. 4A). Other studies of HGT mechanisms have shown transfer rates to by highly affected by relatedness of bacterial species25, 85. Therefore, to investigate whether EV-mediated gene transfer is influenced by relatedness, donor vesicles from three strains were added to cultures of the 5 recipient species, A. veronii, E. cloacae, E. coli, Chromobacterium violaceum and Pseudomonas aeruginosa. The time to plasmid transfer was measured for each donor/recipient pair, revealing strong species dependence on gene transfer (Fig. 4B–D). A. veronii transfers pLC291 via EVs in less time than the other donor strains. There is also significant variability in recipient acquisition of EV carrying plasmids. P. aeruginosa gains antibiotic resistance via plasmid uptake in the shortest time regardless of the donor species. The data also suggests that A. veronii acquires pLC291 from EVs on a slightly faster time scale than the other three recipients, C. violaceum, E. cloacae and E. coli.
Donor and recipient species influence rates of vesicle mediated gene transfer. (A) Relatedness of recipient species based on 16S rRNA sequence. Donor EVs packed with pLC291 from donor strains A. veronii (B), E. cloacae (C) and E. coli (D) were added to recipients strains A. veronii, C. violaceum, E. cloacae, E. coli, and P. aeruginosa. The time to plasmid transfer was measured by selective plating as in Fig. 2. (E) The transfer rate was not correlated with the relatedness of the donor and recipient strains, as measured by divergence of the 16S rRNA sequence. Error bars signify standard deviation from 4 replicates.
To quantify if gene transfer times correlate with the relatedness of the donor and recipient species, the time to transfer was plotted against the 16S rRNA divergence, as shown in Fig. 4E, revealing no clear trend in relatedness and transfer time. Our findings indicate that certain species of bacteria produce DNA packed vesicles that are more readily transferred than others and that specific species have a greater capacity to uptake DNA in vesicles.
Discussion
Horizontal gene transfer enables microbes to rapidly evolve and adapt to complex and constantly changing environments. Large genetic modifications attributed to ubiquitous gene transfer have been found to be widespread across populations of bacteria demonstrating its importance and pervasive nature. Three well-studied, canonical mechanisms, transformation, transduction, and conjugation, contribute significantly to the genetic diversity that is shared between species. Yet, the level and extent to which different HGT events shape microbial genomes is still being studied12, 22. It is probable that other mechanisms contribute to the general abundance of gene exchange, given known barriers to gene exchange in these textbook exchange pathways20. In this study, we look at the role of a recently discovered mechanism for HGT, vesicle-mediated transfer, and its capacity to facilitate intra- and interspecies gene exchange48,49,50, 62. Membrane vesicles have been previously reported to carry DNA and have been demonstrated to mediate HGT within species and between one pair of species57, 70, 73. Here we examine the transfer of multiple types of plasmids between several species of Gram-negative bacteria, finding that plasmid type influences both DNA packaging and transfer rates, and that gene transfer rate depends on the identity of the donor and recipient species.
EVs from E. coli are capable of packaging several non-specialized plasmids. Unlike conjugation, which requires specific origins of transfer and the conjugation machinery conferred on the plasmid33, we demonstrate plasmids with three different origins encoding no genes or sequences specialized for gene transfer were successfully loaded into harvested EVs. The ability to package a range of plasmids suggests the capacity for vesicle-mediated gene transfer to widely contribute to gene transfer within populations, although not all plasmids transferred at the same rate.
The efficiency of loading of plasmids into vesicles was strongly dependent on plasmid identity. pUC19 had 2–3 orders of magnitude more plasmid per vesicle than both pZS2501 and pLC291. We use Fig. S5 to we estimate the loading percentages of each plasmid type into vesicles, and find an average of 3.62, 0.18, and 0.04 plasmids loaded per vesicle for pUC19, pLC291, and pZS2501 respectively. Estimated loading percentages follow the ranking of plasmid copy number, the copy number of pUC19 is twice that of pLC291 and almost 10 times that of pZS2501, see Table S1, however plasmid loading was not directly proportional to copy number. Characteristics such as plasmid size, protein binding, or the location of each plasmid type within the cell may influence vesicle loading. Recent work has demonstrated complex systems for plasmid organization within cells that are influenced by cell cycle and growth rate85, 86. Studies in extracellular vesicle production in a range of bacterial species have also demonstrated the influence of cell cycle on vesicle production87. More work is needed to understand the rules for plasmid packaging into vesicles. It remains an open question whether or not plasmid packaging is a random process, or conversely if plasmids or bacteria evolved mechanisms for increased packaging efficiencies.
Plasmid features also play a role in the rate of gene transfer (Fig. 2B and C). Surprisingly when adjusted for plasmid loading, the transfer rate of plasmids containing pUC19 was more than 100 times slower than the other plasmids. It is unclear how genetic cargo would impact transfer rates. Potentially, genetic cargo modulates vesicle properties related to interactions within donor strains and can also affect recipient uptake. One complication in interpreting transfer rates is that the distribution of plasmid copy numbers within the vesicles is unknown. Although we estimate an average loading of 3.6 plasmids per vesicle in the case of pUC19 compared to 0.04 plasmids per vesicle with pZS2501, some vesicles might be empty and others might contain many plasmids. It is possible the plasmid origin plays a role in transfer times due to the dependence of transfer on both plasmid acquisition and maintenance, however, all three plasmids were stably maintained with the E. coli host. Also the selection marker did not significantly influence transfer times, see Fig. S6. Although the biological significance is yet to be defined, our results point to vesicle mediated horizontal gene transfer to be dependent on genetic content.
To what extent vesicle-mediated gene transfer contributes to gene exchange in wild populations is still unresolved, although these results suggest that the rate of gene transfer via vesicles is not prohibitively slow. Stationary phase cultures reached ratios of nearly one vesicle for every cell, or 0.25 μg vesicle protein per mL of culture. At these ratios the time to a gene transfer event should be on the order of hours for 1 mL of culture (see Supplementary information for details). It should be noted that vesicles harvested from cells in other growth phases may have different properties and dynamics88, 89. The details of vesicle biogenesis are still an active area of research61, 90, 91. The time needed for gene transfer determines the number of recipient cells within a population, predicted to be a critical factor in the fixation of horizontally transfer genes92. The selection coefficient of transferred genes also plays a critical role, especially when gene transfer events are rare92. Understanding the cellular characteristics that set both selection criteria and transfer rates of a given gene will help predict the impact of vesicle-mediated gene transfer on evolutionary dynamics.
As a gene transfer mechanisms, vesicle mediated transfer is surprisingly similar to transduction in many aspects. Harvested P1 phage is estimated to contain 109 to 1010 plaque-forming units/mL93, as compared to 109 vesicles/mL. Approximately 0.1% of P1 phage particles contain transducing regions94,95,96,97, whereas we measured as low as 4% packing of plasmids in vesicles. To transfer a genomic region through P1 transduction typically takes several hours94, and vesicle-mediated transfer occurs over a few hours. It is not yet clear if transduction and vesicle-mediated transfer are equally prevalent mechanisms of gene transfer, and further quantitative comparisons between the rates and specificity of transduction and vesicle-mediated transport are warranted.
Vesicle-mediated transfer overcomes barriers observed in other forms of gene transfer. Fulsundar et al. recently reported the ability of EVs from A. baylyi to facilitate interspecies gene exchange to E. coli 23. In our study, we expand on this observation by extensively investigating the scope of vesicle-mediated interspecies gene exchange using three donor bacterial species and five recipient species. Plasmid identity exhibited a greater effect on DNA packaging into EVs than the species origin of vesicle production (Figs 1D and 3D). The donor and recipient species also seem to set the overall transfer rate. Some species, such as A. veronii, make vesicles that facilitate HGT better than the other two donor species tested. A similar trend is seen with the identity of the recipient species, with P. aeruginosa taking up resistance genes from all three donors strains faster than the first measurement. Although the mechanism that leads to one species being a better donor than another is unknown, specific characteristics of some species confer a greater capacity to transfer DNA via EVs. Future work to identify bacterial features that can affect the ability of particular vesicles to mediate DNA exchange more readily and efficiently would be important to better understand gene exchange patterns in the wild.
Global patterns of interspecies gene exchange have shown that relatedness strongly influences gene transfer rates. Gene maintenance and expression subsequent to transfer contribute to successful HGT and scale with relatedness17, 85. However, the initial mechanism to transport genes between cells also contribute to patterns of interspecies exchange. Transduction and conjugation depend on the relatedness of the donor and recipient species17, 85. Here we found no clear correlation between relatedness of the donor and recipient and transfer rates. The two recipients strains that were the most distantly related had the slowest and fastest uptake rates, C. violaceum and P. aeruginosa respectively. All the bacteria used in this study were proteobacteria, and future work should examine the possibility for vesicle-mediated gene exchange between more diverse species. However, given that in other contexts vesicles enable molecular exchange between very distantly related organisms including bacteria and eukaryotic hosts13, 15, 16, 18, it seems plausible that EVs strongly contribute to gene exchange patterns in natural communities, particularly exchange of non-specialized genetic material between distantly related bacteria. Vesicles also serve to protect genetic material from degradation, which should increase both the rate and spatial range of gene exchange69, 71. Alves et al. demonstrated enzymes protected in EVs maintain enzyme activity65. Quantifying the dynamics of gene transfer via vesicles in mixed populations of multiple donor and recipient strains would aid in understanding gene flow within diverse bacterial communities.
Materials and Methods
Bacterial Strains and Growth Conditions
All bacterial strains used are listed in Table S2 95,96,97. Bacteria were grown in Luria-Bertani (LB) broth (Difco, Sparks, MD) at 37 °C with shaking at 200 rpm. Antibiotics were added to liquid cultures as needed for plasmid maintenance. A. veronii, E. cloacae and E. coli were transformed by electroporation with plasmids listed in Table S1. Following transformation, A. veronii, E. cloacae and E. coli were grown on LB agar plates containing either 35 μg ml−1 chloramphenicol, 50 μg ml−1 kanamycin or 50 μg ml−1 carbenicillin.
Isolation and purification of EVs
EVs were isolated from liquid cultures of A. veronii, E. cloacae and E. coli as previously described81 with some modifications. 400 μl of overnight culture was used to inoculate 400 ml of LB broth containing selective antibiotic. Liquid cultures were grown at 37 °C with shaking at 200 rpm for 16–20 h. Cells were pelleted by centrifugation at 1,200 × g at 4 °C for 30 min. The supernatants were decanted and vacuum filtrated through ExpressPlus 0.45 μm pore-size polyethersulfone (PES) bottle top filter (Millipore, Billerica, MA) to remove remaining cells and cellular debris. Vesicles were collected from the supernatant of 400 ml of liquid culture by ultra-centrifugation at 60,000 × g (Ti 45 rotor; Beckman Instruments, Inc., Fullerton, CA) at 4 °C for 1.5–2 h. Pellets were resuspended in 15 ml of PBS followed by an additional centrifugation of the supernatant at 160,000 × g (Ti 70i rotor; Beckman Instruments, Inc., Fullerton, CA) at 4 °C for 1.5–2 h. The pelleted vesicles were resuspended in 1 ml of phosphate buffered saline (PBS) and stored at 4 °C. Vesicle preparations were treated with 100 ng ml−1 of DNase I at 37 °C for 20 min followed by deactivation of the DNase at 80 °C for 10 min. Vesicle preparations were also plated on LB agar to check for the presence of bacterial cells. At room temperature it has been reported that vesicles are stable for 1 week and over a month at −20 °C69. In our experiments vesicles were used within 1 week of harvesting.
EV protein concentration
Vesicle concentrations was quantified using SDS-Polyacrylamide gel electrophoresis. Vesicle preparations were treated with 6xSDS loading buffer and boiled for 10 min at 100 °C and run on a 10% SDS-PAGE gel (Bio-Rad Laboratories, Hercules, CA), stained for 15 min with Coomassie Brilliant Blue Stain, and destained in H2O, methanol, and acetic acid (50/40/10 v/v/v) overnight. Protein concentrations were determined using ImageJ from a standard curve generated by a BSA protein concentration gradient.
EV size characterization using Dynamic Light Scattering (DLS)
DLS was used to characterize the size of purified EVs. Purified EVs were analyzed using a Wyatt Technology DynaPro Titan (Wyatt Technology Corp., Santa Barbara, CA) equipped with a 0–50 mW laser at 830 nm as a light source. The scattered photons were detected at 90°.
Real-Time PCR
DNA concentration in purified EVs was determined using real-time PCR, Bio-Rad DNA Engine Opticon 2 System for Real-Time PCR Detection (Bio-Rad Laboratories, Hercules, CA), using SYBR Green (Thermo Fisher Scientific Inc., Waltham, MA). Briefly, the reaction consisted of 2 μL of EVs, 0.2 μM of primers, and 1 U of Phusion High-Fidelity DNA Polymerase (New England BioLabs Inc., Ipswich, MA) in a final volume of 45 μL. Each of 35 cycles was denatured at 98 °C for 10 s, annealing at 60 °C for 20 s and extension at 72 °C for 15 s.
EV-mediated gene transfer
Gene transfer experiments were modified from previously published work7, 25. The recipient strains, A. veronii, C. violaceum, E. cloacae, E. coli, and P. aeruginosa, were grown in 4 mL LB broth (Difco, Sparks, MD) at 37 °C with shaking at 200 rpm to early log phase, OD600 0.2, ~1–2 h. Then at time 0 h, 0.01 mg purified vesicles was added. Every hour, 200 μL of culture was removed and plated on LB agar plates containing either 35 μg ml−1 chloramphenicol, 50 μg ml−1 kanamycin or 50 μg ml−1 carbenicillin dependent on plasmid resistance. 100 μg ml−1 kanamycin was used for plating P. aeruginosa. After 16 h of incubation at 37 °C, plates were counted and scored for CFUs. The bacterial colonies that acquired antibiotic resistance were re-select on antibiotic selection plates and screened for the presence of the plasmid using PCR. Gain of resistance not associated with plasmid transfer was not observed.
Statistical Analysis
All two-tailed P values were obtained using unpaired t test to compare the means with standard deviations of two groups with n > 3.
References
Eisen, J. A. Horizontal gene transfer among microbial genomes: new insights from complete genome analysis. Curr. Opin. Genet. Dev. 10, 606–611 (2000).
de la Cruz, F. & Davies, J. Horizontal gene transfer and the origin of species: lessons from bacteria. Trends Microbiol. 8, 128–33 (2000).
Andam, C. P. & Gogarten, J. P. Biased gene transfer in microbial evolution. Nat. Rev. Microbiol. 9, 543–55 (2011).
Soucy, S. M., Huang, J. & Gogarten, J. P. Horizontal gene transfer: building the web of life. Nat. Rev. Genet. 16, 472–82 (2015).
Aminov, R. I. Horizontal gene exchange in environmental microbiota. Front. Microbiol. 2, 158 (2011).
Lorenz, M. G. & Wackernagel, W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 58, 563–602 (1994).
Jiang, S. C. & Paul, J. H. Gene transfer by transduction in the marine environment. Appl. Environ. Microbiol. 64, 2780–7 (1998).
Roberts, A. P. & Kreth, J. The impact of horizontal gene transfer on the adaptive ability of the human oral microbiome. Front. Cell. Infect. Microbiol. 4, 124 (2014).
Smillie, C. S. et al. Ecology drives a global network of gene exchange connecting the human microbiome. Nature 480, 241–4 (2011).
Arber, W. Horizontal Gene Transfer among Bacteria and Its Role in Biological Evolution. Life (Basel, Switzerland) 4, 217–24 (2014).
Andam, C. P., Fournier, G. P. & Gogarten, J. P. Multilevel populations and the evolution of antibiotic resistance through horizontal gene transfer. FEMS Microbiol. Rev. 35, 756–67 (2011).
Townsend, J. P., Bøhn, T. & Nielsen, K. M. Assessing the probability of detection of horizontal gene transfer events in bacterial populations. Front. Microbiol. 3, 27 (2012).
Syvanen, M. Cross-species gene transfer; implications for a new theory of evolution. J. Theor. Biol. 112, 333–43 (1985).
Heinemann, J. A. & Sprague, G. F. Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature 340, 205–9 (1989).
Sprague, G. F. Genetic exchange between kingdoms. Curr. Opin. Genet. Dev. 1, 530–3 (1991).
Genereux, D. P. & Logsdon, J. M. Much ado about bacteria-to-vertebrate lateral gene transfer. Trends Genet. 19, 191–5 (2003).
Fournier, G. P., Huang, J. & Gogarten, J. P. Horizontal gene transfer from extinct and extant lineages: biological innovation and the coral of life. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364, 2229–39 (2009).
Lacroix, B. & Citovsky, V. Transfer of DNA from Bacteria to Eukaryotes. MBio 7 (2016).
Polz, M. F., Alm, E. J. & Hanage, W. P. Horizontal gene transfer and the evolution of bacterial and archaeal population structure. Trends Genet. 29, 170–5 (2013).
Thomas, C. M. & Nielsen, K. M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 3, 711–21 (2005).
Boto, L. Horizontal gene transfer in evolution: facts and challenges. Proc. Biol. Sci. 277, 819–27 (2010).
Zaneveld, J. R., Nemergut, D. R. & Knight, R. Are all horizontal gene transfers created equal? Prospects for mechanism-based studies of HGT patterns. Microbiology 154, 1–15 (2008).
Dubnau, D. DNA uptake in bacteria. Annu. Rev. Microbiol. 53, 217–44 (1999).
Chen, I. & Dubnau, D. DNA uptake during bacterial transformation. Nat. Rev. Microbiol. 2, 241–9 (2004).
Domingues, S. et al. Natural transformation facilitates transfer of transposons, integrons and gene cassettes between bacterial species. PLoS Pathog. 8, e1002837 (2012).
Seitz, P. & Blokesch, M. Cues and regulatory pathways involved in natural competence and transformation in pathogenic and environmental Gram-negative bacteria. FEMS Microbiol. Rev. 37, 336–63 (2013).
Overballe-Petersen, S. et al. Bacterial natural transformation by highly fragmented and damaged DNA. Proc. Natl. Acad. Sci. USA 110, 19860–5 (2013).
Solomon, J. M. & Grossman, A. D. Who’s competent and when: regulation of natural genetic competence in bacteria. Trends Genet. 12, 150–5 (1996).
Claverys, J.-P. & Martin, B. Bacterial "competence" genes: signatures of active transformation, or only remnants? Trends Microbiol. 11, 161–5 (2003).
Sun, D., Zhang, Y. & Shi, Y. Advances in the molecular mechanism of natural bacterial transformation–a review. Wei Sheng Wu Xue Bao 52, 6–11 (2012).
Mell, J. C. & Redfield, R. J. Natural competence and the evolution of DNA uptake specificity. J. Bacteriol. 196, 1471–83 (2014).
Haase, J., Lurz, R., Grahn, A. M., Bamford, D. H. & Lanka, E. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J. Bacteriol. 177, 4779–91 (1995).
Llosa, M., Gomis-Rüth, F. X., Coll, M. & de la Cruz Fd, F. Bacterial conjugation: a two-step mechanism for DNA transport. Mol. Microbiol. 45, 1–8 (2002).
Lawley, T. D., Klimke, W. A., Gubbins, M. J. & Frost, L. S. F factor conjugation is a true type IV secretion system. FEMS Microbiol. Lett. 224, 1–15 (2003).
Schröder, G. & Lanka, E. The mating pair formation system of conjugative plasmids-A versatile secretion machinery for transfer of proteins and DNA. Plasmid 54, 1–25 (2005).
Gomis-Rüth, F. X. & Coll, M. Cut and move: protein machinery for DNA processing in bacterial conjugation. Curr. Opin. Struct. Biol. 16, 744–52 (2006).
Derbyshire, K. M. & Gray, T. A. Distributive Conjugal Transfer: New Insights into Horizontal Gene Transfer and Genetic Exchange in Mycobacteria. Microbiol. Spectr. 2 (2014).
Cabezón, E., Ripoll-Rozada, J., Peña, A., de la Cruz, F. & Arechaga, I. Towards an integrated model of bacterial conjugation. FEMS Microbiol. Rev. 39, 81–95 (2015).
Grohmann, E., Muth, G. & Espinosa, M. Conjugative plasmid transfer in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67, 277–301, table of contents (2003).
Harmer, C. J. & Hall, R. M. The A to Z of A/C plasmids. Plasmid 80, 63–82 (2015).
Carraro, N., Rivard, N., Ceccarelli, D., Colwell, R. R. & Burrus, V. IncA/C Conjugative Plasmids Mobilize a New Family of Multidrug Resistance Islands in Clinical Vibrio cholerae Non-O1/Non-O139 Isolates from Haiti. MBio 7 (2016).
Brüssow, H., Canchaya, C. & Hardt, W.-D. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68, 560–602, table of contents (2004).
Canchaya, C., Fournous, G., Chibani-Chennoufi, S., Dillmann, M. L. & Brüssow, H. Phage as agents of lateral gene transfer. Curr. Opin. Microbiol. 6, 417–24 (2003).
Fortier, L.-C. & Sekulovic, O. Importance of prophages to evolution and virulence of bacterial pathogens. Virulence 4, 354–65 (2013).
Stevens, R. H. Transduction-mediated horizontal gene transfer in the oral microbiome. Front. Cell. Infect. Microbiol. 5, 12 (2015).
Kurland, C. G., Canback, B. & Berg, O. G. Horizontal gene transfer: a critical view. Proc. Natl. Acad. Sci. USA 100, 9658–62 (2003).
Biller, S. J. et al. Bacterial vesicles in marine ecosystems. Science 343, 183–6 (2014).
Bishop, D. G. & Work, E. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem. J. 96, 567–76 (1965).
Das, J. & Chatterjee, S. N. Formation of protoplasts from Vibrio cholerae. Bull. Calcutta Sch. Trop. Med. 14, 130–1 (1966).
Dorward, D. W. & Garon, C. F. DNA Is Packaged within Membrane-Derived Vesicles of Gram-Negative but Not Gram-Positive Bacteria. Appl. Environ. Microbiol. 56, 1960–2 (1990).
Pérez-Cruz, C., Delgado, L., López-Iglesias, C. & Mercade, E. Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria. PLoS One 10, e0116896 (2015).
Mashburn-Warren, L., McLean, R. J. C. & Whiteley, M. Gram-negative outer membrane vesicles: beyond the cell surface. Geobiology 6, 214–9 (2008).
Brown, L., Wolf, J. M., Prados-Rosales, R. & Casadevall, A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 13, 620–30 (2015).
Li, Z., Clarke, A. J. & Beveridge, T. J. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 180, 5478–83 (1998).
Kim, J. H., Lee, J., Park, J. & Gho, Y. S. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 40, 97–104 (2015).
Schertzer, J. W. & Whiteley, M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. MBio 3, e00297-11- (2012).
Pérez-Cruz, C. et al. New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA content. Appl. Environ. Microbiol. 79, 1874–81 (2013).
Haurat, M. F., Elhenawy, W. & Feldman, M. F. Prokaryotic membrane vesicles: new insights on biogenesis and biological roles. Biol. Chem. 396, 95–109 (2015).
Hasegawa, Y., Futamata, H. & Tashiro, Y. Complexities of cell-to-cell communication through membrane vesicles: implications for selective interaction of membrane vesicles with microbial cells. Front. Microbiol. 6, 633 (2015).
Avila-Calderón, E. D. et al. Roles of bacterial membrane vesicles. Arch. Microbiol. 197, 1–10 (2015).
Kulp, A. & Kuehn, M. J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64, 163–84 (2010).
Berleman, J. & Auer, M. The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ. Microbiol. 15, 347–54 (2013).
Haurat, M. F. et al. Selective sorting of cargo proteins into bacterial membrane vesicles. J. Biol. Chem. 286, 1269–76 (2011).
Ballok, A. E., Filkins, L. M., Bomberger, J. M., Stanton, B. A. & O’Toole, G. A. Epoxide-mediated differential packaging of Cif and other virulence factors into outer membrane vesicles. J. Bacteriol. 196, 3633–42 (2014).
Alves, N. J. et al. Bacterial Nanobioreactors–Directing Enzyme Packaging into Bacterial Outer Membrane Vesicles. ACS Appl. Mater. Interfaces 7, 24963–72 (2015).
Yoon, H. Bacterial outer membrane vesicles as a delivery system for virulence regulation. J. Microbiol. Biotechnol., doi:10.4014/jmb.1604.04080 (2016).
Ho, M.-H., Chen, C.-H., Goodwin, J. S., Wang, B.-Y. & Xie, H. Functional Advantages of Porphyromonas gingivalis Vesicles. PLoS One 10, e0123448 (2015).
Olsen, I. & Amano, A. Outer membrane vesicles - offensive weapons or good Samaritans? J. Oral Microbiol. 7, 27468 (2015).
Fulsundar, S. et al. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl. Environ. Microbiol. 80, 3469–83 (2014).
Rumbo, C. et al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55, 3084–90 (2011).
Renelli, M., Matias, V., Lo, R. Y. & Beveridge, T. J. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 150, 2161–9 (2004).
Yaron, S., Kolling, G. L., Simon, L. & Matthews, K. R. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl. Environ. Microbiol. 66, 4414–20 (2000).
Grande, R. et al. Helicobacter pylori ATCC 43629/NCTC 11639 Outer Membrane Vesicles (OMVs) from Biofilm and Planktonic Phase Associated with Extracellular DNA (eDNA). Front. Microbiol. 6, 1369 (2015).
Norrander, J., Kempe, T. & Messing, J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene 26, 101–6 (1983).
Chubiz, L. M., Purswani, J., Carroll, S. M. & Marx, C. J. A novel pair of inducible expression vectors for use in Methylobacterium extorquens. BMC Res. Notes 6, 183 (2013).
Ingram, L. C., Richmond, M. H. & Sykes, R. B. Molecular characterization of the R factors implicated in the carbenicillin resistance of a sequence of Pseudomonas aeruginosa strains isolated from burns. Antimicrob. Agents Chemother. 3, 279–88 (1973).
Betlach, M. et al. A restriction endonuclease analysis of the bacterial plasmid controlling the ecoRI restriction and modification of DNA. Fed. Proc. 35, 2037–43 (1976).
Boedicker, J. Q., Garcia, H. G., Johnson, S. & Phillips, R. DNA sequence-dependent mechanics and protein-assisted bending in repressor-mediated loop formation. Phys. Biol. 10, 66005 (2013).
Silver, R. P. & Cohen, S. N. Nonchromosomal antibiotic resistance in bacteria. V. Isolation and characterization of R factor mutants exhibiting temperature-sensitive repression of fertility. J. Bacteriol. 110, 1082–8 (1972).
McBroom, A. J., Johnson, A. P., Vemulapalli, S. & Kuehn, M. J. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 188, 5385–92 (2006).
Klimentová, J. & Stulík, J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol. Res. 170, 1–9 (2015).
Neidhardt, F. C. (Frederick C. & Curtiss, R. Escherichia coli and Salmonella: cellular and molecular biology. (ASM Press, 1996).
Vázquez-Juárez, R. C., Gómez-Chiarri, M., Barrera-Saldaña, H., Hernández, N. & Ascencio, F. The major Aeromonas veronii outer membrane protein: gene cloning and sequence analysis. Curr. Microbiol. 51, 372–8 (2005).
Souza, V., Rocha, M., Valera, A. & Eguiarte, L. E. Genetic structure of natural populations of Escherichia coli in wild hosts on different continents. Appl. Environ. Microbiol. 65, 3373–85 (1999).
Andam, C. P. & Gogarten, J. P. Biased gene transfer and its implications for the concept of lineage. Biol. Direct 6, 47 (2011).
Pogliano, J. Dynamic cellular location of bacterial plasmids. Curr. Opin. Microbiol. 5, 586–90 (2002).
Fulsundar, S. et al. Molecular characterization of outer membrane vesicles released from Acinetobacter radioresistens and their potential roles in pathogenesis. Microb. Pathog. 83–84, 12–22.
McCaig, W. D., Koller, A. & Thanassi, D. G. Production of outer membrane vesicles and outer membrane tubes by Francisella novicida. J. Bacteriol. 195, 1120–32 (2013).
Schwechheimer, C., Kulp, A. & Kuehn, M. J. Modulation of bacterial outer membrane vesicle production by envelope structure and content. BMC Microbiol. 14, 324 (2014).
Schwechheimer, C. & Kuehn, M. J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–19 (2015).
Turnbull, L. et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 7, 11220 (2016).
Pettersen, A.-K. et al. Modeling suggests frequency estimates are not informative for predicting the long-term effect of horizontal gene transfer in bacteria. Environ. Biosafety Res. 4, 223–233 (2005).
Battaglioli, E. J. et al. Isolation of generalized transducing bacteriophages for uropathogenic strains of Escherichia coli. Appl. Environ. Microbiol. 77, 6630–5 (2011).
Thomason, L. C., Costantino, N. & Court, D. L. In Current Protocols in Molecular Biology Chapter 1, 1.17.1–1.17.8 (John Wiley & Sons, Inc., 2007).
Boedicker, J. Q., Garcia, H. G. & Phillips, R. Theoretical and Experimental Dissection of DNA Loop-Mediated Repression. Phys. Rev. Lett. 110, 18101 (2013).
Kreamer, N. N., Phillips, R., Newman, D. K. & Boedicker, J. Q. Predicting the impact of promoter variability on regulatory outputs. Sci. Rep. 5, 18238 (2015).
Guo, X. & Boedicker, J. Q. The Contribution of High-Order Metabolic Interactions to the Global Activity of a Four-Species Microbial Community. PLoS Comput. Biol. 12, e1005079 (2016).
Acknowledgements
The authors would like to thank Vadim Cherezov and Ming-Yue Lee for experimental assistance and well as Steven Finkel for helpful discussions.
Author information
Authors and Affiliations
Contributions
F.T. and J.B. conceived and designed the study, F.T. conducted the experiments and analyzed the results, F.T. wrote the manuscript that was edited by J.B.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tran, F., Boedicker, J.Q. Genetic cargo and bacterial species set the rate of vesicle-mediated horizontal gene transfer. Sci Rep 7, 8813 (2017). https://doi.org/10.1038/s41598-017-07447-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07447-7
This article is cited by
-
Composition and functions of bacterial membrane vesicles
Nature Reviews Microbiology (2023)
-
Unravelling the DNA sequences carried by Streptomyces coelicolor membrane vesicles
Scientific Reports (2022)
-
Co-expression of double-stranded RNA and viral capsid protein in the novel engineered Escherichia coli DualX-B15(DE3) strain
BMC Microbiology (2021)
-
Outer membrane vesicles mediated horizontal transfer of an aerobic denitrification gene between Escherichia coli
Biodegradation (2021)
-
iTRAQ®-based quantitative proteomics reveals the proteomic profiling of methicillin-resistant Staphylococcus aureus-derived extracellular vesicles after exposure to imipenem
Folia Microbiologica (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.