Abstract
Arsenic is globally infamous for inducing immunosuppression associated with prevalence of opportunistic infection in exposed population, although the mechanism remains elusive. In this study, we investigate the effect of arsenic exposure on thymocyte lineage commitment and the involvement of regulatory T cells (Treg) in arsenic-induced immunosuppression. Male Balb/c mice were exposed to 0.038, 0.38 and 3.8 ppm sodium arsenite for 7, 15 and 30 days through oral gavage. Arsenic exposure promoted CD4 lineage commitment in a dose dependent manner supported by the expression of ThPOK in thymus. Arsenic also increased splenic CD4+ T cells and promoted their differentiation into Treg cells. In parallel, arsenic exposure induced immunosuppression characterized by low cytokine secretion from splenocytes and increased susceptibility to Mycobacterium fortuitum (M. fortuitum) infection. Therefore, we linked arsenic-induced rise in Treg cells with suppressed Th1 and Th2 related cytokines, which has been reversed by inhibition of Treg cells in-vivo using wortmannin. Other parameters like body weight, kidney and liver function, histoanatomy of thymus and spleen as well as thymocyte and splenocytes viability were unaltered by arsenic exposure. Taken together our findings indicated that environmentally relevant dose of arsenic enhanced differentiation of Treg cells which in turn induce immunosuppression in experimental animals.
Similar content being viewed by others
Introduction
Arsenic is a well known environmental toxicant which became globally infamous for its toxic effect in every sphere of life. The major natural source of arsenic is ground water which disseminate in the living system by different anthropogenic activity1. More than 200 million people globally are exposed to arsenic contaminated ground water at a level more than the permissible limit (<10 ppb)2. Protection against low dose of arsenic exposure is challenging because of its omnipresence therefore, the concern of health effect is shifting towards even lower doses3. In human and various animal models, arsenic has been found to alter immune response either by severe suppression or activation which may eventually lead to increased susceptibility to pathogen or hypersensitivity disorder respectively4,5,6,7,8,9. Arsenic-induced immunosuppression leads to increased susceptibility to opportunistic infections like tuberculosis as observed in arsenic exposed areas in Chile10. Due to infectious diseases, mortality rates were found to be increased in arsenic exposed populations11.
Arsenic has been reported to impair thymic development in infants concurrent with enhanced morbidity which seems to be the outcome of possible immunosuppression12. Arsenic exposure altered the relative distribution of different T cell subpopulation (CD4+, CD8+, Th1, Th2, Th17, Treg) in exposed population as well as in-vitro arsenic exposed human PBMCs13,14,15,16,17,18. Mitogen induced T-cell proliferative response and cytokine secretion was also found to be suppressed in arsenic exposed population15, 19 and in-vitro arsenic exposed human PBMCs14, 16, 17. Similar response was observed in mouse model20,21,22.
Arsenic has been shown to suppress T cell cytokine secretion by interrupting T cell receptor signaling cascade21, 23. However, the role of Treg cells in the inhibition of T cell cytokine cannot be ignored24, 25. Interestingly, there have been no reports yet showing the involvement of arsenic-induced increased Treg cells in the suppression of T cell cytokine secretion. Therefore, we envisage looking in to the role of Treg cells in arsenic-induced suppression of T cell cytokine production.
In the present study, we examined the hypothesis that exposure to environmentally relevant dose of arsenic may increase Treg cell population which in turn alter functional status of T cells leading to immunosuppression. To test this hypothesis, we investigated the effect of environmentally relevant doses of arsenic on thymocyte differentiation into CD4+ and CD8+ lineage. Subsequently, relative distribution of CD4+ and CD8+ population in spleen was checked. Functional alteration was detected by measuring Th1 and Th2 related cytokine in splenic culture supernatant. Arsenic - induced immunosuppression was validated by pathogen challenge assay using Mycobacterium fortuitum. We have also explored the effect of arsenic exposure on Treg cells and its role in altering the T cell function. Finally, the involvement of Treg cells in arsenic-induced immunosuppression was confirmed by suppressing Treg cells using wortmannin in-vivo. To rule out the involvement of other factors; body weight, viability of thymocytes and splenocytes, blood level of AST, ALT and Urea as well as histoanatomy of thymus and spleen were checked. Here, we tried to delineate the role of Treg cells in arsenic-induced suppression of T cell cytokine secretion which leads to severe immunosuppression.
Results
Arsenic exposure enhanced CD4+ T-cell lineage in thymus
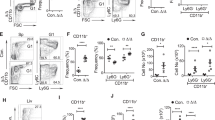
The percent distribution of CD4−CD8− (DN: Double negative), CD4+CD8+ (DP: Double positive), CD4+CD8− (CD4+) and CD4−CD8+ (CD8+) sub-population was determined following 7, 15 and 30 days of arsenic exposure. Following 7 day exposure to 0.038 and 0.38 ppm arsenic, no significant alteration in DN and DP sub-population was observed as compared to control group, while 3.8 ppm arsenic increased the percentage of DN cells from 3.9% (control) to 9.0% (p < 0.01) and decreased DP cells from 86.8% (control) to 76.2% (p < 0.01). The percentage of CD4+ T cells increased in a dose dependent manner with a significant increase from 5.5% to 8.2% (p < 0.05) and 11.1% (p < 0.01) in exposure to 0.38 and 3.8 ppm arsenic respectively for 7 days. Whereas the percentage of CD8+ T cells was decreased insignificantly in all arsenic exposed groups as compare to control group. Any significant alteration in CD4+ and CD8+ cells was not observed following 15days of arsenic exposure, however, 3.8 ppm arsenic increased the percentage of DN cells from 5.7% (control) to 9.0% (p < 0.05) while DP cells decreased from 82.6% to 73.1% (p < 0.05). T cell sub-population distribution did not show any significant alteration following 30 days arsenic exposure. In contrary 3.8 ppm of arsenic increased DN cells from 8.8 to 22.9 (p < 0.05) and CD4+ cells from 10.4 to 18.2 (p < 0.05) while decreased the DP cells significantly to 51.1 from 75.9 (p < 0.05) (Fig. 1a,b). Expression of c-kit, as a measure of influx of DN cells into thymus from bone marrow, was significantly increased by 3.8 ppm arsenic which corresponds to significant rise in DN cells in thymus following 30 day arsenic exposure (Supplementary Fig. S1). Interestingly, the rise in CD4+ cells was not due to apoptosis in CD8+ cells following 30 days exposure to arsenic (Supplementary Fig. S2). Therefore, exposure to arsenic resulted in influx of DN cells into thymus from bone marrow and enhanced commitment of DP cells towards CD4+ cell type without significant changes in CD8+ cells.
Effect of arsenic exposure on CD4+ T cell lineage in thymus. Thymocytes (0.2 × 106) isolated from mice treated with arsenic (0.00, 0.038, 0.38 and 3.8 ppm) orally for 7, 15 and 30 days were stained with PE conjugated anti-CD4 monoclonal antibody and PerCP conjugated anti-CD8 monoclonal antibody. The PE and PerCP fluorescence were measured using flow cytometer. (a) Percentage of CD4+, CD8+, DN (Double negative) and DP (Double Positive) thymocytes at 7, 15 and 30 days. (b) Each representative dot plot reflects thymocytes stained with CD4 and CD8 antibodies. Exposure to arsenic promoted commitment of double positive thymocytes into CD4+ T cells. Values are mean ± S.E. (n = 9 per group). *p < 0.05, **p < 0.01 and ***p < 0.001 compared to control.
Arsenic exposure altered the expression of ThPOK and RunX3 in thymus
ThPOK and RunX3 are master regulator of CD4 and CD8 lineage differentiation in thymus. ThPOK promotes CD4 commitment by preventing RunX3 directed CD8 lineage differentiation in DP cells26. ThPOK was found to be increased significantly following 7 and 30 days of 3.8 ppm arsenic exposure (p < 0.01) while 15 days exposure group did not show any alteration (Fig. 2a). However, the expression level of CD8+ lineage specific transcription factor RunX3 remained unaltered throughout the exposure regimen (Fig. 2b). Alteration in expression level of both transcription factors corresponds to the immunophenotyping data shown in Fig. 1.
Effect of arsenic exposure on the expression of ThPOK and RunX3 in thymus. Thymus dissected from mice treated with arsenic (0.00, 0.038, 0.38 and 3.8 ppm) orally for 7, 15 and 30 days were homogenized for protein preparation and examined for expression of ThPOK (a) and RunX3 (b) transcription factors. Representative western blots and densitometric analysis (normalized by GAPDH) reflect significant rise in ThPOK expression following 7 and 30 days of 3.8 ppm arsenic exposure. Values represented as bar graph showing mean ± SE. (n = 3 per group) *p < 0.05, **p < 0.01 and ***p < 0.001 compared to control. The original full-length western blots for ThPOK, RunX3 and corresponding GAPDH are provided in supplementary information sheet from Fig. S3 to Fig. S8.
Arsenic-induced enhanced CD4+ T cell was reflected in spleen
At 7 day, a dose dependent increase in CD4+ cells was observed being significant following 3.8 ppm exposure (20.7%; p < 0.01), however, CD8+ population was unaffected. Following 15 days of expoures, neither of CD4+ nor CD8+ population was altered. Further exposure till 30 days increased CD4+ population to 24.9% in 3.8 ppm exposure group compared to 14.0% in control group (p < 0.01), whereas an insignificant increase was observed in 0.038 and 0.38 ppm exposure group. Therefore, enhanced CD4+ commitment in thymus had also been reflected in spleen (Fig. 3a,b).
Effect of arsenic exposure on splenic CD4+ T cells. Splenocytes (0.2 × 106) isolated from mice treated with arsenic (0.00, 0.038, 0.38 and 3.8 ppm) orally for 7, 15 and 30 days were stained with PE conjugated anti-CD4 monoclonal antibody and PerCP conjugated anti-CD8 monoclonal antibody. The PE and PerCP fluorescence were measured using flow cytometer. (a) Percentage of CD4+ and CD8+ T cells in splenocytes at 7, 15 and 30 days. (b) Each representative dot plot reflects splenocytes stained CD4 and CD8 antibodies. Arsenic promoted percent distribution of CD4+ T cells in spleen. Values are mean ± S.E. (n = 9 per group). *p < 0.05, **p < 0.01 and ***p < 0.001 compared to control.
Arsenic exposure suppressed splenic cytokine profile
Th1 (IFN-γ, IL-12 and TNF-α) and Th2 (IL-4, IL-5 and IL-10) related cytokines were measured in ConA stimulated splenocyte culture supernatant. Arsenic exposure at all the doses and time points altered cytokine secretion to some extent. Level of IFN-γ was a little irregular following 7 days exposure but 15 and 30 days of arsenic exposure suppressed IFN-γ level in all doses significantly (p < 0.001 or <0.01). Almost same pattern was observed in Th1 cytokine TNF-α as well as Th2 cytokines IL-4 and IL-5. None of the doses affected another Th1 cytokine, IL-12 production significantly except 30 days exposure. Th2 cytokine, IL-10 was also suppressed in all the exposure groups and time points except 0.038 ppm dose in 7 and 15 days exposure groups (Fig. 4a,b). Therefore, subchronic exposure to arsenic suppressed Th1 and Th2 related cytokine production from ConA activated splenocytes.
Effect of arsenic exposure on the mitogen induced splenic cytokine profile. Splenocytes (0.1 × 106/200 µl/well) isolated from mice treated with arsenic (0.00, 0.038, 0.38 and 3.8 ppm) orally for 7, 15 and 30 days were incubated in 5% CO2 incubator at 37 °C for 72 h in the presence or absence of ConA (2.5 µg/ml). Levels of (a) Th1 (IFN- γ, IL-12 and TNF-α) and (b) Th2 (IL-4, IL-5 and IL-10) related cytokines in splenocytes culture supernatant was estimated; plated in triplicate from pooled samples of each treatment group. Fluorescence associated with different antibody-coated magnetic beads was read under Bio-Plex MAGPIX multiplex reader. The cytokine levels are expressed as percent of control. Values are mean ± S.E. (n = 3 per group). *p < 0.05, **p < 0.01 and ***p < 0.001 compared to control.
Effect of arsenic exposure on splenic Treg cell
To investigate the possible reason for arsenic-induced T cell cytokine suppression, splenic Treg cells distribution was studied in 30 days arsenic exposed groups. Interestingly, Treg cell population (CD4+CD25+) was found to be increased from 4.65% to 5.35% in 0.038 ppm exposure group and significantly (p < 0.01) increased to 7.39% and 6.2% following 0.38 and 3.8 ppm of arsenic exposure (Fig. 5a,b). Increased level of Treg cells in spleen was confirmed by measuring transcription factor FoxP3 expression,which controls T cell differentiation into Treg cells27. Significant rise in FoxP3 expression was observed in spleen of 0.38 and 3.8 ppm exposed group (p < 0.001) (Fig. 5c). Level of Treg cell associated cytokine TGF-β in ConA stimulated splenocyte culture supernatant was measured to be significantly higher in 0.38 and 3.8 ppm arsenic exposed groups (p < 0.001) (Fig. 5d).
Effect of arsenic exposure on Treg cell. Splenocytes isolated from mice treated with arsenic (0.00, 0.038, 0.38 and 3.8 ppm) orally for 30 days were processed to isolate CD4+ and CD8+ T cells using CD4 and CD8 specific magnetic particles by positive selection. Purity of splenic T cells was 85 to 90%. Isolated splenic T cells were stained with PE conjugated CD4, PerCP conjugated CD8 and PeCy7 conjugated CD25 antibody for 1 h at 37 °C. PE stained CD4+ T cells were gated for CD25+ cells. Each representative dot plot (a) and bar graph (b) reflects percent population of CD4+CD25+ T cells in total parent T cell population. (c) Representative western blot and densitometric analysis showing level of FoxP3 in spleen dissected from each treatment group. (d) Levels of TGF-β in splenocyte culture supernatants expressed as percent of control. Values are mean ± S.E. (n = 3-5 per group). *p < 0.05, **p < 0.01 and ***p < 0.001 compared to control. The original full-length western blots for FoxP3 and GAPDH are provided in supplementary information sheet in Fig. S9.
Inhibition of Treg cells reversed arsenic-induced cytokine suppression
To confirm the involvement of Treg cells in T cell cytokine suppression upon arsenic exposure, we inhibited Treg population by in-vivo wortmannin treatment. We found that arsenic-induced rise in splenic Treg population from 7.8% to 32.2% was restricted to 12.1% following wortmannin treatment in 30 days arsenic exposed group (p > 0.05). However in wortmanin alone treated group, Treg population decreased to 6.1% as compare to control group (p > 0.05) (Fig. 6A,B). Viability of splenocytes from different exposure groups was found to be unaltered (data not shown). Treg inhibition by wortmanin was confirmed by examining significantly decreased FoxP3 mRNA expression in arsenic exposed splenic T cells (Fig. 6C). In arsenic exposed mice, inhibition of Treg cells was associated with suppressed TGF-β production from ConA activated spenocytes as compared to control (p < 0.001) and arsenic exposed group (p < 0.001) (Fig. 6D). Inhibition in Treg cells and TGF-β cytokine production in arsenic exposed group resulted in significantly high secretion of IFN-γ and IL-4 cytokine compared to control and only arsenic treated group (p < 0.001) (Fig. 6E).
Effect of wortmannin on arsenic-induced altered splenic cytokine profile. Splenocytes isolated from mice treated with DMSO as vehicle, 0.38 ppm arsenic, 0.38 ppm arsenic along with wortmannin and only wortmannin group were processed to isolate CD4+ T cells using CD4 magnetic particles by positive selection. Isolated CD4+ T cells were stained with PE conjugated CD4 and PeCy7 conjugated CD25 antibody for 1 h at 37 °C. Each representative dot plot (A) and Bar graph (B) reflects percent population of CD4+CD25+ T cells in total parent CD4 population. (C) FoxP3 transcript level in isolated CD4+ T cells. (D) Levels of TGF-β, (E) IFN-γ and IL-4 in splenocyte culture supernatant expressed as percent of control. Values are mean ± S.E. (n = 3–5 per group). *p < 0.05, **p < 0.01 and ***p < 0.001 compared to control. Ctrl; Control; DMSO; Dimethyl sulfoxide; As; 0.38 ppm arsenic; WM; Wortmannin.
Effect of arsenic exposure on pathogen clearance
To validate arsenic - induced immune suppression, we challenged 30 day 0.38 ppm arsenic exposed mice with M. fortuitum. Bacterial load in kidney was persistently higher following 3, 7, 15 and 21 DPI as compared to arsenic unexposed group. In arsenic exposed group, CFU increased from 4.92 Log10 at 1 DPI to 6.05 Log10 after 15 DPI and found to be higher than arsenic unexposed group which was 5.15 Log10 at 15 DPI (p < 0.05). Following 21 DPI, CFU in arsenic exposed group reduced to 5.79 Log10 which was significantly higher than arsenic unexposed group with 4.8 Log10 (p < 0.01) CFU (Fig. 7a). Rise in bacterial load also reflected morphologically in the form of prominent and large white patches of M. fortuitum infection on the kidney of arsenic exposed group following 15 DPI as compared to light and small patches on the kidney of arsenic unexposed group (Fig. 7b).
Effect of arsenic exposure on pathogen clearance. Mice exposed to 0.38 ppm arsenic for 30 days were infected with 107 of pathogen and sacrificed following 3, 7, 15 and 21 days post infection (DPI). Dissected kidney from infected mice were homogenized and plated for colony forming unit (CFU) estimation. (a) The number of M. fortuitum CFU in kidney represented as mean ± S.E of Log10 (n = 3 per group). (b) Representative photomicrographs of visible M. fortuitum patches on kidney following 15DPI. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to M. fortuitum group.
Effect of arsenic exposure on general parameters
Body weight : Exposure of mice to arsenic resulted in no significant changes in body weight of mice with time. In the control group receiving water alone, body weight increased from 29.3 ± 1.3 to 33.9 ± 1.7 g. All the arsenic exposed groups showed similar changes in body weight with time as in control (Fig. 8a). Lymphocyte viability: Following 72 h ex-vivo culture of thymocytes and splenocytes, no significant changes in viability was observed in any of the arsenic exposure group compared to control group (Fig. 8b,c). Liver and kidney function: Serum level of AST, ALT (liver function) and Urea (Kidney function) were estimated. No significant alteration was observed between control and different exposure group following 30 days (Fig. 8d,e). Thymic and splenic histopathology: Exposure to arsenic was not found to affect histo-architecture of thymus and spleen by any dose of arsenic upon 30 days of exposure. No major difference in the histoarchitecture was observed between control and exposed group (Fig. 8f,g).
Effect of arsenic exposure on general parameters. Following arsenic exposure mice body weight was recorded weekly. (a) Line graph represents the changes in body weight. Values presented as mean ± S.E at each time point (n = 5–8 per group). (b) Thymocytes and (c) splenocytes (0.1 × 106 cells/200 µl/well) isolated from mice treated with arsenic (0.00, 0.038, 0.38 and 3.8 ppm) orally for 7, 15 and 30 days were cultured at 37 °C in 5% CO2 incubator and viability was checked following 72 h incubation using Presto blue reagent. Fluorescence was measured at 544 (ex.) and 610 (em.) expressed as percent of control (n = 3–5 per group). Serum level of (d) AST, ALT and (e) urea expressed as mean ± S.E. (n = 3 per group). Representative micrographs of histo-architecture of (f) thymus and (g) spleen obtained at 10x magnification (scale bar = 60 µm) from control and 30 day exposed groups.
Discussion
The present study was carried out in male mouse model to avoid gender specific difference in immune response28. However, the use of mouse model has always been questioned for human relevance. Since mice evolved in a quite different environment to humans and have been exposed to different antigens and their immune systems might therefore be expected to have evolved in subtly different ways but the basic development and differentiation process of immune cells is similar29. Although they have fine differences but exhibit profound functional similarities in immune response to xenobiotics thereby making it a good model system to study immune response which can be judiciously extrapolated for human concern20,21,22.
In the present study we aimed to check whether low level arsenic exposure can alter the differentiation of thymocytes into CD4/CD830. We also intended to study whether alteration in T cell differentiation has any role in arsenic - induced immunosuppression, which is yet to be explained clearly. Alteration in thymocyte differentiation may affect the balance in the ratio of different subset of T cells in the peripheral system and thereby can influence the immune response31. Therefore, arsenic associated immunotoxic effect is of important concern. To our knowledge, this is the first report demonstrating the effect of arsenic exposure on CD4/CD8 differentiation in thymus. Although few studies reported xenobiotic-induced alteration in thymic lymphocyte composition resulted from death of thymocytes32, 33, but the present study showed that arsenic altered thymocyte differentiation without affecting its viability or histoanatomy of thymus.
Arsenic exposure promoted CD4 lineage differentiation without any major alteration in CD8 population. High influx of DN cells replenished the pool of DP population in thymus following enhanced differentiation towards CD4+ cells. Changes observed in percent distribution of thymocyte subsets following 7 days of arsenic exposure were not visible after 15 days of exposure. Continuous arsenic exposure rules out the possible involvement of arsenic deprivation for the observed recovery in 15 days arsenic exposed group. Therefore, some active process, which we are not sure about, may be involved in bringing back the regular thymocyte distribution. However, the homeostatic regulation was disturbed again following 30 days of arsenic exposure resulting in a significantly high production of CD4+ cells and high influx of DN cells in thymus in 3.8 ppm exposed group (Fig. 1).
This was further confirmed by examining the expression pattern of CD4 and CD8 lineage specific transcription factors ThPOK and RunX3 respectively34. Higher expression of ThPOK following 3.8 ppm arsenic at 7 and 30 day of exposure supported the increased differentiation into CD4+ population in thymus. In contrary, the unaltered expression of RunX3 supported the unaffected CD8 population (Fig. 2). These observations highlighted that the process of thymocyte differentiation into CD4+ or CD8+ in thymus is very sensitive to arsenic.
Once DP cells differentiated into either CD4+ or CD8+ cells they reach secondary lymphoid organs for further maturation process. Therefore, percent distribution of CD4+ and CD8+ cells were analyzed in spleen following arsenic exposure. Higher CD4+ population was reflected in spleen following 7 and 30 days of arsenic exposure (Fig. 3). However, low CD4+ population in splenic lymphocytes was reported following 30 days exposure of 1 ppm arsenic to male CD57BL6N mice21. Therefore, the differential response of CD4+ cells may be due to either dose difference or species specificity21, 35, 36.
Arsenic has been shown to affect Th1 associated cytokines in arsenic exposed human lymphocytes1, 37. In our study, 7 days exposure to low dose of arsenic (0.038 and 0.38 ppm) promoted Th2 related cytokines in general which might reflect asthma and allergic diseases like symptoms38. In contrast, higher dose of arsenic (3.8 ppm) induced Th1 response which might contribute in the induction of autoimmune diseases like symptoms39. Following 15 days of arsenic exposure, cytokine production exhibited a decreasing pattern compared to 7 days exposed group except IL-12. Since percent distribution of CD4+ cells was not altered following 15 day arsenic exposure therefore, reflecting a time and dose dependent functional suppression of T cells40. Longer exposure to arsenic for 30 days however enhanced CD4+ population in spleen but interestingly altered their functional status which has been reflected in drastically reduced secretion of Th1 and Th2 related cytokines from ConA-activated splenocytes (Fig. 4). This finding is consistent with previous studies showing suppressed production of IFN-γ, IL-4, IL-5, IL-10 and TNF-α in arsenic exposed human population as well as in mice model15, 19, 21, 41. Reduced secretion of cytokines may result in inefficient immune response against pathogen which may enhance the possibility of opportunistic infections. Previous studies have also reported high risk for development of respiratory tract infections8, 42, chronic lung diseases27 in arsenic exposed population. Therefore, elucidating the mechanism of immunosuppression may help intervening the arsenic-induced disease susceptibility. However, defective T cell receptor signaling cascade in response to arsenic exposure may lead to suppressed cytokine production21 but the involvement of Treg cells in the suppression of cytokine production cannot be ignored24, 25.
In the present study, we found that 30 day exposure to arsenic (0.38 ppm and 3.8 ppm) increased number of splenic Treg cells expressed as CD4+CD25+ cells and FoxP3 expression (Fig. 5a–c). Similar increase in Treg cells following arsenic exposure in - vitro 43 as well as higher expression of Treg differentiation supporting IL-2RB gene in arsenic exposed lymphocytes had been observed23, 44. Unlike Th1/Th2 related cytokines, exposure to arsenic enhanced TGF-β secretion from activated splenocytes (Fig. 5d) which stimulates Treg cell differentiation by promoting FoxP3 expression45 and simultaneously inhibits other cytokine production46.
Therefore, most likely the suppressive effect of arsenic is associated with the formation of high Treg cells and consequent higher level of TGF-β secretion. Involvement of Treg cells in the event of cytokine suppression was confirmed by inhibiting Treg production in mice by targeting PI3K-AKT pathway using wortmannin47. Since 30 days exposure to 0.38 ppm arsenic increased Treg population significantly hence we selected this group for Treg reversal study. Interestingly, wortmannin treatment significantly brought down the arsenic-induced increased Treg population, FoxP3 mRNA expression in splenic T cells and TGF-β production from splenocytes. Inhibition of Treg cells led to enhanced production of typical Th1 cytokine IFN-γ & Th2 cytokine IL-4 in arsenic exposed splenocytes culture supernatant. These results confirmed the participation of Treg cells in suppressing cytokine secretion following arsenic exposure (Fig. 6). These findings made us to conclude that environmentally relevant concentration of arsenic induced immunosuppression by increasing Treg cells in mice.
Arsenic-induced immunosuppression may promote infectious diseases like tuberculosis10. In our study, the immunosuppression was validated using M. fortuitum. It is a non tubercular opportunistic pathogen, but may become infectious under immune compromised state. Growth kinetics and spread of M. fortuitum in mouse model is well studied following tail vein injection48 and it is well accepted as an alternative model pathogen and injection through tail vein is a regular process in experimental scenario. Once it reaches blood it will spread and infect an immune compromised subject49, 50. In the present study, increased bacterial load reflected high persistence of pathogen due to inefficient clearance from kidney (Fig. 7)51. Longer survival of pathogen can be due to impaired macrophage function as reflected by suppressed TNF-α and IL-12 production from arsenic exposed splenocytes as shown in (Fig. 3)52, 53. Ineffective macrophage induction for TNF-α and IL-12 production can be resulted from low level of IFN-γ secretion from T cells following arsenic exposure54. These results confirmed that suppressed cytokine production from splenocytes following arsenic exposure induced immuosuppression which enhanced the prevalence of opportunistic infections7, 55 and corroborate the evidences of rising tuberculosis rate with high arsenic exposure levels in Chile10.
To rule out the possibility of involvement of other factors in the event of arsenic-induced immunosuppression alteration in the body weight, thymic and splenic viability, histoarchitecture as well as liver and kidney function were tested following arsenic exposure. None of the parameters were found to be altered significantly compared to control group, thereby confirming the noninvolvement of other physiological parameters (Fig. 8).
In conclusion, the present study clearly showed that exposure to environmentally relevant doses of arsenic disturb thymocyte differentiation as reflected by enhanced commitment into CD4 lineage in thymus. It has also been clearly demonstrated that arsenic exposure affected T cell cytokine secretion by up regulating Treg population thereby induced immunosuppression (Fig. 9). Since mycobacterium is known to exploit Treg cell mediated immune suppression for their survival56 therefore these results also direct us to understand the participation of Treg cells in supporting mycobacterium survival and increasing the risk of mycobacterail disease in arsenic exposed individuals10.
Schematic representation of arsenic-induced Treg cells mediated immnosuppression. Exposure to arsenic promoted commitment of DP cells into CD4+ T cells in thymus. Simultaneously arsenic enhanced differentiation of splenic CD4+ T cells into Treg cells. Increased Treg cells secreted high amount of TGF-β which subsequently inhibited Th1 and Th2 related cytokines production thereby leading to immunosuppression.
Methods
Reagents and antibodies
Cell culture and real time PCR reagents were purchased from thermo fisher scientific. Fluorochrome tagged antibodies for flowcytometry and T cell isolation kit were procured from BD Bioscience. Antibodies and reagents for cytokine multiplex assay were from millipore. Sodium arsenite (NaAsO2) and other general laboratory reagensts were procured from Sigma Aldrich.
Animals and arsenic exposure
Four to five weeks old male Balb/c mice were procured from the CSIR-Indian Institute of Toxicology Research (CSIR-IITR) animal facility. All The protocol for the study was approved by the Institutional Animal Ethics Committee of CSIR-IITR, Lucknow, India, and all experiments have been carried out in accordance with the guidelines laid down by the committee for the purpose of control and supervision of experiments on animals (CPCSEA), Ministry of Environment and Forests (Government of India), New Delhi, India. Mice were housed in ventilated polypropylene cages with food and water supplied ad libitum and acclimatized for one week (25 ± 2 °C room temp with 12 h day light cycle) before arsenic exposure started. Animals were divided into 12 groups containing 15 animals (5 mice in each cage) in each group (0.0, 0.038, 0.380, 3.800 ppm sodium arsenite and three time points: 7, 15, 30 days). Sodium arsenite was dissolved in distilled water and the treatment given daily afternoon through oral gavage (≈100 μl). Among them 0.38 ppm is most frequently found in public water sources in India57 and being used in other studies58. Weights of mice were monitored weekly to examine changes in body weight. Following arsenic exposure regimen animals were euthanized using chloroform.
Wortmanin treatment
Mice were intraperitoneally injected with 40 µg wortmanin diluted in 100 μl PBS from a stock solution (4 mg/ml in DMSO). Wortmannin given every alternate day starting from day 24 upto day 30 of arsenic exposure47.
Thymic and splenic cells preparation
Thymic and splenic single cell suspension was prepared following standard method, resuspended in complete medium (RPMI 1640, 2 mM glutamine, 1% antibiotic-antimycotic solution 10% heat inactivated FBS, 50 µM β-mercaptoethanol, 25 mM HEPES, 25 mM glucose, 1 mM Sodium pyruvate) and enumerated using hemocytometer.
Cell viability assay
Thymocytes and splenocytes were seeded at a density of 0.1 × 106 cells/200 µl/ well in 96 well culture plates and incubated in a 5% CO2 incubator at 37 °C for 72 h. Cell viability was determined using Presto blue reagent (Life Technologies) following manufacturer’s protocol in a microplate reader (FLUOstar Omega, BMG).
Flow cytometric analysis of T lymphocytes
Thymocytes and splenocytes (0.2 × 106) were stained in 200 µl PBS with PE-conjugated CD4 and PerCP-conjugated CD8 monoclonal antibodies. Splenic T cells were isolated by using BD IMag anti-mouse CD4+ and CD8+ magnetic beads through positive selection under magnetic field. Isolated splenic T cells stained with PEcy7 conjugated CD25 monoclonal antibody to detect Treg cells. Purity of isolated CD4+ and CD8+ cells was found to be 85–90%. For Treg inhibition study, we isolated only CD4+ T cells using anti-CD4-coated magnetic beads. All the samples were run on BD-FACSCANTO II flowcytometer. Data of ten thousand events were collected and analysed using BD-FACSDiva software.
Real time PCR
Total RNA was extracted from isolated splenic CD4+ T cells with TRIzol reagent followed by cDNA preparation using high capacity cDNA reverse transcription kit. Quantitative real time PCR (RT-PCR) was performed on the QuantStudio 6 Flex instrument with SYBR Green for FoxP3 gene. Hypoxanthine-guanine phosphor ribosyl transferase (HPRT) was used for normalization.
Immunoblot analysis
Protein was isolated from whole tissue lysate of thymus and spleen following standard protocol and estimated using Bradford reagent (Bio-Rad). Protein samples (100 µg) were mixed with loading buffer (5x), ran on 10% SDS-PAGE, transferred to PVDF membrane (Pall Corporation, USA) and probed overnight with Anti-ZFP67 and Anti-RUNX3 rabbit polyclonal antibody (Sigma), FoxP3 mouse monoclonal antibody (Merck-Millipore, USA) at 4 °C and developed with HRP-conjugated goat anti-rabbit and anti-mouse IgG (Santa Cruz Biotech, CA, USA) using chemi-luminescence substrate (Merck-Millipore, USA) under ImageQuant LAS 500 system (GE Healthcare, USA). The densitometric analysis was done using Image-J. Band intensities were normalized with GAPDH band intensity. Primary antibodies were used in 1:2000 and secondary antibodies were used in 1:3000 dilution.
Cytokine assay
Splenocytes were seeded at a density of 0.1 × 106 cells/200 µl/ well in 96 well culture plates and incubated in the absence or presence of concanavalin A (ConA) (2.5 µg/ml; Sigma) in a 5% CO2 incubator at 37 °C for 72 h. Culture supernatants were collected and pooled from each treatment group, and secreted cytokines were measured in supernatant plated in triplicate using a Milliplex mouse cytokine assay kit as previously described59 in Bio-Plex MAGPIX multiplex reader (Bio-Rad). Fluorescence associated with different antibody-coated magnetic beads was read to quantitate respective cytokines. The Th1 and Th2 related cytokines measured were Interferon-γ (IFNγ), IL12, Tumor necrosis factor-α (TNFα), IL4, IL5, IL10 and Tumor growth factor- β (TGF β).
M. fortuitum infection for resistance assay
Following 30 day of exposure to 0.38 ppm arsenic, mice were injected via tail vein with 107 bacteria per 100 µl of PBS. After 1,3,7,15 and 21 days post infection (DPI) (Silva et al. 2010) kidney was aseptically removed and homogenized in normal saline with 0.1% tween 20. Bacterial load in kidney was determined by counting colony forming unit (CFU) on OADC (oleic acid, albumin, dextrose, catalase; Difco) supplemented Middlebrook 7H10 agar (Difco) plates. Colonies were counted after 3 days of incubation at 37 °C. All the microbiological works were done at CSIR-Central Drug Research Institute, India.
Histopathology
Thymus and spleen from control and 30 day arsenic treated mice were isolated from heparinized-PBS perfused mice and fixed in 4% paraformaldehyde for 48 h at 4 °C. Paraformaldehyde-fixed tissues were dehydrated through graded alcohol, embedded in paraffin and routine microtomy carried out to obtain 5 µm thick sections. The sections were stained with hematoxylin and eosin followed by microscopic examination.
Biochemical analysis of serum
Blood samples collected through cardiac puncture were allowed to clot at room temperature for about 60 minutes. Samples were centrifuged at 2000 g for 15 minutes at 4 °C and serum collected in micro-centrifuge tube. Levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and urea in serum were measured in an Automated Biochemical Clinical Auto Analyzer (ChemWell, USA) using kit from SPINREACT (Spain).
Statistical analysis
Statistical analysis was done by GraphPad Prism (GraphPad Software Inc., San Diego, CA) using One way analysis of variance (ANOVA) followed by Newman-Keuls test with 95% confidence intervals and p value < 0.05 was considered significant.
References
Wu, M. M., Chiou, H. Y., Ho, I. C., Chen, C. J. & Lee, T. C. Gene expression of inflammatory molecules in circulating lymphocytes from arsenic-exposed human subjects. Environmental health perspectives 111, 1429–1438 (2003).
Naujokas, M. F. et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environmental Health Perspectives (Online) 121, 295 (2013).
Schmidt, C. W. Low-dose arsenic: in search of a risk threshold. Environmental health perspectives 122, A130–A134 (2014).
Lemarie, A., Morzadec, C., Bourdonnay, E., Fardel, O. & Vernhet, L. Human macrophages constitute targets for immunotoxic inorganic arsenic. The Journal of Immunology 177, 3019–3027 (2006).
Nayak, A. S., Lage, C. R. & Kim, C. H. Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio). Toxicological sciences 98, 118–124 (2007).
Kozul, C. D. et al. Chronic Exposure to Arsenic in the Drinking Water Alters the Expression of Immune Response Genes in Mouse Lung. Environmental Health Perspectives 117, 1108–1115 (2009).
Ramsey, K. A., Foong, R. E., Sly, P. D., Larcombe, A. N. & Zosky, G. R. Early life arsenic exposure and acute and long-term responses to influenza A infection in mice. Environmental Health Perspectives 121, 1187–1193 (2013).
Rahman, A., Vahter, M., Ekstrom, E. C. & Persson, L. A. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environmental Health Perspectives 119, 719–724 (2011).
Martin-Chouly, C. et al. Inorganic arsenic alters expression of immune and stress response genes in activated primary human T lymphocytes. Molecular Immunology 48, 956–965 (2011).
Smith, A. H. et al. Evidence from Chile that arsenic in drinking water may increase mortality from pulmonary tuberculosis. American Journal of Epidemiology 173, 414–420 (2010).
Sohel, N. et al. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology 20, 824–830 (2009).
Raqib, R. et al. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicology Letters 185, 197–202 (2009).
Vega, L., Montes de Oca, P. V., Saavedra, R. & Ostrosky-Wegman, P. Helper T cell subpopulations from women are more susceptible to the toxic effect of sodium arsenite in vitro. Toxicology 199, 121–128 (2004).
Tenorio, E. P. & Saavedra, R. Differential effect of sodium arsenite during the activation of human CD4+ and CD8+ T lymphocytes. International Immunopharmacology 5, 1853–1869 (2005).
Soto-Pena, G. A. et al. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. The FASEB journal 20, 779–781 (2006).
Morzadec, C., Bouezzedine, F., Macoch, M., Fardel, O. & Vernhet, L. Inorganic arsenic impairs proliferation and cytokine expression in human primary T lymphocytes. Toxicology 300, 46–56 (2012).
Morzadec, C. et al. Inorganic arsenic represses interleukin-17A expression in human activated Th17 lymphocytes. Toxicology and Applied Pharmacology 262, 217–222 (2012).
Burchiel, S. W. et al. Differential susceptibility of human peripheral blood T cells to suppression by environmental levels of sodium arsenite and monomethylarsonous acid. PLoS One 9, e109192 (2014).
Biswas, R. et al. Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Human & Experimental Toxicology 27, 381–386 (2008).
Sikorski, E. E., McCay, J. A., White, K. L., Bradley, S. G. & Munson, A. E. Immunotoxicity of the Semiconductor Gallium Arsenide in Female B6C3F1 Mice. Toxicological Sciences 13, 843–858 (1989).
Soto-Pena, G. A. & Vega, L. Arsenic interferes with the signaling transduction pathway of T cell receptor activation by increasing basal and induced phosphorylation of Lck and Fyn in spleen cells. Toxicology and Applied Pharmacology 230, 216–226 (2008).
Cho, Y. et al. Age-related effects of sodium arsenite on splenocyte proliferation and Th1/Th2 cytokine production. Archives of Pharmacal Research 35, 375–382 (2012).
Andrew, A. S. et al. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a US population. Environmental Health Perspectives 116, 524–531 (2008).
Rubtsov, Y. P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558 (2008).
Shin, D. S. et al. Regulatory T cells suppress CD4+ T cells through NFAT‐dependent transcriptional mechanisms. EMBO reports 15, 991–999 (2014).
Luckey, M. A. et al. The transcription factor ThPOK suppresses Runx3 and imposes CD4+ lineage fate by inducing the SOCS suppressors of cytokine signaling. Nature Immunology 15, 638–645 (2008).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nature Reviews Immunology 16, 626–638 (2016).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. The Journal of Immunology 172, 2731–2738 (2004).
Drela, N. Xenobiotic-induced alterations in thymocyte development. APMIS 114, 399–419 (2006).
Lu, W. et al. CD4: CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. Journal of the International AIDS Society 18, 20052 (2015).
Pieters, R. et al. Selective inhibition of immature CD4-CD8+ thymocyte proliferation, but not differentiation, by the thymus atrophy-inducing compound di-n-butyltin dichloride. Immunology 81, 261–267 (1994).
Pathak, N., Mitra, S. & Khandelwal, S. Cadmium Induces Thymocyte Apoptosis via Caspase‐Dependent and Caspase‐Independent Pathways. Journal of biochemical and molecular toxicology 27, 193–203 (2013).
Egawa, T., Tillman, R. E., Naoe, Y., Taniuchi, I. & Littman, D. R. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. The Journal of experimental medicine 204, 1945–1957 (2007).
Becker, S. M. & McCoy, K. L. Gallium Arsenide Selectively Up-Regulates Inflammatory Cytokine Expression at Exposure Site. Journal of Pharmacology and Experimental Therapeutics 307, 1045–1053 (2003).
Dangleben, N. L., Skibola, C. F. & Smith, M. T. Arsenic immunotoxicity: a review. Environmental Health 12, 1 (2013).
Engström, K. et al. Transcriptomics and methylomics of CD4-positive T cells in arsenic-exposed women. Archives of toxicology 91, 2067–2078 (2017).
Singh, V. K., Mehrotra, S. & Agarwal, S. S. The paradigm of Th1 and Th2 cytokines. Immunologic Research 20, 147–161 (1999).
Druet, P., Sheela, R. & Pelletier, L. Th1 and Th2 cells in autoimmunity. Clinical and Experimental Immunology 101, 9–12 (1995).
Ferrario, D., Gribaldo, L. & Hartung, T. Arsenic Exposure and Immunotoxicity: a Review Including the Possible Influence of Age and Sex. Current environmental health reports 3, 1–12 (2016).
Ahmed, S. et al. Arsenic exposure and cell-mediated immunity in pre-school children in rural Bangladesh. Toxicological sciences 141, 166–175 (2014).
Farzan, S. F. et al. Infant infections and respiratory symptoms in relation to in utero arsenic exposure in a US cohort. Environmental Health Perspectives (Online) 124, 840–847 (2016).
Smith, A. H. et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environmental health perspectives 114, 1293–1296 (2006).
Hernandez-Castro, B. et al. Effect of arsenic on regulatory T cells. Journal of clinical immunology 29, 461–469 (2009).
Burchill, M. A., Yang, J., Vogtenhuber, C., Blazar, B. R. & Farrar, M. A. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. The Journal of Immunology 178, 280–290 (2007).
Chen, W. et al. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. The Journal of experimental medicine 198, 1875–1886 (2003).
Yoshimura, A. & Muto, G. TGF-β function in immune suppression. Current Topics in Microbiology and Immunology 350, 127–147 (2010).
Abu-Eid, R. et al. Selective inhibition of regulatory T cells by targeting the PI3K–Akt pathway. Cancer immunology research 2, 1080–1089 (2014).
Parti, R. P., Srivastava, S., Gachhui, R., Srivastava, K. K. & Srivastava, R. Murine infection model for Mycobacterium fortuitum. Microbes and infection 7, 349–355 (2005).
Rodriguez-Coste, M. A., Chirca, I., Steed, L. L. & Salgado, C. D. Epidemiology of Rapidly Growing Mycobacteria Bloodstream Infections. The American journal of the medical sciences 351, 253–258 (2016).
El Helou, G., Viola, G. M., Hachem, R., Han, X. Y. & Raad, I. I. Rapidly growing mycobacterial bloodstream infections. The Lancet Infectious Diseases 13, 166–174 (2013).
Simonet, M., Berche, P., Fauchere, J. & Veron, M. Impaired resistance to Listeria monocytogenes in mice chronically exposed to cadmium. Immunology 53, 155 (1984).
Flynn, J. et al. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. The Journal of Immunology 155, 2515–2524 (1995).
Cavalcanti, Y. V. N., Brelaz, M. C. A., Neves, J. Kd. A. L., Ferraz, J. C. & Pereira, V. R. A. Role of TNF-alpha, IFN-gamma, and IL-10 in the development of pulmonary tuberculosis. Pulmonary medicine 2012, 745483 (2012).
Jouanguy, E. et al. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Current opinion in immunology 11, 346–351 (1999).
Kozul, C. D., Ely, K. H., Enelow, R. I. & Hamilton, J. W. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environmental Health Perspectives 117, 1441–1447 (2009).
Kursar, M. et al. Cutting Edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. The Journal of Immunology 178, 2661–2665 (2007).
Jadhav, S. H., Sarkar, S. N., Ram, G. C. & Tripathi, H. C. Immunosuppressive Effect of Subchronic Exposure to a Mixture of Eight Heavy Metals, Found as Groundwater Contaminants in Different Areas of India, Through Drinking Water in Male Rats. Arch. Environ. Contam. Toxicol. 53, 450–458 (2007).
Rai, A., Maurya, S. K., Khare, P., Shrivastava, A. & Bandyopadhyay, S. Characterization of developmental neurotoxicity of As, Cd and Pb mixture: synergistic action of metal mixture in glial and neuronal functions. Toxicological sciences 118, 586–601 (2010).
Singh, V., Mitra, S., Sharma, A. K., Gera, R. & Ghosh, D. Isolation and characterization of microglia from adult mouse brain: selected applications for ex vivo evaluation of immunotoxicological alterations following in vivo xenobiotic exposure. Chemical Research in Toxicology 27, 895–903 (2014).
Acknowledgements
This work was supported by CSIR network project, INDEPTH (Integrated NextGen Approaches in Health, Disease and Environmental Toxicity) and NanoSHE (Nanomaterials: Applications and Impact on Safety, Health and Environment); R.G. was supported by UGC-Senior Research Fellowship, V.S. was supported by CSIR Senior Research Fellowship. A.K.S. was supported by NanoSHE. CSIR-IITR manuscript number is 3428.
Author information
Authors and Affiliations
Contributions
D.G. and R.G. designed the study and wrote the paper. R.G. and V.S. performed different experiments. S.M. contributed in flow cytometry related experiments. A.K.S. helped in primary culture related experiments. A.S. and A.D. contributed in M. fortuitum related experiments. D.S. and P.J. performed immunohistochemistry of thymus and spleen. M.K. performed biochemical analysis of blood serum. S.P. helped in writing the manuscript. All authors analyzed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gera, R., Singh, V., Mitra, S. et al. Arsenic exposure impels CD4 commitment in thymus and suppress T cell cytokine secretion by increasing regulatory T cells. Sci Rep 7, 7140 (2017). https://doi.org/10.1038/s41598-017-07271-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07271-z
This article is cited by
-
Co-administration of L-Ascorbic Acid and α-Tocopherol Alleviates Arsenic-Induced Immunotoxicities in the Thymus and Spleen by Dwindling Oxidative Stress-Induced Inflammation
Biological Trace Element Research (2024)
-
Exposure of Arsenic Associated with Cellular Turnover and Apoptosis Profile in the Bone Marrow of Mice Including Stem/Progenitor Population
Proceedings of the Zoological Society (2024)
-
Arsenic exposure to mouse visceral leishmaniasis model through their drinking water linked to the disease exacerbation via modulation in host protective immunity: a preclinical study
Scientific Reports (2023)
-
Formaldehyde exposure induces differentiation of regulatory T cells via the NFAT-mediated T cell receptor signalling pathway in Yucatan minipigs
Scientific Reports (2022)
-
High efficiency of magnetite nanoparticles for the arsenic removal from an aqueous solution and natural water taken from Tambo River in Peru
Journal of Environmental Health Science and Engineering (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.