Abstract
Animals use pheromones as a conspecific chemical language to respond appropriately to environmental changes. The soil nematode Caenorhabditis elegans secretes ascaroside pheromones throughout the lifecycle, which influences entry into dauer phase in early larvae, in addition to sexual attraction and aggregation. In adult hermaphrodites, pheromone sensory signals perceived by worms usually elicit repulsion as an initial behavioral signature. However, the molecular mechanisms underlying neuronal pheromone sensory process from perception to repulsion in adult hermaphrodites remain poorly understood. Here, we show that pheromone signals perceived by GPA-3 is conveyed through glutamatergic neurotransmission in which neuronal DAF-16/FoxO plays an important modulatory role by controlling glutaminase gene expression. We further provide evidence that this modulatory role for DAF-16/FoxO seems to be conserved evolutionarily by electro-physiological study in mouse primary hippocampal neurons that are responsible for glutamatergic neurotransmission. These findings provide the basis for understanding the nematode pheromone signaling, which seems crucial for adaptation of adult hermaphrodites to changes in environmental condition for survival.
Similar content being viewed by others
Introduction
Pheromones serve as a chemical language through which organisms of the same species communicate in response to environmental changes, including the presence of stress, different sexes and food scarcity. The soil nematode Caenorhabditis elegans, one of the most genetically well-understood metazoans secretes pheromones termed daumones or ascaroside pheromones throughout the lifecycle. For instance, the nematode ascaroside pheromones have been known to signal worms to enter dauer phase, a non-aging state, under unfavorable growth conditions 1–5. In addition, these ascaroside pheromones (pheromones) are involved in diverse biological processes (e.g., sexual attraction, aggregation, and fungal traps) depending on their developmental stage (early larvae vs. adults) and sex (hermaphrodites vs. male) 6–9. Especially, it is well known that these pheromones act as a signal to the nematode that the surrounding environment is an unfavorable condition. Thus, when pheromone signals are recognized, young larvae enter the dauer phase, a non-aging state, for a long-term survival1,2,3,4. However, when adult hermaphrodites sense the pheromones, they elicit repulsion response as an initial behavioral output10. Although this repulsion serves as a signature of pheromone sensory process, molecular mechanism underlying pheromone sensory process after an initial perception remains less characterized.
In search of pheromone signaling perception, there have been a few reports on the putative pheromone receptors that are demonstrated to mediate neuronal responses to ascr#1–3 in ASK neurons11 (SRBC64/66) or receptors in ASI neurons that respond to ascr#212 (DAF-37/38). Additionally, Bargmann group reported the identification of srg-36/37 genes encode G-protein-coupled receptors for ascr#5 (C3)13. Despite decades-long research on pheromone function, its perception, the molecular pathway in adult hermaphrodites that starts from an initial pheromone perception to elicit behavioral outputs as a repulsive response is not fully understood. Here, we show that pheromone sensory signals are likely conveyed through glutamatergic neurotransmission in which neuronal DAF-16/FoxO plays an important modulatory role.
Results and Discussion
Perception of pheromone sensory signaling by GPA-3 via insulin/IGF-1 pathway
To identify some molecular components involved in perception of pheromones, we screened G-protein subunit genes by assessing the chemotaxis index of C. elegans that had been exposed to three major ascaroside pheromones (daumones 1–3 or ascr#1–3) as a measure of perception of pheromones8 (Fig. S1a). These three pheromones have been known as most abundant and highly active on pheromone functions among those identified so far3, 5, 14, 15. In our experiment, we used both plate-based assays and drop assays in our study. For instance, the plate-based chemotaxis assay10 was used to determine the stationary response to the aversive chemicals at the period of certain times (duration), whereas the drop assay was used especially when the rescuing transgenic animals were not stable lines16. Transmission rate of the extrachromosomal arrays in transgenic worms are variable between lines. Some siblings from transgenic worms tend to lose transgene. Thus transgenic animals, which only showed myo-3P::dsRed marker (proof of carrying transgene) were individually picked and tested for the drop assay. However, these two assays produced essentially the same results against all three pheromones.
When we determined net movement rather than individual real-time movements of worms across 1 h (Fig. S1a), N2 wild type worms showed a dose- and time-dependent repulsion response to all three individual pheromones (Fig. S1b,c), to which late larvae (L4) and young adult worms responding more strongly than early larvae (L1) (Fig. S1d). Notably, all three pheromones elicited a similar pattern and intensity of repulsion responses. Therefore, we used a single pheromone rather than blend of their combination for the plate-based chemotaxis assay in most cases. It appears that they share the common repulsion behavior in response to any of three pheromones. Because the daumone 1 (ascr#1) induced the repulsion of hermaphrodites as well as induction of dauers and fungal traps3, 9, 14, we used mostly daumone 1 in chemotaxis screening of G-protein subunit mutant strains and related experiments.
As the initial pheromone sensory process relies on G-proteins (e.g., GPA-2 and 3)17, we further sought to define the specific Gα subunit that primarily transfers the initial detection signals of the three major pheromones. Of the 17 Gα subunits examined, only gpa-3 mutant worms showed defective repulsion responses to all three pheromones (Figs S1e and S2a), suggesting that gpa-3 is the major G-protein subunit gene that is involved in the perception of these pheromones. Previously, we reported that gpa-3 negatively controls both insulin/IGF-1 signaling (IIS) and TGF-beta signaling18. Thus, to identify the downstream cell signaling pathway involved in GPA-3 signaling, we assessed repulsion responses in worms with mutations in downstream effectors of IIS or TGF-β pathways. Interestingly, daf-16/FoxO mutant worms showed reduced repulsion responses to all three pheromones (Fig. S2b), whereas daf-3/Smad and daf-5/SnoSki mutant worms showed similar repulsion responses as wild-type worms (Fig. S2c). These results suggest that at least part of the IIS pathway, but not the TGF-β pathway, participates in pheromone sensory signaling in hermaphrodites (Fig. S2b,c). It also indicated that DAF-16/FoxO may play an important role in pheromone sensory signaling process.
Role of neuronal DAF-16/FoxO in glutamatergic neurotransmission of pheromone sensory signals
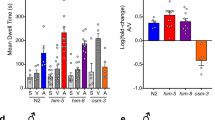
Since DAF-2 normally suppresses nuclear localization of DAF-16/FoxO19, we examined whether daf-16/FoxO and/or its isoforms participate in the pheromone sensory process. To this end, daf-2(e1370);daf-16/FoxO (mgDf50) double mutants were subjected to rescue experiment by microinjection of those constructs containing each daf-16/FoxO isoform20. All isoforms examined rescued the repulsion responses of daf-16/FoxO mutants, suggesting their common roles in conveying pheromone sensory signals (Fig. 1a). Notably, the rescue effect by the daf-16b/FoxO isoform20 suggests that neuronal DAF-16/FoxO might play an important role (activation or suppression) in conveying pheromone signals. To test whether the tissue specificity of DAF-16/FoxO is important in conveying pheromone signals, we performed rescue experiments using ges-1 promoter-driven intestine-specific daf-16/FoxO and unc-119 promoter-driven pan-neuronal daf-16/FoxO. Whereas intestine-specific DAF-16/FoxO did not rescue repulsion responses, neuron-specific DAF-16/FoxO recovered the repulsion responses of daf-16/FoxO mutants (Fig. 1b), indicating a tissue-specific (neuronal) expression of DAF-16/FoxO seems critical in conveying pheromone sensory signals in head neurons.
Glutamate signaling mediates pheromone sensory signals to produce repulsion response through the insulin/IGF-1 signaling. (a) Rescuing the daf-2(e1370);daf-16/FoxO (mgDf50) phenotype with different daf-16/FoxO isoforms (wild type, n = 145; daf-2;daf-16/FoxO, n = 196; daf-2;daf-16/FoxO;DAF-16/FoxOa, n = 146; daf-2;daf-16/FoxO;DAF-16/FoxOdf, n = 218; daf-2;daf-16;DAF-16/FoxOb, n = 133). (b) Tissue-specific rescue of daf-2;daf-16/FoxO phenotype (wild type, n = 149; daf-2;daf-16/FoxO, n = 225; daf-2;daf-16/FoxO;ges-1P::DAF-16/FoxO, n = 150; daf-2;daf-16/FoxO;unc-119P::DAF-16/FoxO, n = 142). DAF-16/FoxO cDNA was expressed under control of intestine- (ges-1 P) or pan-neuronal-specific (unc-119 P) promoters. (c) eat-4(ky5) mutants deficient in glutamate transporter showed defective repulsion responses. (d) Genetic epistasis between daf-2 and eat-4 mutants (wild type, n = 58; eat-4, n = 47; daf-2, n = 68; daf-2 eat-4, n = 62). (e) DAF-16/FoxO in glutamatergic neurons rescued daf-2;daf-16/FoxO repulsion responses (wild type, n = 79; daf-2;daf-16/FoxO, n = 94; daf-2;daf-16/FoxO; eat-4P::daf-16/FoxO, n = 80). DAF-16/FoxO cDNA was expressed under control of the eat-4 promoter. *P<0.05, ns: not significant compared to wild type. **P<0.05 compared to daf-2;daf-16/FoxO. Significance was determined using two-tailed, unpaired t-tests. In these experiments, daumone 1 (1 μM) was singly used in isolation.
To identify neurotransmitters required for the transmission of pheromone sensory signals and to define the role of neuronal daf-16/FoxO, we tested several strains with mutations of genes involved in neurotransmitter signaling for their elicitation of repulsive behaviors. They are; eat-4 (glutamate transporter), tph-1 (serotonin biosynthesis), cat-2 (dopamine biosynthesis), egl-3 (pro-neuropeptide processing), cha-1 (acetylcholine biosynthesis), and unc-49 GABA receptor. Of these, only eat-4(ky5) mutants showed defective repulsion responses (Fig. 1c), suggesting that neuronal pheromone signals may share (or converted to) glutamate signals to elicit repulsion responses. When we repeated this experiment with another allele of eat-4 (ad819) mutant, the results remained essentially the same (data not shown) (data in Fig. 1c).
We next assessed the genetic epistatic relationship between glutamate and daf-2 IIS by comparing single daf-2 or eat-4 mutants with daf-2(e1370) eat-4(ky5) double mutants. Whereas daf-2 mutants, in which DAF-16/FoxO is highly activated, showed strong repulsion responses similar to those of wild-type worms, daf-2 eat-4 double mutants showed defective repulsion responses similar to those of eat-4 single mutants (Fig. 1d), suggesting that eat-4- mediated glutamatergic signaling may occur downstream of daf-2 activity. However, we cannot exclude the possibility that daf-2 signaling could be in parallel with glutamatergic signaling by modulating other related metabolic pathways. Next, to test whether daf-16/FoxO functions in glutamatergic cell autonomously or not, we examined if daf-16/FoxO expression in eat-4-expressing neurons is enough for the recovery of repulsion behavior in daf-16 mutants by generating transgenic worms in which daf-16 is expressed under control of the eat-4 promoter in daf-2(e1370);daf-16(mgDf50) mutants. Interestingly, eat-4 promoter-driven daf-16/FoxO rescued the daf-16/FoxO mutant phenotype (Fig. 1e), providing evidence of a cell-autonomous function of DAF-16/FoxO in controlling the glutamatergic neurotransmission central to pheromone sensory signaling. In addition, when we examined whether DAF-16 influences eat-4 expression, we found that the transcript levels of eat-4 remained unchanged in daf-16 mutants (Fig. S2d). We also found that the reduced daf-16/FoxO response in daf-16(mgDf50) single mutant was comparable to that of daf-2; daf-16/FoxO double mutants (Fig. S3). Taken together, our data suggests that daf-2 signaling maybe genetically upstream of glutamatergic signaling not by regulating expression of eat-4 expression level but presumably by altering another components in glutamate signaling pathway.
Neuronal DAF-16/FoxO controls glutaminase gene expression
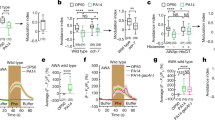
We next addressed a question as to what would be the potential role of neuronal DAF-16/FoxO in conveying the pheromone sensory signals through the glutamate neurotransmission to elicit repulsion behavior. The levels of neuronal glutamate are tightly regulated by glutaminase activity in conjunction with energy metabolism in astrocytes of mammalian brain21. In fact, mammalian brain is a high-energy demand organ and glucose is the primary source of energy. The C. elegans genome contains three glutaminase genes: glna-1, glna-2, and glna-3. To test whether DAF-16/FoxO modulates glutaminase gene expression thereby conveying pheromone sensory signals, we examined the relative expression of these genes and found that only glna-3 expression was reduced in daf-16/FoxO mutants (Fig. 2a), which was also supported by RNAi knockdown results (Fig. 2b). We also found that the expression of DAF-16/FoxO in glna-3-expressing neurons rescued the repulsion responses of daf-2 (e1370); daf-16/FoxO (mgDf50) mutants (Fig. 2c). And the reduced response of daf-16 mutant was fully rescued by overexpression of glna-3(glna-3P::glna-3), which suggests that glna-3 acts downstream of daf-16 to regulate the pheromone response (Fig. 2d, Fig. S7a). In this rescue experiment, independent transgenic lines were also tested and they all showed essentially the same results (Fig. S7a). Taken together, these results strengthen the notion that glutamatergic neuronal activity responsible for conveying pheromone sensory signals to elicit repulsion behavior appears to be regulated at the level of glna-3 expression by neuronal DAF-16/FoxO. To corroborate the involvement of glutamate receptors in conveying pheromone sensory signals, we next tested worms with mutation of mgl-1, a homolog of the human type II metabotropic receptor GRM3, which is predicted to locate at the presynaptic glutamate neuron that inhibits glutamate release22. As expected, mgl-1(tm1811) mutants elicited a stronger repulsion response than wild-type worms (Fig. S4), perhaps due to enhanced presynaptic glutamate release, indicating the potential role of MGL-1 as a gate for pheromone-elicited glutamatergic repulsion responses. However, it remains to be determined whether additional downstream components of glutamate signaling (e.g., glr-1, mgl-2, and nmr-2) in post-synaptic neurons contribute to repulsion responses. Because the mgl-1(tm1811) strain exhibited a hypersensitive phenotype at a lower concentration of pheromone (i.e., >1.0 nM), we normalized the repulsion responses of mgl-1 mutants to those of wild-type worms.
DAF-16/FoxO regulates glna-3 expression. (a) Glutaminase gene transcript levels in daf-16/FoxO mutants. Bars represent the mean of three independent biological replicates. *P<0.05, ns: not significant compared to wild type. (b) RNAi against glna-3 in a neuronal RNAi-sensitive strain (unc-119P::sid-1). F1 animals were hatched and grown on control or glna-3 RNAi plates. F1 young adults were transferred to new RNAi plates and allowed to lay eggs, and F2 young adults were tested (Ctrl RNAi, n = 148; glna-3 RNAi, n = 163). *P<0.05 compared to Ctrl RNAi (c) DAF-16/FoxO in glna-3-expressing neurons rescued the daf-2;daf-16/FoxO phenotype (wild type, n = 88; daf-2;daf-16/FoxO, n = 101; daf-2;daf-16/FoxO; glna-3P::DAF-16/FoxO, n = 82). DAF-16/FoxO cDNA was expressed under control of the glna-3 promoter. *P<0.05 compared to wild type, **P<0.05 compared to daf-2;daf-16/FoxO. (d) glna-3P::glna-3 rescued daf-16/FoxO mutant phenotype (wild type, n = 150; daf-16/FoxO, n = 149; daf-16/FoxO;glna-3P::glna-3, n = 150). *:daf-16(-) vs daf-16(-); glna-3P::daf-16. Bars represent the mean of three independent biological replicates. Significance was determined using two-tailed, unpaired t-tests.
Cellular and transcriptional expression of glna-3
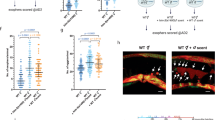
With respect to glna-3-expressing neurons, we observed that glna-3P::gfp expression, driven by a 1422-bp segment in the 5′ upstream region of the glna-3 gene, was localized in head neurons (Fig. 3a,b). Specifically, glna-3P::gfp expression and a dye filling assay, which stains chemosensory amphid neurons, showed that glna-3 is expressed in AWB neurons (Fig. 3a), consistent with previous findings that eat-4/vGlut1 is expressed in AWB neurons23. By contrast, ASI, ADL, ASK, ASH, and ASJ neurons did not express glna-3P::gfp (Fig. 3b).
Expression pattern of glna-3P:gfp and ChIP analysis of DAF-16/FoxO::GFP bound to the upstream region of glna-3 gene (a) and (b) Expression pattern of glna-3P::gfp. Worms were also stained with DiI dye to visualize chemosensory amphid neurons. (c) str-1P::glna-3 rescued daf-16/FoxO mutant phenotype (wild type, n=160; daf-16/FoxO, n=155; str-1P::glna-3; daf-16/FoxO, n=150.) *P<0.05 compared to wild type, **P<0.05 compared to daf-16/FoxO. (d) Putative DAF-16/FoxO binding sites in the 5′ upstream region and first intron of the glna-3 gene. (e) ChIP of DAF-16/FoxO::GFP with anti-GFP antibody in daf-2;DAF-16/FoxO and daf-2;DAF-16/FoxO;DAF-16/FoxO::gfp mutants. Bars represent the mean of three independent biological replicates. *P<0.05 compared to daf-2;DAF-16/FoxO. Significance was determined using two-tailed, unpaired t-tests.
However, the glna-3P::gfp reporter used in our study24 was expressed in a limited number of head neurons compared to that of previously reported23. This is presumably because our promoter construct did not include the first intron sequence. At least three pairs of amphid neurons (ASH, ADL, and AWB) are required for detecting either attractants or repellents25, with the AWB neuron being required for repulsion responses to 2-nonanone26. To address whether AWB neurons are involved in transmitting dauer pheromone-mediated hermaphrodite repulsion behavior, glna-3 was specifically expressed in daf-16 mutant under the control of AWB specific str-1 promoter26 (Figs 3c, S7b). The str-1P::glna-3 partially rescued the reduced repulsion phenotype of daf-16 mutant, suggesting that AWB neuron, at least in part, plays a role in pheromone-induced repulsion response. We further tested lim-4(ky403) and ceh-37 (ok272) mutants. In lim-4 and che-37 mutants, neuronal cell fate of AWB neurons is altered, as a result, AWB neurons adopt AWC neuronal characteristics27, 28. Our study showed that the lim-4 and ceh-37 mutants conferred reduced repulsion behavior upon exogenous dauer pheromone (Fig. S5). Thus, it is likely that pheromone sensing signal that is initially perceived by GPA-3 is transmitted through AWB glutamatergic neuron where neuronal DAF-16/FoxO modulates glutaminase gene expression, resulting in elicitation of repulsion behavior. Of course, we cannot exclude the possibility that other neurons may also be involved in this process.
These results also raised additional questions: (1) What are the molecular mechanisms by which DAF-16/FoxO transcriptionally regulates glna-3 expression? (2) Similar to nematode DAF-16 /FoxO, can the corresponding mammalian mFoxO3 regulate glutamate transmission in the hippocampus, a specific expression site of mFoxO3, a close homolog of daf-16 29. To answer the first question as to the transcriptional regulation mechanism by which DAF-16/FoxO controls glna-3 expression, we examined seven predicted putative DAF-16/FoxO binding domains (BDs) located within the 5′ upstream and first intron region of the glna-3 gene (Fig. 3d) for their binding to DAF-16/FoxO. To determine whether DAF-16/FoxO could bind to these sites, we performed chromatin immunoprecipitation (ChIP) in daf-2(e1370); daf-16(mgDf50); daf-16/FoxO::gfp animals using anti-GFP antibody. ChIP assay showed that DAF-16/FoxO binding is more enriched in BD1 (upstream) and BD6/7 (intron region) of the glna-3 gene regulatory region, suggesting that neuronal DAF-16/FoxO may regulate glna-3 transcription by binding to at least these two upstream regions of the glna-3 gene in neurons (Fig. 3e). This result is also supported by a recent report that glna-3 levels were up-regulated in daf-2 mutants compared to daf-2;daf-16/FoxO double mutants30. Together, it is suggested that the DAF-16/FoxO transcription factor may modulate neuronal glutamate homeostasis by regulating glutaminase expression, which subsequently produces the repulsion behavior in response to the exogenous pheromones. However, it remains to further delineate the interactions between DAF-16/FoxO and the specific DNA sequences within the BD1 and BD6/7 of the glna-3 gene.
A conserved modulatory role of DAF-16/FoxO in glutamatergic neurotransmission
To answer the second question as to conservation of FoxO function between nematodes and mammals, we performed electrophysiological experiments in mice. Whereas C. elegans has only one FoxO transcription factor (DAF-16/FoxO), humans and mice have four FoxO transcription factors (FoxO1, 3, 4, and 6). DAF-16/FoxO shares the highest sequence homology with mammalian FoxO329, whereas the expression pattern of FoxO6 is enriched in brain tissues31. To examine similarities between mammalian FoxO (mFoxO) and nematode DAF-16/FoxO in glutamatergic transmission regulatory function that is crucial for pheromone sensory transmission, we knocked down both mFoxO3 and mFoxO6 expression in cultures of mouse primary hippocampal neurons, which are known to express both mFoxO3 and mFoxO632, by shRNA-mediated viral infection. Our experiment was also based on the earlier report that neurons in hippocampus mainly release glutamate and GABA33. After shRNA constructs were initially tested in NIH/3T3 cell lines before viral packaging, we chose two shRNA constructs for each gene (Fig. S6). Whole-cell patch recordings were obtained from primary hippocampal neurons 5 days after infection with scrambled adeno-associated virus (AAV-Scr), AAV-shFoxO3, or AAV-shFoxO6 at 10 days in vitro 33. The efficiency of viral infection was confirmed by mCherry expression as a marker of AAV vector (Fig. 4a). Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded in the presence of picrotoxin (50 μM) to exclude inhibitory postsynaptic currents (Fig. 4b). Both sEPSCs and large-amplitude burst oscillations were observed in primary hippocampal neurons, as previously reported32. The frequency of sEPSCs was reduced in AAV-shFoxO3-infected neurons compared with control, AAV-Scr-, or AAV-shFoxO6-infected neurons, whereas the frequency of sEPSCs was unchanged in AAV-shFoxO6 infected neurons (Fig. 4c). There were no differences between groups in sEPSC amplitude (Fig. 4d). AAV-shFoxO3-infected neurons also showed reduced burst oscillation frequency (Fig. 4e) and amplitude (Fig. 4f ). These results indicate that the specific knockdown of mFoxO3 suppresses glutamatergic transmission in mammalian neurons, which is consistent with our results in C. elegans. Our findings demonstrate that FoxO plays a conserved pivotal role in maintaining glutamate homeostasis in the mouse hippocampus and the head of C. elegans.
Knockdown of FoxO3 reduced sEPSCs, burst oscillations in mouse primary hippocampal neurons. (a) Primary hippocampal neurons without (control) or with infection of AAV-Scr, AAV-shFoxO3, or AAV-shFoxO6 in bright-field (top) and mCherry fluorescence (bottom) images. AAV-infected groups had increased infection rates (38.1%, AAV-Scr; 42.5%, AAV-shFoxO3; 56.7% AAV-shFoxO6). (b) Representative traces of sEPSCs and burst oscillations from primary hippocampal neurons held at −70 mV in voltage clamping mode in the presence of picrotoxin. The part of each trace in the left panel marked with an upper line is enlarged in the right panel. (c) AAV-shFoxO3 neurons (n = 8) exhibited fewer sEPSCs than control (n = 6), AAV-Scr (n = 6), or AAV-shFoxO6 (n = 6) neurons. (d) There were no differences in sEPSC amplitude. (e) and (f) Burst oscillation frequency and amplitude were reduced in AAV-shFoxO3 neurons. *P<0.05, **p<0.01, ***p<0.001.
Conclusions and perspectives
In this work, we demonstrate how information contained in pheromones is processed internally by neural circuit to yield behavioral response. Furthermore, we provide a previously unexplored basic framework for neuronal components that are likely involved in neurotransmission of pheromone signals and a potential modulatory role of neuronal DAF-16/FoxO in this process. The potential components that participate in pheromone sensory processing leading to repulsion behavior include, but are not limited to, GPA-3, EAT-4, DAF-16/FoxO, GLNA-3, and MGL-1 (Fig. 5). Because gpa-3 is not expressed in AWB neurons17, we may draw the conclusion that once pheromones are sensed in gpa-3 expressing neurons such as ASI, ADL, or ASK, their signals are conveyed to glna-3 expressing AWB neurons to elicit repulsion behaviors (Fig. 5). Moreover, this sensory process appears to be modulated by evolutionally conserved neuronal DAF-16/mFoxO3. Given that eliciting repulsion behaviors may be important for various pheromone activities1, 2, 4, our work on the identification of pheromone sensory signaling pathway may mark a major breakthrough in this field. This work could also stimulate investigations on general pheromone signaling in animals including mammals as well as its potential application to related neuronal disorders. As many important regulatory functions of FoxO across species are being explored, it may also be possible to conduct integrated studies that link neuronal FoxO-mediated pheromone sensation to neurological diseases caused by disturbances in glutamatergic neurotransmission in humans such as Alzheimer’s disease.
A proposed model of neuronal DAF-16/FoxO-mediated pheromone sensory signal transduction pathway. The pathway from pheromone perception to repulsion behavior includes at least five components: GPA-3, DAF-16/FoxO, GLNA-3, EAT4, and MGL-1. By binding to putative pheromone receptors (not shown), pheromones may stimulate GPA-3 and subsequently activate glutamatergic neurotransmission in AWB neurons, which is transcriptionally modulated by neuronal DAF-16/FoxO via GLNA-3 activation. The production of glutamate signals is likely gated by MGL-1/mGRM3.–unconfirmed relation; ─ confirmed relation.
Methods
C. elegans strains and culture
C. elegans were cultured using standard techniques34. The strains used in this work were N2 Bristol (wild-type), DAF-16/FoxO(mu86), DAF-16/FoxO(m26), DAF-16/FoxO(mgDf50); daf-2(e1370), DAF-16/FoxO(mgDf50); daf-2(e1370) unc-119(ed3); lpIs12[DAF-16/FoxOa::RFP + unc-119(+)], DAF-16/FoxO(mgDf50); daf-2(e1370) unc-119(ed3); lpIs13[DAF-16/FoxOb::CFP + unc-119(+)], DAF-16/FoxO(mgDf50); daf-2(e1370) unc-119(ed3); lpIs14[DAF-16/FoxOf::GFP + unc-119(+)], gpa-1(pk15), gpa-2(pk16), gpa-3(pk35), gpa-4(pk381), gpa-5(pk376), gpa-6(pk480), gpa-8(pk345), gpa-9(pk438), gpa-10(pk362), gpa-11(pk349), gpa-12(pk322), gpa-13(pk1270), gpa-14(pk342), gpa-15(pk477), goa-1(n1134), odr-3(n2105), eat-4(ad819), mgl-1(tm1811), tbh-1(n3247), cmk-1(ok287), osm-6(p811), cat-2(e1112), egl-3(gk238), cha-1(n2411), che-37(ok272), lim-4(ky403) and unc-49(e382), DAF-16/FoxO(mu86); ykpEx025[ glna-3P::glna-3 + myo-3P::rfp], and DAF-16/FoxO(mu86); ykpEx026[ str-1P::glna-3 + myo-3P::RFP]. Worms were grown on nematode growth media seeded with E. coli OP50 as a food source.
Transgenic worms
Rescue constructs of DAF-16/FoxO were generated by PCR fusion of the regulatory regions of unc-119 (1200 bp), eat-4 (2196 bp), or glna-3 (1422 bp) upstream of the start codon of DAF-16/FoxO::gfp amplified from the TJ356 strain. Transgenes were microinjected in the germline of daf-2; daf-16/FoxO mutants with myo-3P::dsRed as a transgene marker. NC1478, a strain harboring wdEx584[glna-3P::gfp, unc-119(+)]; unc-119(ed3), was gift from Dr. David Miller III. Rescue construct of glna-3 and AWB neuron-specific glna-3 rescue construct were also generated by PCR fusion of the 1422 bp upstream regions of glna-3 or 4000 bp upstream region of str-1 gene26 to glna-3 cDNA including 3′ UTR. Each transgenes were microinjected in the germline of daf-16(mu86) mutants with Pmyo-3::RFP as a transgene marker, to generate glna-3 rescue worms and AWB neuron-specific glna-3 rescue worms.
Ascaroside Pheromones
All ascaroside pheromones (daumones 1–3 or ascr#1–3) were chemically synthesized and characterized at our laboratory as previously described2, 5, 15. Pheromones were dissolved in absolute ethanol and prepared in a stock solution (10 mM with ethanol). A serial dilution of pheromones were diluted into in M13 buffer in Eppendorf tubes to the final concentration of pheromone for the plate-based chemotaxis assay or drop assay (see below).
Chemotaxis assay
For the plate-based chemotaxis assay, L1-synchronized worms were collected and grown to the young adult stage, washed three times with S-basal buffer to remove E. coli, and transferred to the center of a plate using aspirator tube assemblies for calibrated microcapillary pipettes (Sigma, St. Louis, MO). Chemotaxis index values were determined by counting worms that moved to different zones of the plate according to the following formula (see Fig. S1a): (A−B)/(A + B), where A is the number of worms that moved to zone A (containing pheromone [1 μM]) and B is the number of worms that moved to zone B (containing EtOH only). Unless otherwise indicated, chemotaxis index values were calculated 1 h after placement on the plate. In this assay, any anesthetizing drugs were not used. Worms that crawled up the side of the plate were not counted. To avoid errors in measurement due to reduced movement, we used strains with no motility deficits. Each data point represents 100–150 worms. Statistics were performed using GraphPad Prism 5. The drop assay for ascaroside pheromone-induced repulsion behavior was previously described16. For drop assay, worms were stage-synchronized with egg-preparation assay in prior to the assay. Twenty young adult animals (total 140–150 worms in each assay) were moved onto unseeded NGM plate (55 mm diameter) at 20 °C with the platinum wire. A serial dilution of pheromones (stock of 10 mM with ethanol) diluted into in M13 buffer in Eppendorf tubes to the final concentration of 1 μM of pheromone. Glass capillary was utilized to deliver pheromone to the head of a forward moving worm, then, scored the positive and negative responses. The repulsion behavior was monitored by putting a small drop of the ascaroside pheromone ahead of the forward moving worm and observed the two to three turns of backward movements as ‘repulsive’ and the fraction of worms ‘repulsive’ was calculated by comparing the with buffer controls. The synthetic daumone 1 used for the mgl-1 mutant behavior was a different batch of other experiments.
DiI staining and Microscopy
DiI staining was performed to visualize ciliated chemosensory neurons as described previously in Michael Koelle’s protocol (www.wormatlas.org/EMmethods/DiDiO.htm), with minor modifications. Briefly, DiI (1.1′-dilinoleyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, Molecular Probes) stock solution was prepared in 2 mg/ml concentration in dimethyl formamide, stored at −20 °C. The DiI stock solution was diluted 1:200 in M9 and 150 μl of solution was put in a glass tube, where L2 worms were transferred and DiI-stained for 2 hours at 20 °C. After staining, worms were washed with M9 and transferred to NGM plate to crawl on a bacterial lawn for 1 hour to destain. Worms were visualized by using confocal microscope LSM 700 (Carl Zeiss). Images were analyzed with Carl Zeiss Zen 2.1 (Ver. 11.0) software.
shRNA design and vector
pLKO.1-puro constructs containing scrambled (SHC002, Sigma), shFoxO3 (TRCN0000071616, Sigma), or shFoxO6 (TRCN000008777, Sigma) sequences were transfected into NIH/3T3 cells to confirm knock-down efficiency. The mouse shFoxO3 nucleotide targeted the FoxO3 sequence from 1441 to 1461 bp (5′-CGGCACCATGAATCTGAATGA-3′, NM_019740.2), and the mouse shFoxO6 nucleotide targeted the FoxO6 sequence from 830 to 850 bp (5′-CCTCGCCACTCATGTACCCAA-3′, NM_194060.1).
For AAV packaging, scrambled, shFoxO3, or shFoxO6 sequences were cloned into pAAV-U6-shRNA-CMV-mCherry vector by the site-directed mutagenesis method (Enzynomics). Each construct was synthesized using complementary primers; scrambled: 5′-AGAGATTGGTGCTCTTCATCTTGTTGTTTTTTCTCGAGTACTAGGA-3′ (sense), 5′-TGAATTGGTGCTCTTCATCTTGTTGAAACAAGGCTTTTCTCCAAG-3′ (antisense); shFoxO3: 5′-AGAGATCATTCAGATTCATGGTGCCGTTTTTTCTCGAGTACTAGGA-3′ (sense), 5′-TGAATCATTCAGATTCATGGTGCCGAAACAAGGCTTTTCTCCAAG-3′ (antisense); shFoxO6: 5′-AGAGATTGGGTACATGAGTGGCGAGGTTTTTTCTCGAGTACTAGGA-3′ (sense), 5′-TGAATTGGGTACATGAGTGGCGAGGAAACAAGGCTTTTCTCCAAG-3′ (antisense).
Primary hippocampal neuron cultures
For primary hippocampal neuron cultures, hippocampi were isolated from mice on postnatal day 0–2 and maintained in ice-cold Ca2+- and Mg2+-free Hank’s balanced salt solution (HBSS). They were then incubated with HBSS containing trypsin (0.15 mg/ml) and L-cystein (0.5 mg/ml) for 20 min at 37 °C and triturated into single cells. After centrifugation, cells were suspended in Neurobasal A medium with B-27 supplement and 2 mM glutamine and then plated on coverslips coated with poly-D-lysine (1 mg/ml) at a concentration of 5 × 105 cells/ml. Half of the medium was replaced every 4 days. Neuronal cultures were infected with AAV-Scr, AAV-shFoxO3, or AAV-shFoxO6 at 10 days in vitro and used for experiments at 15 days in vitro.
qRT-PCR analysis
Total RNA was isolated from age-synchronized young adult worms using Trizol reagent (Invitrogen) followed by clean-up with RNeasy spin columns (Qiagen, Valencia, CA). cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche) and used for qRT-PCR. All the relative expression data of worms by qRT-PCR was normalized by act-2 gene expression.
Electrophysiology
This experiment was performed as previously described35. Primary hippocampal neurons isolated from mice and cultured on coverslips were placed in a recording chamber (Warner Instrument, Hamden, CT) mounted to an upright microscope (EX51WI, Olympus, Japan) and camera (ORCA-R2, Hamamatsu, Japan). The recording chamber was perfused continually with artificial cerebrospinal fluid containing (in mM) 124 NaCl, 3 KCl, 1.3 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 2.4 CaCl2−2H2O, and 10 glucose aerated with 95% O2/5% CO2 at room temperature. Borosilicate glass capillaries (GC150F-10, Warner Instrument Corp., Hamden, CT) for fabricating patch electrodes (4 to 6 MΩ) were made using a pipet puller (P-97, Sutter Instrument, Novato, CA). Synaptic currents were measured in whole-cell configuration and amplified using Multiclamp 700B (Molecular Devices, Sunnyvale, CA). Data acquisition was performed using a Digitizer 1440 A (Molecular Devices) and Clampex 10.3 (Molecular Devices). Analysis of data was conducted using Clampfit 10.3 (Molecular Devices) and the MiniAnalysis program (Synaptosoft, Fort Lee, NJ). The intracellular pipette solution for voltage-clamp recordings contained (in mM) 130 CsCl, 10 MgCl2, 10 HEPES, 5 Mg-ATP, 5 QX-314, 0.5 Na-GTP, and 0.1 EGTA, at pH 7.3 and 282 mOsm. For measurement of bursting, membrane potential was held at −70 mV, and 50 μM picrotoxin (Sigma) was added to the bath for 5 min.
References
Golden, J. W. & Riddle, D. L. A Pheromone influences larval development in the nematode Caenorhabditis elegans. Science 218, 578–580 (1982).
Jeong, P. Y. et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 433, 541–545 (2005).
Butcher, R. A., Fujita, M., Schroeder, F. C. & Clardy, J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat. Chem Biol. 3, 420–422 (2007).
Fielnbach, N. & Antebi, A. C. elegans dauer formation and the molecular basis of plasticity. Genes and Dev. 22, 2149–2165 (2008).
Kim, K. Y. et al. Development of a Method to Quantitate Nematode Pheromone for Study of Small-Molecule Metabolism in Caenorhabditis elegans. Anal. Chem. 85, 2681–2688 (2013).
Srinivasan, J. et al. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 454, 1115–1118 (2008).
von Reuss, S. H. et al. Comparative Metabolomics Reveals Biogenesis of Ascarosides, a Modular Library of Small-Molecule Signals in C. elegans. J. Am. Chem. Soc. 134, 1817–1824 (2012).
Srinivasan, J. et al. A Modular Library of Small Molecule Signals Regulates Social Behaviors in Caenorhabditis elegans. Plos Biology 10, e1001237 (2012).
Hsueh, Y. P., Mahanti, P., Schroeder, F. C. & Sternberg, P. W. Nematode-trapping fungi eavesdrop on nematode pheromones. Curr Biol. 23, 83–86 (2013).
Macosko, E. Z. et al. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458, 1171–1175 (2009).
Kim, K. et al. Two Chemoreceptors Mediate Developmental Effects of Dauer Pheromone in C. elegans. Science 326, 994–998 (2009).
Park, D. et al. Interaction of structure-specific and promiscuous G-protein-coupled receptors mediates small-molecule signaling in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 109, 9917–9922 (2012).
McGrath, P. T. et al. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature 477, 321–325 (2011).
Joo, H.-J. et al. Caenorhabditis elegans utilizes dauer pheromone biosynthesis to dispose of toxic peroxisomal fatty acids for cellular homoeostasis. Biochem. J. 422, 61–71 (2009).
Joo, H.-J. et al. Contribution of the peroxisomal acox gene to the dynamic balance of daumone production in Caenorhabditis elegans. J. Biol. Chem. 285, 29319–29325 (2010).
Jang, H. & Bargmann, C. I. Acute behavioral responses to pheromones in C. elegans (adult behaviors: attraction, repulsion). Methods in Molecular Biology (Clifton, NJ) 1068, 285–292 (2013).
Zwaal, R. R., Mendel, J. E., Sternberg, P. W. & Plasterk, R. H. Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans Dauer-inducing pheromone. Genetics 145, 715–727 (1997).
Hahm, J.-H., Kim, S. & Paik, Y.-K. Endogenous cGMP regulates adult longevity via the insulin signaling pathway in Caenorhabditis elegans. Aging Cell 8, 473–483 (2009).
Henderson, S. T. & Johnson, T. E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 11, 1975–1980 (2001).
Kwon, E.-S., Narasimhan, D., Yen, K. & Tissenbaum, H. A. A new DAF-16/FOXO isoform regulates longevity. Nature 466, 498–502 (2010).
Belanger, M., Allaman, I. & Magistretti, P. J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Met. 14, 724–738 (2011).
Dillon, J., Hopper, N. A., Holden-Dye, L. & O’Connor, V. Molecular characterization of the metabotropic glutamate receptor family in Caenorhabditis elegans. Biochem. Soc. Trans. 34, 942–948 (2006).
Serrano-Saiz, E. et al. Modular Control of Glutamatergic Neuronal Identity in C. elegans by Distinct Homeodomain Proteins. Cell 155, 659–673 (2013).
Watson, J. D. et al. Complementary RNA amplification methods enhance microarray identification of transcripts expressed in the C. elegans nervous system. BMC genomics 9, 84 (2008).
Bargmann, C. I. Chemosensation in C. elegans. WormBook 1–29, doi:10.1895/wormbook.1.123.1 (2006).
Troemel, E. R., Kimmel, B. E. & Bargmann, C. I. Reprogramming chemotaxis responses: Sensory neurons define olfactory preferences in C. elegans. Cell 91, 161–9 (1997).
Sagasti, A., Hobert, O., Troemel, E. R., Ruvkun, G. & Bargmann, C. I. Alternative olfactory neuron fates are specified by the LIM homeobox gene lim-4. Genes Dev. 13, 1794–1806 (1999).
Lanjuin, A., VanHoven, M. K., Bargmann, C. I., Thompson, J. K. & Sengupta, P. Otx/otd homeobox genes specify distinct sensory neuron identities in C. elegans. Dev. Cell 5, 621–633 (2003).
Ogg, S. et al. The Fork head transcription factor DAF-16/FOXO transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994–999 (1997).
Kaletsky, R. et al. The C. elegans adult neuronal IIS/FOXO transcriptome reveals adult phenotype regulators. Nature 529, 92–96 (2016).
Hoekman, M. F. M., Jacobs, F. M. J., Smidt, M. P. & Burbach, J. P. H. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr. Patterns 6, 134–140 (2006).
Bacci, A., Verderio, C., Pravettoni, E. & Matteoli, M. Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurons in culture. Eur. J. Neurosci. 11, 389–397 (1999).
Kullmann D. The Hippocampus Book, Oxford University Press, 203–241 (2007).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Halder, D. et al. Combining Suppression of Stemness with Lineage-Specific Induction Leads to Conversion of Pluripotent Cells into Functional Neurons. Chem Biol. 22, 1512–1520 (2015).
Acknowledgements
We thank the Caenorhabditis Genetics Center (funded by the NIH Office of Research Infrastructure Programs (P40 OD10440)) for providing mutant worms. We thank Dr. David Miller III for the gift of glna-3P::gfp transgenic animals. We thank Dr. E.-S. Kwon for his gifts of DAF-16/FoxO isoforms and related double mutants for this work. This work was supported by the National Research Foundation of Korea to YKP (2011-0028112) and DP (2013R1A1A2009033).
Author information
Authors and Affiliations
Contributions
Y.K.P. conceived of the project; D.P., J.H.H., G.E.H., G.E.C., H.J., S.P., and S.H.K. performed experiments and analyzed the data; H.K. provided reagents; Y.K.P., J.H.H., D.P., H.J., and E.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, D., Hahm, JH., Park, S. et al. A conserved neuronal DAF-16/FoxO plays an important role in conveying pheromone signals to elicit repulsion behavior in Caenorhabditis elegans . Sci Rep 7, 7260 (2017). https://doi.org/10.1038/s41598-017-07313-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07313-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.